Abstract
In metazoans, bone morphogenetic proteins (BMPs) direct a myriad of developmental and adult homeostatic events through their heterotetrameric type I and type II receptor complexes. We examined 3 existing and 12 newly generated mutations in the Drosophila type I receptor gene, saxophone (sax), the ortholog of the human Activin Receptor-Like Kinase1 and -2 (ALK1/ACVRL1 and ALK2/ACVR1) genes. Our genetic analyses identified two distinct classes of sax alleles. The first class consists of homozygous viable gain-of-function (GOF) alleles that exhibit (1) synthetic lethality in combination with mutations in BMP pathway components, and (2) significant maternal effect lethality that can be rescued by an increased dosage of the BMP encoding gene, dpp+. In contrast, the second class consists of alleles that are recessive lethal and do not exhibit lethality in combination with mutations in other BMP pathway components. The alleles in this second class are clearly loss-of-function (LOF) with both complete and partial loss-of-function mutations represented. We find that one allele in the second class of recessive lethals exhibits dominant-negative behavior, albeit distinct from the GOF activity of the first class of viable alleles. On the basis of the fact that the first class of viable alleles can be reverted to lethality and on our ability to independently generate recessive lethal sax mutations, our analysis demonstrates that sax is an essential gene. Consistent with this conclusion, we find that a normal sax transcript is produced by saxP, a viable allele previously reported to be null, and that this allele can be reverted to lethality. Interestingly, we determine that two mutations in the first class of sax alleles show the same amino acid substitutions as mutations in the human receptors ALK1/ACVRl-1 and ACVR1/ALK2, responsible for cases of hereditary hemorrhagic telangiectasia type 2 (HHT2) and fibrodysplasia ossificans progressiva (FOP), respectively. Finally, the data presented here identify different functional requirements for the Sax receptor, support the proposal that Sax participates in a heteromeric receptor complex, and provide a mechanistic framework for future investigations into disease states that arise from defects in BMP/TGF-β signaling.
THE type I serine-threonine receptors of the transforming growth factor-β (TGF-β)/bone morphogenetic protein (BMP) signaling pathway are critical for the transduction and specificity of signals initiated by the secreted ligands of this superfamily. A dimeric ligand elicits a vast range of biological responses by binding to the extracellular domain of a heterotetrameric receptor complex comprising two type I and two type II serine/threonine kinase receptors that then transduce a signal by phosphorylation of intracellular transcriptional regulators (reviewed by Yamashita et al. 1994; Weis-Garcia and Massague 1996; Kirsch et al. 2000a,b; Shi and Massague 2003; ten Dijke and Hill 2004). Both receptor types have a cysteine-rich, ligand-binding extracellular domain, a single transmembrane domain, an intracellaular kinase domain, and associated regulatory domains. Additionally, type I receptors contain a glycine-serine repeat (GS) domain, which is required for full kinase activation (Franzen et al. 1995). When complexed, the constitutive kinase activity of the type II receptor transphosphorylates the type I GS domain, activating the type I receptor, which binds and phosphorylates primarily receptor-mediated Smad proteins (R-Smad) (Carcamo et al. 1994; Wrana et al. 1994; Chen et al. 1998b; Macias-Silva et al. 1998; Chen and Massague 1999). Mutations in the human type I receptors ALK1/ACVRL1, ALK2/ACVR1, ALK3/BMPR1A,ALK4/ACVR1B, ALK5/TGFβR1, and ALK6/BMPR1B have been identified and in each case are associated with a disease and/or developmental syndrome with very specific manifestations, e.g., hereditary hemorrhagic telangiectasia type 2 (HHT2) (ALK1/ACVRL1), fibrodysplasia ossificans progressiva (FOP) (ALK2/ACVR1), juvenile polyposis syndrome (ALK3/BMPR1A), pancreatic adenocarcinoma (ALK4/ACVR1B), Loeys-Dietz syndrome (ALK5/TGFβR1), and brachydactyly type A2 (ALK6/BMPR1B) (Howe et al. 2001; Su et al. 2001; Zhou et al. 2001; Kim et al. 2003; Loeys et al. 2005; Lehmann et al. 2003, 2006; Abdalla and Letarte 2006; Bayrak-Toydemir et al. 2006; Shore et al. 2006; Wehner et al. 2006; Olivieri et al. 2007). In general, the specific effects of each mutation are not well understood in terms of signal transduction and functional consequences. In this report, we describe the identification of new mutations and the functional characterization of two distinct classes of saxophone (sax) alleles in the Drosophila melanogaster ortholog of human ALK1/ACVRL1 and ALK2/ACVR1 type I receptors.
In addition to saxophone (sax), two other Drosophila type I receptors are encoded by the thick veins (tkv) and baboon (babo) genes (Childs et al. 1993; Brummel et al. 1994, 1999; Nellen et al. 1994; Penton et al. 1994; Xie et al. 1994). Like the ligands, the Drosophila type I receptors have both overlapping and distinct domains of expression and functions that are essential to the development of a variety of tissues and organs (Schupbach and Wieschaus 1989; Affolter et al. 1994; Brummel et al. 1994, 1999; Nellen et al. 1994; Penton et al. 1994; Terracol and Lengyel 1994; Xie et al. 1994; Bangi and Wharton 2006b). Tkv and Sax are both essential mediators of BMP signaling, where the loss of tkv results in a complete loss of the phosphorylated form of the BMP-specific R-Smad protein, Mad (pMad), while loss of sax leads to reduced pMad levels (Singer et al. 1997; Dorfman and Shilo 2001; Bangi and Wharton 2006b).
The Drosophila orthologs of vertebrate BMP2/4 and BMP5/6/7/8 ligands are encoded by decapentaplegic (dpp) and glass bottom boat (gbb), respectively (Padgett et al. 1987; Wharton et al. 1991; Doctor et al. 1992). A third more divergent Drosophila BMP encoded by screw (scw), (Arora et al. 1994) appears to be specifically required for embryonic dorsal/ventral patterning (reviewed by O'connor et al. 2006). Tkv acts as a high affinity Dpp receptor on the basis of data from genetic and biochemical studies while Sax exhibits a higher affinity for Scw and Gbb (Haerry et al. 1998). In two well-characterized developmental processes, Tkv and Sax receptors have been shown to mediate signaling from the Drosophila BMPs, Dpp and Scw, in embryonic dorsal/ventral patterning, and Dpp and Gbb in the generation of a BMP activity gradient required for patterning the wing imaginal disc (Brummel et al. 1994; Nellen et al. 1994; Burke and Basler 1996; Zecca et al. 1996; Singer et al. 1997; Chen et al. 1998a; Khalsa et al. 1998; Neul and Ferguson 1998; Nguyen et al. 1998; Ray and Wharton 2001; O'connor et al. 2006; Bangi and Wharton 2006a,b).
Several other sax loss-of-function studies indicate that Sax also contributes to patterning the anterior eggshell, as well as to the maintenance of germline stem cell divisions (Schupbach and Wieschaus 1989; Xie et al. 1994; Twombly et al. 1996; Xie and Spradling 1998). The contribution of Sax to overall BMP signaling appears to be more complex than originally thought as indicated by its ability to play both a positive and a negative role in signaling (Bangi and Wharton 2006b). The ability of Sax to mediate signaling requires the presence of Tkv and thus, the molecular basis of Sax's dual role has been proposed to depend on the combination of type I receptors that make up the signaling complex, whereby Tkv–Sax complexes promote signaling while Sax–Sax complexes bind ligand but are unable to transduce a signal.
Differential signaling output dependent on type I receptor composition has also been suggested for ALK1 and ALK5 (Goumans et al. 2003; Finnson et al. 2008). In most contexts, human TGF-β signals through the ALK5 receptor; however, in endothelial cells and chondrocytes ALK1 is also involved in the transduction of TGF-β signals (Chen and Massague 1999; Oh et al. 2000; Goumans et al. 2003; Finnson et al. 2008). In response to TGFβ1, ALK5 is essential for ALK1 kinase activation and the phosphorylation of Smad1/5, while ALK5 alone acts in Smad3 phosphorylation. ALK1 acts to inhibit TGFβ1-induced Smad3 phosphorylation and thus, the presence of different levels of ALK1 and ALK5 in both endothelial cells and chondrocytes will likely have a dramatic influence on overall signaling output.
Prior to the work reported here, a limited number of sax mutations were available for the study of Drosophila BMP receptor function. The original sax alleles, sax1 and sax2, were identified as maternal effect lethal (MEL) mutations, in which the maternal genotype is the overriding factor in determining the mutant phenotype of the progeny (Brummel et al. 1994; Nellen et al. 1994; Penton et al. 1994; Xie et al. 1994). To gain a better understanding of the range of in vivo functions of sax, we undertook genetic screens to isolate new mutant alleles. Here, we report the generation of such sax alleles, their characterization and their interactions with other BMP signaling pathway elements. We show that the preexisting alleles, sax1 and sax2, as well as saxP, are mutations that behave in a manner distinct from deficiencies that lack the sax coding region. In fact, the MEL of these alleles can be reverted to recessive lethal alleles and thus, we classify sax1, sax2, and saxP alleles as gain-of-function (GOF) alleles indicating that the product produced by these alleles has a function beyond that of the wild-type protein. In contrast to the behavior of these GOF alleles, the new sax mutations are partial or complete loss-of-function (LOF) on the basis of their similarity to deficiencies in a number of assays. Our studies provide insight into the functional consequences of different types of receptor mutations and will aid in understanding the molecular mechanisms by which Sax-like type I receptors contribute to signaling and the effects of specific lesions associated with human disease.
MATERIALS AND METHODS
Fly strains and culture conditions:
All strains used in this study are referenced in FlyBase (2003) and cultured by standard methods. Canton-S and y1 Df(1)67c23 strains represent wild type. All dpphr strains are described previously in (Wharton et al. 1993, 1996). Dp(dpp+) refers to Dp(2;2)DTD48, dppdho, or Dp(2;2)VT1, dpp+ (also designated Dp(2;2)B16), which are both tetrasomic for dpp+, and Madnull, refers to Df(2L)JS17. The sax1 mutation is no longer homozygous viable, presumably due to a secondary, unassociated lethal mutation on the same chromosome. Df(2R)P32 (43A3-43F6) and Df(2R)H23 (43C1-43F2) both lack sax function (Brummel et al. 1994; Nellen et al. 1994; Xie et al. 1994) while Df(2R)ST1 (42B3-43E18) retains sax function. The inducible sax+ rescue construct, P[hs-sax], contains a sax-RA cDNA with the potential to encode both Sax-PA and Sax-PB (Brummel et al. 1994).
Genetic and phenotypic studies:
Maternal sax1 and sax2 assays:
For the lethal phase: Females of a given sax genotype were separately crossed to males of indicated genotypes (Figure 1, Tables 2, and 3). Resulting embryos were harvested 8–12 hr after egg lay (AEL) and transferred to a grid on standard fly culture media. Embryonic lethality and viability was assessed at 12, 24, and 36 hr AEL and larval viability at 10–15 days AEL. Adult eclosion was assessed for up to 18 days AEL. Embryonic lethal phenotypes were examined as Hoyer's mounted embryonic/first instar larval cuticle preparations and as live embryos under halocarbon oil.
Figure 1.—
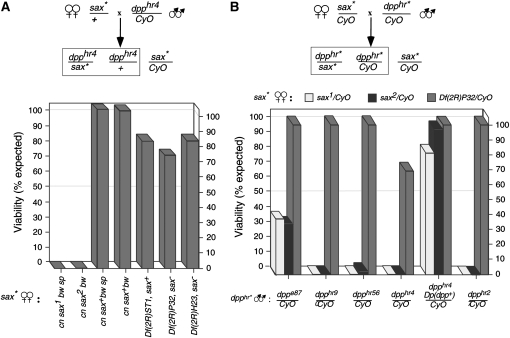
sax1 and sax2 maternal enhancement of dpp mutant progeny. Females heterozygous for the maternal effect mutations, sax1 or sax2, dominantly interact with dpphr alleles. Percentage of survivorship of dpphr/+ adults (boxed genotypes) is shown on the y-axis. (A) Females heterozygous for sax1 or sax2, the parental chromosomes (cn bw sp and cn bw) or deficiencies that retain (Df(2R)ST1) or lack (Df(2R)P32, Df(2R)H23) sax function were tested for maternal enhancement of dpphr4/+ lethality. The number of progeny examined was ≥577. (B) Survivorship of different dpphr alleles when crossed with females heterozygous for a given sax mutation is shown as the percentage of expected dpphr heterozygous progeny. The female genotypes are indicated by different bar shading. dpp alleles are listed along the x-axis. The number of progeny examined from each cross was ≥200.
TABLE 2.
Summary of new sax alleles
| Allele | Mutagen | Screen selection | Cytology |
|---|---|---|---|
| Extant | |||
| sax1 | EMS | Recessive female sterile (MEL)a | Normal |
| sax2 | EMS | Recessive female sterile (MEL)a | Normal |
| saxP | P element | 2nd chromosome lethalb | ND |
| New | |||
| sax1rv1 | γ-ray | Reversion of sax1 DME | Normal |
| sax1rv2 | γ-ray | Reversion of sax1 MEL | Dp(2;1)43C-F; 46B3-14;20 |
| sax1rv5 | EMS | Reversion of Mad sax1 MEL | Normal |
| Df(2R)sax-H9 | Hobo | Df(2R)P32 lethal | Df(2R)43F1-2 |
| Df(2R)sax-H30 | Hobo | Df(2R)P32 lethal | Df(2R)43F1-2 |
| sax3 | EMS | Df(2R)H23 lethal | Normal |
| sax4 | EMS | Df(2R)H23 lethal | Normal |
| sax5 | EMS | Df(2R)H23 lethal | Normal |
| sax6 | EMS | Df(2R)H23 lethal | Normal |
| saxPE5 | P excision | Df(2R)sax-H9 lethal | ND |
| saxPE7 | P excision | Df(2R)sax-H9 lethal | ND |
| saxPE10 | P excision | Df(2R)sax-H9 lethal | ND |
MEL, maternal effect lethality; DME, dominant maternal enhancement (of dpp mutant phenotypes).
Isolated by Schupbach and Weischaus (1989).
Isolated by Török et al. (1993) and this allele, and its homozygous viable phenotypes are described in detail by Nellen et al. (1994).
TABLE 3.
Molecular lesions associated with sax alleles
| Allele | Nucleotide change | Codon change | saxn/Df(sax) phenotype |
|---|---|---|---|
| Newly described alleles | |||
| sax3 | CAG→TAG | Gln 121 stop | Lethal* |
| sax4 | CAA→TAA | Gln 114 stop | Lethal |
| sax5 | GCC→GAC | Ala 289 Asp | Lethal |
| sax6 | CGC→CAG | Arg 541 His | Lethal |
| saxPb | P[lacW] insertion | After aa 34 of Sax-PA | Viable |
| Previously described alleles | |||
| sax1a | ACC→ATC | Thr 434 Ile | Viable |
| sax2a | GGA→GAA | Gly 412 Glu | Viable |
saxn, new sax allele; *sax3/Df(sax) exhibits weak (0.6–2%) viability.
Isolated by Schupbach and Weischaus (1989).
Isolated by Török et al. 1993; previously described in Nellen et al. 1994 as a P-element insertion after the first 36 amino acid residues of the SAX protein.
Maternal loss-of-function assays:
Genotypes and crosses were performed by standard methods as described in Twombly et al. (1996). Briefly, germline clones were induced by heat shock of late third instar larvae and early pupae of the genotype P{hs-FLP}/Y; P{>w+>} P{ovoD1=18}32X9/P{>w+>} sax5 sha1. Several hundred such females possibly containing sax5 sha1 homozygous germline clones were mated to appropriate tester males and progeny were processed as described above.
Zygotic loss-of-function studies:
Heterozygous saxA/+ females were mated to males heterozygous for a second allele (saxB/SM6a) and the progeny were counted. All crosses were tested in two assays: (1) lethal phase and (2) zygotic lethal phenotype. Lethal phase was treated as above. For lethal phenotypes, sax3, sax4, sax5, and sax6 were mated to Df(2R)H23 males. All strains utilized CyO, P{ry[+t7.2]=en1}wg[en11], permitting the unambiguous identification of hemizigous larvae. Fifty larvae of each hemizygous genotype were examined for morphological abnormalities. Larval and pupal samples in 50% glycerol were documented with bright field and Nomarsky photomicroscopy.
Amnioserosa cell counts:
Progeny from sax mutant females were crossed to Canton-S males and the resulting embryos were harvested from a 4- to 7-hr egg lay and aged 9 hrs before fixation. Anti-Krüppel antibody labeling was performed as described previously in Wharton et al. (1993). In triplicate, 20 embryos were scored for each genotype. For each 20, the four high and four low counts were removed, then the mean was determined. This was necessary to uncover statistically different means, in an assay that has considerable variation.
Genetic interactions studies:
For maternal interactions with dpp, females of the relevant sax* genotype were mated to heterozygous dpphr males. Cultures were neither overcrowded nor sparse. All progeny were scored through day 18. The percentage of survivorship of dpphr was calculated as {[(sax*/ dpphr + dpphr/CyO) ÷ 2] ÷ (sax*/CyO)} × 100. Control crosses, performed identically, reversed the sex of the genotypes. For dominant maternal Mad–sax interactions, the lethality of the progeny from Mad* sax/Mad+ sax* females were determined as described for lethal phase studies. The zygotic phenotypes of Df(2L)JS17 sax1/dppd12 adults were scored by examination of each leg of the adult for the presence of tarsal claws. For zygotic interactions with dpp in the wing, dpphr56/SM6a females were crossed to dppd5*/SM6a males, where * is sax1, sax4, sax5 or Df(2R)H23. Wings of dpp mutant progeny were mounted in DPX mountant (EM Sciences) and scored for patterning defects in the longitudional veins. Images were collected on a Nikon FXA with a SPOT-RT camera (Diagnostic Instruments).
sax complementation and rescue:
Percentage of survivorship was calculated as ([(saxA/saxB) ÷ [(saxA/SM6a + saxB/SM6a)÷2]) × 100. Crosses were performed in both directions. The viability of sax3 hemizygotes utilized sax deficiencies and sax1 revertants. All rescue experiments were performed as per Brummel et al. (1994). Percentage of rescue equals {sax*/Df(2R)sax ÷ [(sax*/SM6a + Df(2R)sax/SM6a) ÷ 2]}×100.
Genetic screens for sax alleles:
sax1 reversion screen:
G0 irradiated (4000 rads, Cs137) cn1 sax1 bw1 sp/SM6a males were mated to ncoSco cn1 sax2 bw1/SM6a females. Cross A: 1362 [5 G1 sax1*/ncoSco sax2 females] were mated to wild-type males to test for a reduction in the expected 100% MEL. Cross B: 1920 [5 G1 sax1*/SM6a females] were crossed to dpphr4 sp/SM6a males to test for the loss of maternal enhancement of dpp mutations [scored for presence of Cy+ (dpphr4/sax1 *) progeny]. In the absence of a sax1 revertant, few or no progeny resulted from cross A, while a candidate sax1* reversion was indicated by the presence of ≥15 progeny. The presence of a sax1* reversion in cross B led to Cy+ (dpphr4/sax1*) progeny.
Hobo element mutagenesis screen:
The homozygous viable, fertile enhancer trap H{Lw2}SW283 (Smith et al. 1993) was mobilized using the Hobo transposase source, CyO-P{HBL1}2 (Calvi and Gelbart 1994). H{Lw2}SW283 complements all sax mutations. Twenty-four thousand G1 progeny were scored for changes in w+ eye color, resulting in 214 independent mobilizations that were tested for zygotic lethality and MEL in trans to Df(2R)P32 and sax1rv1, respectively. Two independently derived zygotic lethal 2nd chromosome mutations were recovered, which retained the original Hobo and a new insertion with an adjacent deletion (data not shown). These lines were further mobilized and no reversion was observed.
Mad-sax1 reversion screen:
A double mutant, Df(2L)JS17 cn1 sax1, chromosome (referred to as Df(Mad)sax1) was generated and balanced over a CyO containing a dpp+ rescue transgene (Wharton et al. 1993), which zygotically rescues the embryonic lethality of this strain. Male Df(2L)JS17 cn1 sax1/CyO23 flies were EMS treated (Lewis and Bacher 1968) and crossed to wild-type females. Resulting G1 Df(2L)JS17 cn1 sax1/+ + females were tested for fertility. In the absence of a reversion event, few adult progeny were observed, while a revertant resulted in a modest number of progeny (≥15).
F2 lethal screen:
Isogenized nub1 b1 pr1 males were EMS treated and crossed to a balancer strain. Individual F1 males were crossed to tester sax1rv1 and Df(2R)sax-H9 females. A test sax rescue cross was performed at 25°, which exhibits 10–15% rescue (Brummel et al. 1994). Lethal mutations that could be rescued were assayed for viability over Df(2R)H23 and Df(2R)ST1.
saxP excision screen:
The P-element insertion was mobilized using CyO,P[HBL1]2. Female y Df(1)67c23; In(2LR)Gla/CyO,P[HBL1]2 were mated to Df(1)67c23; saxP/SM6a males and the F1 Cy, Gla+ male progeny were recovered. These y Df(1)67c23; saxP/CyO,P[HBL1]2 males were mated to Df(1)67c23; In(2LR)Gla/SM6a females in individual culture vials and Cy, Gla+, w− male progeny were recovered and stocked by crosses to Df(1)67c23; In(2LR)Gla/SM6a females. Of 50 F1 males crossed, 42 w− excision lines (saxPE) were recovered and tested for lethality in trans to the sax4, sax5, and Df(2R)H23 chromosomes.
Molecular analysis and DNA sequencing:
Genomic DNA was isolated from larvae hemizygous (sax*/Df(2R)H23) for sax mutations, sax3, sax4, sax5, and sax6, according to standard procedures (Wharton et al. 1996). Regions of the sax gene were amplified by PCR and sequenced using the dsDNA cycle sequencing system (GIBCO BRL). PCR-induced sequence changes were eliminated by sequencing two or more independent PCR amplifications. The entire coding sequence of all the alleles was determined. The primer pairs used were (5′ to 3′) TAGGCTCGGACAAATAAC and CATTAGCTATGGACAGGC3 or TGATGACGCACTACTATC and GTCTTGTACTTGGATTAG.
For RT–PCR, RNA was isolated from yw1118 and yw; FRTG13 saxP homozygous third instar larvae (n = 10) using QIAGEN RNeasy and treated with Promega RQ1 DNase before cDNA synthesis. cDNA was synthesized using MLV RTase (Sigma) and oligo (dT)12-18 primer (Invitrogen). RT–PCR analysis was conducted using the following primers: (sax 9396 fwd) GCTGTGCCGGTGATTA CTG and (sax 11119 rev) GTCTTGTACTTGGATTAG; (P{lacW} 9661 fwd) GGATCTTC TTGAGATCC and (P{lacW}10648 fwd) GGATGTCTCTTGCCGACGGG; (8103) CGTTTCTGCTGTACAATAATGCCAG and (10228) GCCCATTAGCTATGGACAGGC.
The sax5 mutation was generated in a sax-RA cDNA clone (gift from Mike O'Connor) by primer extension using PfuUltra DNA Polymerase (Stratagene) with the following primer GGCGAAAGCATCGACGTGAAGATAT.
Cell-based BMP signaling assay:
A cell-based BMP signaling assay using S2 cells has been described previously (Muller et al. 2003; Bangi and Wharton 2006b) and depends on the endogenous expression of BMP signaling components. In this assay, a reporter construct expressing lacZ is controlled by a Su(H) transcriptional activation response element as well as a brk transcriptional silencer element (Su(H)/brk-lacZ). Transcription is activated by cotransfection of the reporter construct with plasmids encoding Su(H) and an activated form of Notch (N*). Activation of BMP signaling leads to repression of lacZ expression due to the presence of the brk silencer element, and thus, a reduction in β-galactosidase activity. BMP signaling levels are inversely correlated with the level of β-galactosidase activity. Plasmids containing the coding sequences of tkv (pAcpA-tkv1-FLAG), sax (pAcpA-sax-FLAG), or sax5 (pAW-sax5) were cotransfected with Su(H), N*, Su(H)/brk-lacZ, and luciferase plasmids, all under the control of the actin 5C promoter using Effectene Transfection (QIAGEN). β-Galactosidase values were measured using the dual luciferase assay system (Dual-Light, Applied Biosystems) and normalized to luciferase for each sample.
RESULTS
Maternal contribution of sax1 or sax2 affect BMP signaling:
The saxophone (sax) gene was originally identified by genetic screens for mutations resulting in recessive MEL (Schupbach and Wieschaus 1989). When crossed to wild-type males, females homozygous for a sax mutation, sax1 or sax2 (sax1/sax1, sax2/sax2, or sax1/sax2), produce phenotypically normal eggs that die as embryos (Table 1, compare crosses F, H and G, O). Cuticle preparations indicate that these lethal embryos exhibit a weakly ventralized phenotype, reminiscent of weak embryonic lethal dpp alleles (Schupbach and Wieschaus 1989; Wharton et al. 1993; Brummel et al. 1994). The loss of BMP-dependent dorsal patterning is also supported by the observed reduction in the number of Kr-positive amnioserosa cells (the dorsal most embryonic fate) in embryos laid by sax1/sax2 mutant mothers (Table 1).
TABLE 1.
Maternal effect lethality of sax1 and sax2 mutations
| % lethality
|
Kr+ AS cells
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cross | Female genotype | Male genotype | Embryonic | Larval | Pupal | Total | n | No.b |
| A | Df(2R)ST1/+ | +/+ | 11.3 | 5.1 | 4.4 | 20.9 | 273 | |
| B | Df(2R)H23/+ | +/+ | 1.5 | 4.1 | 0.7 | 6.4 | 266 | |
| C | sax1/+ | +/+ | 0 | 0.7 | 1.7 | 2.4 | 293 | |
| D | sax2/+ | +/+ | 1.6 | 3.5 | 0.8 | 5.9 | 254 | |
| E | sax1/+ | sax2/+ | 1 | 0.6 | 1.3 | 2.9 | 521 | |
| F | +/+ | sax1/sax2 | 1 | 2.7 | 0.3 | 4.1 | 293 | |
| G | +/+ | sax2/sax2 | 0.7 | 1.7 | 0.7 | 3.2 | 286 | |
| H | sax1/sax2a | +/+ | 100 | 0 | 0 | 100 | 971 | 28 ± 5 |
| I | sax1/sax2 | Dp(dpp+)/Dp(dpp+) | 37.7 | 45.8 | 6 | 89.5 | 284 | |
| J | sax1/Df(2R)ST1 | +/+ | 4.4 | 2.9 | 1.4 | 8.8 | 274 | |
| K | sax1/Df(2R)ST1 | Dp(dpp+)/Dp(dpp+) | 10.6 | 7.6 | 1 | 19.2 | 198 | |
| L | sax1/Df(2R)H23 | +/+ | 85.2 | 12.4 | 0.3 | 97.9 | 290 | |
| M | sax1/Df(2R)H23 | Dp(dpp+)/Dp(dpp+) | 8.2 | 4.9 | 4.1 | 17.2 | 245 | |
| N | sax1/Df(2R)P32 | +/+ | 91.7 | 7.2 | 0.7 | 99.7 | 279 | 42 ± 4 |
| O | sax2/sax2 | +/+ | 81.7 | 14.3 | 0.8 | 96.8 | 252 | |
| P | sax2/sax2 | Dp(dpp+)/Dp(dpp+) | 9.7 | 8.9 | 3.1 | 21.8 | 257 | |
| Q | sax2/Df(2R)ST1 | +/+ | 2.2 | 11.9 | 4.1 | 18.4 | 267 | |
| R | sax2/Df(2R)ST1 | Dp(dpp+)/Dp(dpp+) | 11 | 10.5 | 2.9 | 24.4 | 209 | |
| S | sax2/Df(2R)H23 | +/+ | 41.7 | 37.1 | 6.7 | 85.5 | 283 | |
| T | sax2/Df(2R)H23 | Dp(dpp+)/Dp(dpp+) | 5 | 5 | 0 | 10 | 238 | |
| U | sax2/Df(2R)P32 | +/+ | 64.8 | 27.7 | 3.7 | 96.1 | 267 | 69 ± 5 |
Boldface genotypes and lethality values highlight the distinct behavior of sax*/sax* or sax*/sax* vs. sax*/Df(2R)H23 or sax*/Df(2R)H23 females. Underlined values indicate the failure of Df(2R)ST1 to uncover the sax maternal effect lethality.
sax1/sax1 homozygotes are not shown due to the presence of a second site, unrelated, lethal on the chromosome, acquired since the isolatio of sax1.
Compared to 164 ± 23 Kr+ aminoserosa (AS) cells present in wild-type embryos (Raftery et al. 1995).
MEL of sax1 and sax2 rescued by dpp+:
We find that this MEL induced by sax1 or sax2 can be significantly rescued if the fathers possess two extra copies of the wild-type dpp gene (Table 1, crosses I and P), such that all progeny carry three copies of dpp+. This rescue of MEL suggests that an increase in zygotic dpp+ copy number leads to an increase in ligand level and a subsequent increase in BMP signaling that compensates for a loss of maternal sax function.
Interestingly, we discovered that when homozygous, sax1 and sax2 mothers induce a higher percentage of embryonic lethality than when either allele is in trans to a sax deficiency (i.e., sax1/Df(2R)P32 or sax1/Df(2R)H23) (Table 1, crosses L, N, S, U). This finding raised the possibility that sax1 and sax2 are GOF alleles and not standard null, LOF alleles. Both sax1 and sax2 are missense mutations that alter conserved amino acid residues within the kinase domain of the Sax receptor (Brummel et al. 1994), possibly producing a defective Sax receptor protein that is in some way detrimental to normal BMP signaling in the early embryo. The MEL induced by hemizygous sax1 or sax2 is also rescued by dpp duplications (Table 1, crosses M and T), again indicating that the lethality induced by aberrant sax function in the mother can be compensated for by increased BMP signaling. The higher embryonic lethality produced by homozygous sax1 or sax2 mutant mothers could reflect the fact that BMP signaling in the early embryo is particularly sensitive to the absolute level of a defective Sax protein.
Mutant sax maternal contribution reduces dpp function:
We further investigated the possibility that sax1 and sax2 negatively impact BMP signaling by examining the ability of these alleles to enhance dpp haplolethality (Brummel et al. 1994; Nellen et al. 1994; Penton et al. 1994; Xie et al. 1994). dpphr4/+ males crossed to +/+ females result in viable heterozygous dpphr4/+ progeny (Figure 1A). However, when dpphr4/+ males are crossed to sax1/+ or sax2/+ females, all the resulting dpphr4/+ progeny die. Importantly, deficiencies known to lack the sax coding sequences show little maternal enhancement of dpphr4/+ lethality, as is also true of deficiencies that do not uncover sax function (Df(2R)ST1) (Figure 1) (Brummel et al. 1994; Nellen et al. 1994; Nicholls and Gelbart 1998). To test if this effect was specific to dpphr4, we examined a series of dpphr alleles, dppe87, dpphr56, dpph90, dpphr4, and dpphr27, which represents decreasing Dpp activity (respectively) and lesions in three different essential regions of the gene (Wharton et al. 1993, 1996). We found that both sax1/+ and sax2/+ females are strong maternal enhancers of all dpphr mutations tested. We also found that this maternal enhancement of dpp haplolethality by sax1 or sax2 is rescued by a dpp duplication, suggesting that an increase in dpp levels can overcome the lethality caused by defective maternal sax function (Figure 1A).
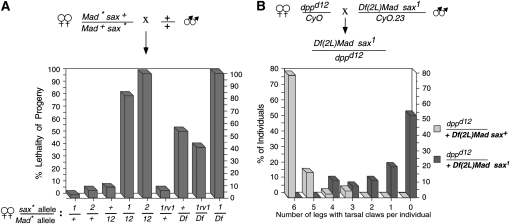
Dominant Mad-sax genetic interaction dominant interactions have been observed between sax1 and sax2 and mutations in Mothers against dpp (Mad) and Medea (Med), two genes encoding the R-Smad and co-Smad intracellular mediators of BMP signaling, respectively (reviewed by Raftery and Sutherland 2003) (Figure 1B). Strong MEL is observed when double transheterozygous females Df(Mad) sax+/Mad+sax1, Mad12 sax+/Mad+sax1 or Mad12 sax+/Mad+sax2 are crossed to wild-type males (Figure 2A). In addition to this dominant interaction between Mad-sax in the mother, we also observed a zygotic interaction, at later stages of development, in the adult appendages (Figure 2B). The presence or absence of the most distal element of the adult leg, the tarsal claw, is easily quantified and represents a sensitive assay for dpp function, whose highest activity is required in the distal most elements of adult appendages (Spencer et al. 1982). In dppd12Mad+sax+/dpp+ Df(Mad) sax+ transheterozygotes, 78% of the individuals have a full complement of legs (6) with tarsal claws and the remaining having at least three legs with tarsal claws. By introducing the sax1 mutation into this genotype, 53% of the dppd12Mad+sax+/dpp+Df(Mad) sax1 adults lack all tarsal claws and none (0%) have tarsal claws on all six legs. A similar effect is seen with defects in wing venation (data not shown). Taken together, our data from the MEL studies, the maternal enhancement of dpp mutant genotypes, and the dominant interaction between Mad and sax1 and sax2 alleles, indicate that sax1 and sax2 produce more severe phenotypes than sax deficiencies, consistent with being GOF alleles. Furthermore, the GOF activity of sax1 and sax2 appears to impact the output of Dpp/BMP signaling at multiple stages of development.
Figure 2.—
Dominant Mad–sax interactions. (A) Mad–sax MEL. The percentage of lethality of progeny from females heterozygous for various Mad and sax alleles crossed to wild-type males is depicted by bars. Transheterozygous (Mad +/+ sax) females show significant maternal effect lethality. Female genotypes are listed on the x-axis. n ≥ 300 embryos scored for control crosses and n ≥ 475 embryos for experimental crosses. (B) Mad–sax zygotic enhancement of the dppd12 disk mutation. The number of legs per individual with tarsal claws was quantified in progeny from dppd12/+ females crossed to males bearing a Mad− sax− double mutant chromosome (Df(2L)JS17 sax1/CyO23) (dark bars) or only Mad− (light bars). A lowering of both Mad and sax dosage results in an enhancement of tarsal claw loss associated with a reduction in dpp function.
Genetic screens for new sax alleles:
Given that sax1 and sax2 exhibit GOF activity, we reasoned that it should be possible to revert this behavior such that any potential revertants would behave similarly to deficiencies of sax. We carried out five different genetic screens for (1) revertants of sax1 or sax2 or for (2) the isolation of new sax alleles (Table 2; supporting information, Figure S1; materials and methods). Here, we briefly describe our rationale for each screen and the results. For the screens aimed at identifying sax revertants, we used the observation that sax mutant mothers exhibit 100% MEL (no progeny survive from a cross between sax1/sax2 females and +/+ males), while a cross between Df(sax)/sax2 females and +/+ males results in ∼30–50% survivorship of the embryonic progeny. Similarly, heterozygous sax1/+ and sax2/+ females give rise to no viable dpphr4/+ adults, while Df(sax)/+ females give rise to 85% viable dpphr4/+ adults.
sax1 γ-ray reversion:
In an F2 sax1 γ-ray reversion screen, we simultaneously screened for revertants of sax1/sax2 MEL (cross A) and revertants of the sax1/+ enhancement of dpphr/+ (cross B) (Figure S1, A). A total of 16,410 mutagenized haploid genomes were screened; 6810 by cross A and 9600 by cross B. One revertant from each cross was recovered and designated sax1rv2 and sax1rv1. Both revertants are lethal in trans to sax deficiencies and genetically behave like a sax deficiency. For instance, sax1rv1/sax2 females exhibit MEL comparable to Df(2R)H23/sax2 females. The identification of sax1rv1 and sax1rv2 demonstrate that sax1 is indeed a GOF allele and this function can be eliminated.
Hobo Mobilization Screen:
Bolstered by the recovery of sax1 revertants that were lethal, we chose to perform an insertional mutagenesis using a white+ marked, homozygous viable, Hobo element insertion adjacent to sax (43E10-43E15) (H[J21.31,w+]) as the mutagen (Figure S1, B) (Smith et al. 1993). We expected that the element would either transpose locally or cause a local genomic aberration that may affect the sax coding region. Two strains, Df(2R)sax-H9 and Df(2R)sax-H30, that exhibited lethality over tester chromosomes were identified and each was shown to result in a deletion of the 43F1-2 polytene interval (data not shown).
Maternal Df(Mad)/sax1 reversion screen:
Success in the previous screens led to a third assay based upon the dominant interactions observed between sax1 and sax2 and Mad (Figure 2). While strong MEL is observed when double transheterozygous females, i.e., Df(Mad) sax+/Mad+sax1, are crossed to wild-type males (Figure 2A), we found that females bearing one of the new sax revertants, Df(Mad) sax+/Mad+sax1rv1, exhibit only 40% MEL instead of >90% by Df(Mad) sax+/Mad+sax1 females. Thus, we reasoned that a screen designed to recover mutations that revert the sax GOF interaction with Mad mutants could also result in LOF sax alleles (Figure S1, C). Of ∼8000 mutagenized genomes screened, one revertant was recovered, sax1rv5 and is lethal in trans to sax deficiencies.
F2 lethal screen:
The three screens described above recovered five sax alleles that were lethal. These screens were unbiased in their identification of lethal alleles because they were designed to either revert the maternal effects of the original sax1 mutation or to identify a Hobo mobilization. Nonetheless, we asked whether lethal sax alleles could be recovered independently of a reversion assay and performed a standard F2 lethal screen for mutations in the 43E18-F2 region. Lethal mutations were then tested for rescue by a sax transgene (P{hs-sax}) when in trans to a sax deficiency Df(2R)sax-H9/SM6a). By this method, 5610 mutagenized genomes were screened and four new lethal alleles were recovered, sax3, sax4, sax5, and sax6.
saxP excision screen:
Finally, a P-element excision screen was performed using the saxP allele previously reported to be a placW insertion in sax (Nellen et al. 1994). Forty-two independent white− excisions were selected (saxPE) and 12 were shown to be lethal in trans to three different LOF sax alleles (saxPE/sax4, saxPE/sax5, and saxPE/Df(2R)H23). Three lines saxPE5, saxPE7, and saxPE10 were retained for further examination (Table 2).
Characterization of new sax alleles:
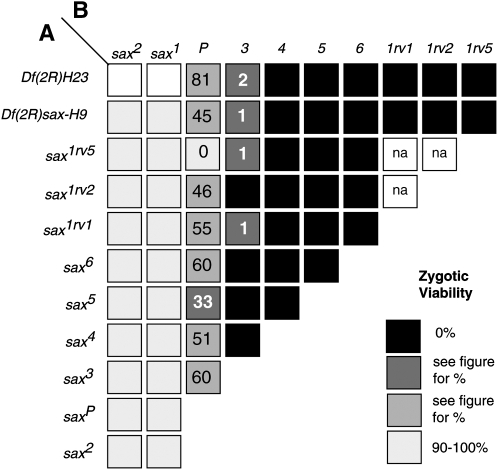
From five genetic screens, a total of 12 new sax alleles were generated (Table 2). All new alleles were first characterized by complementation analysis and all failed to complement Df(2R)H23 (Figure 3). Although lethality was not used as a criteria to select for mutations in two of the screens, lethal sax alleles were still identified and proved to be lethal over other alleles isolated from subsequent screens aimed at specifically selecting lethal alleles, each done in different genetic backgrounds. On the basis of viability studies and the failure to complement a deficiency, two general classes of sax alleles emerged.
Figure 3.—
Interallelic complementation between sax alleles. Females (A) and males (B) heterozygous for the listed sax alleles were crossed and the lethality of sax*A/sax*B progeny was quantified. Shading of boxes indicates the percentage of progeny exhibiting zygotic viability of each allelic combination (key on right). The percentage of viability was calculated as the number of Cy+, divided by the number in the Cy parental class that survived least well (n ≥ 300 adults scored for each cross).
One class consisting of sax1, sax2, and saxP shows little or no lethality when in trans to Df(2R)H23. sax1 and sax2 are not only 100% viable in trans to sax deficiencies but also display 100% viability over all new alleles (termed sax* here) (Figure 3). Similarly, saxP/Df(sax) and saxP/sax* exhibit robust viability. The second class of alleles, consisting of sax3, sax4, sax5, sax6, sax1rv1, sax1rv2, sax1rv5, all saxPE alleles, Df(2R)sax-H9, and Df(2R)sax-H30 show complete lethality in trans to sax deficiencies (sax*/Df(sax)), as well as to one another [with the exception of sax3, which shows a very small percentage (1–2%) of escapers] (Figure 3). To test whether the lethality of the new alleles is in fact due to a loss of sax function, we assayed for the ability of an inducible sax+ transgene (P[hs-sax]) to rescue the lethality associated with these alleles. Eight of 12 new sax alleles were rescued in trans to Df(2R)sax-H9 and in trans to Df(2R)H23 (Table S1). sax1rv2, saxPE7, Df(2R)sax-H9, and Df(2R)sax-H30 failed to be rescued. The failure of these alleles to be rescued reflects the fact that the lesion associated with each of these mutations most likely disrupts more than just the sax locus. This is clearly evident from the cytology of sax1rv2, Df(2R)sax-H9, and Df(2R)sax-H30 (Table 2). Furthermore, Southern blot analysis of saxPE7 indicates that genomic sequences beyond the sax locus have been deleted (data not shown). The rescue of the remaining eight alleles by the sax+ transgene indicates that the lethality associated with these mutations must result from a reduction in sax function.
Molecular lesions associated with new sax alleles:
The molecular lesions associated with sax3, sax4, sax5, and sax6 were identified as single nucleotide changes within the sax coding region (Table 3), indicating that these new alleles specifically affect sax function. The sax3 and sax4 mutations both result in a premature stop codon in the extracellular domain of the Sax protein, while sax5 and sax6 are missense mutations altering highly conserved residues in different regions of the cytoplasmic kinase domain. The sax5 A289D mutation affects a region of the molecule critical for ATP binding. Specific molecular lesions associated with sax1rv1 and sax1rv5 were not identified and while it was determined that saxPE5 and saxPE10 exhibit abnormal restriction patterns 3′ to the saxP insertion site (data not shown), the exact boundaries of the lesions were not determined.
Lethal sax alleles fail to maternally enhance dpp phenotypes:
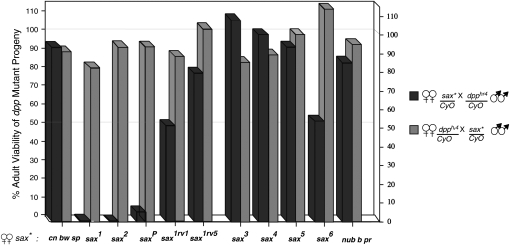
saxP/+ shows a strong maternal enhancement of dpphr4/+ lethality as discussed above for sax1 and sax2 (Figures 1 and 4). saxP/+ females also enhance dpphr56, dpphr90, and dppe87 heteozygotes resulting in 26, 41, and 55% viability, respectively. In contrast, the majority of alleles in the second class of sax mutations (the lethal alleles) show no specific maternal enhancement of dpphr/+ lethality. Of this class, only sax1rv1 and sax6 show a moderate maternal enhancement. Given the importance of dpp in BMP signaling in the embryo, the mutations giving rise to sax1, sax2, and saxP must clearly impact sax function in a manner that more significantly affects this role for dpp in the embryo than the lethal sax alleles do, such that dpphr/+ animals are no longer viable.
Figure 4.—
Maternal enhancement of dpp mutant progeny by all sax alleles. Test crosses between all sax alleles and dpphr4 were performed in both directions, with respect to the genotypes of the females and males. Crosses in which the females were mutant for sax are represented by the solid bars, and the male sax mutant crosses are represented by shaded bars. The genotypes of tested chromosomes are listed below. cn1 bw1 sp1 is the parental chromosome for sax1, sax1rv1, and sax1rv5. nub1 b1 pr1 is the parental chromosome for sax3, sax4, sax5, and sax6. For female sax mutant crosses, the number of progeny scored per cross was ≥550 (except saxP, n = 417). For male sax mutants crosses, the number of progeny scored per cross was ≥345 (except sax2, n = 155; saxPE4, n = 269).
As another measure of the effect of the sax1, sax2, and saxP alleles on embryonic sax function, we examined the possibility that these alleles genetically interact with scw mutations. The haplolethality of dpp is associated with its role in embryonic dorsal–ventral patterning and scw is intimately involved in establishing the BMP activity gradient critical for dorsal–ventral patterning (Arora et al. 1994; Neul and Ferguson 1998; Shimmi et al. 2005). We found that sax1, sax2, and saxP alleles exhibit a dominant maternal enhancement of scwE1 and scwE2 mutations (Table S2) and as with their failure to enhance dpp lethality, the lethal sax alleles do not show this maternal enhancement (data not shown). Interestingly, scwE1 and scwE2 are GOF alleles thought to alter dpp activity (Raftery et al. 1995) and while sax1, sax2, and saxP heterozygous mothers generate synthetic lethality when crossed to these alleles, they show no enhancement when crossed to LOF scw alleles (Table S2; crosses A, B, G, H, M). Given the critical role of Dpp:Scw heterodimers in the generation of dorsal/ventral patterning in the embryo, it is likely that the GOF nature of scwE1 and scwE2 reflects their ability to dominantly influence the function of Dpp when heterodimerized with Scw mutant protein, such that a more significant reduction in the effectiveness of BMP signaling is observed.
We also examined the ability of sax mutations to modify a later dpp function by separately scoring the various aspects of the dppd5/dpphr56 resulting wing phenotype in different sax mutant backgrounds in addition to any effects on adult viability (Table 4). A deficiency of sax leads to a very slight suppression of the dppd5/dpphr56 phenotype as expected from our previous work indicating that Sax receptors can inhibit BMP signaling in the Drosophila wing (Bangi and Wharton 2006b). Consistent with the expectation that the sax4 mutation leads to a complete loss of Sax protein, sax4 behaves identically to the sax deficiency and causes a slight suppression of the dppd5/dpphr56 wing phenotype (Table 4). Interestingly, sax5 does not show this same suppression and in fact shows an enhancement of lethality associated with dppd5/dpphr56 not seen when sax dosage is reduced by a deficiency.
TABLE 4.
Zygotic Enhancement of dpp Phenotypes
| % wings showing phenotype
|
Viability
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | n | Intact | L2 gap | L2 intact | L4 gap | L4–L5 reduced | L4–L5 fused | n | % Exp |
| dppd5 +/dpphr56 + | 168 | 59 | 41 | 100 | 0 | 63 | 38 | 517 | 50 |
| dppd5 Df(2R)H23/dpphr56 + | 135 | 65 | 35 | 100 | 0 | 94 | 6 | ND | ND |
| dppd5 sax4/dpphr56 + | 136 | 62 | 38 | 100 | 0 | 88 | 12 | 521 | 55 |
| dppd5 sax5/dpphr56 + | 105 | 47 | 53 | 99 | 1 | 68 | 32 | 227 | 23 |
All crosses have a genotype (dppd5/dpphr56) with reduced dpp signaling which results in a wing tissue that is “sensitized phenotypically” and responds readily to further perturbation of BMP signaling. Boldface percentages are those of the control class, dppd5 Df(2R)H23/dpphr56 sax+. Importantly, note that the sax4 allele closely mimics the results of the deficiency of sax (underlined).
sax5 exhibits dominant negative behavior:
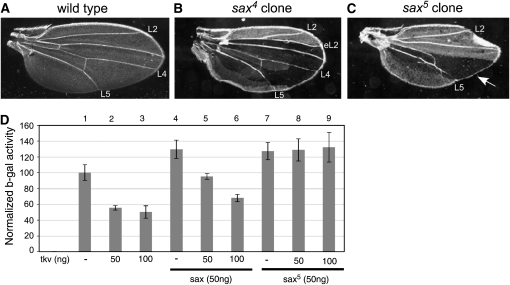
The difference in the ability of sax5 to enhance dpp lethality compared to sax4 or a sax deficiency (Df(2R)H23), prompted us to investigate in more detail the possibility that sax5 may exhibit a mild dominant-negative effect. We had previously examined the role of sax in wing patterning (Bangi and Wharton 2006b) and sought to compare the phenotype associated with a sax4 vs. a sax5 clone in the adult wing. While large posterior clones of sax4 show no wing patterning abnormalities, large clones of sax5 show a significant loss of longitudinal vein 4 (L4) and a narrowing of the L4/L5 intervein, a phenotype associated with a loss of dpp function (Figure 5). This result is consistent with the enhancement of dppd5/dpphr56 lethality by sax5 (Table 4) and supports the conclusion that sax5 is able to negatively impact dpp function.
Figure 5.—
sax5 produces more severe phenotypes than sax4. (A) Dark field image of a wild-type wing. Longitudinal veins 2 (L2), 4 (L4), and 5(L5) are indicated. (B and C) Wings resulting from sax mutant clones as described in Bangi and Wharton (2006b). Clones marked with shv appear dark in images. (B) A sax4 clone encompassing the entire posterior compartment shows no patterning defects. Consistent with previous studies (Singer et al. 1997; Bangi and Wharton 2006b), a small sax4 clone in the anterior compartment leads to an ectopic L2 (eL2) vein. (C) A sax5 clone in the posterior compartment results in the loss of L4 (arrow) and a narrowing of the L4/L5 intervein, a phenotype never seen in an equivalent sax4 clone. The more severe phenotype of sax5 suggests that the presence of a defective Sax receptor is more detrimental to BMP signaling during wing patterning than the complete loss of the Sax receptor. (D) A cell-based BMP signaling assay indicates that the sax5 mutation is able to negatively affect BMP signaling mediated by Tkv. S2 cells were cotransfected with the Su(H)/brk-lacZ reporter construct, Su(H), and N* constructs to stimulate transcription (sample 1), and tkv, and/or sax and sax5 constructs under the control of the actin 5C promoter (samples 2–9). Values depicted are the fold activation of β-galactosidase over the basal activity of the reporter construct alone. All values represent the average of samples measured in triplicate and normalized for transfection efficiency.
We next made use of a cell-based BMP signaling assay to assess the ability of the sax5 mutation to affect BMP signaling. As described previously, lacZ expression in this assay is repressed by BMP signaling in a quantitative manner and thus, β-galactosidase activity is inversely correlated with the level of BMP signaling (Bangi and Wharton 2006b; Muller et al. 2003). As observed previously, Tkv exhibits some degree of signaling when wild-type tkv constructs are transfected into S2 cells alone (samples 2 and 3, Figure 5D), while Sax does not (sample 4, Figure 5D). We have found that S2 cells express gbb (T. Akiyama, unpublished results) and transfection with a wild-type sax construct appears to block endogenous Gbb signaling, likely as a result of ligand being bound by nonsignaling Sax–Sax complexes (Bangi and Wharton 2006b). When cotransfected with tkv, a wild-type sax construct results in the antagonism of signaling in a dose-dependent manner (samples 5 and 6, Figure 5D). In agreement with our genetic analysis of sax5 mutants, cotransfection of tkv with a sax5 construct leads to a complete inhibition of Tkv-mediated BMP signaling (samples 8 and 9, Figure 5D), indicating that the Sax5 protein can completely disrupt successful signaling.
MEL and zygotic lethality induced by lethal sax alleles is not rescued by increased dpp dosage:
We have shown that increasing the dosage of dpp+ can rescue the MEL associated with the GOF sax1 and sax2 alleles. All new sax alleles exhibit significant MEL (Table S3), therefore, we tested for the ability of increased dpp+ dosage to rescue sax3, sax4, sax5, sax6, and saxP, in trans to sax1rv1. As Figure S2 shows, increasing the dosage of dpp+ to three copies in sax*/sax1rv1animals fails to rescue all but saxP and sax3. No rescue of the other new alleles was observed even in the presence of four copies of dpp+ (data not shown). The rescue of saxP and sax3 may indicate that they retain some residual wild-type sax function.
We would predict that the sax3 mutation should generate a truncated Sax protein (121 amino acids of the extracellular domain) that is likely to be unstable and, therefore, should lack all sax function. However as shown in Figure 3, sax3 unexpectedly exhibits a very low percentage of viability (0.6–2%) in trans to Df(2R)H23, sax1rv1, sax1rv5, and Df(2R)sax-H9. Hemizygous adult escapers display wing venation defects (50% penetrance) similar to saxP/Df while females display 100% MEL with severely reduced egg production, averaging 5.7 eggs/female (n = 54 females). In all other assays, sax3 is indistinguishable from sax4, a mutation expected to truncate the Sax protein after amino acid 114. We tested the possibility that a suppressor mutation was induced at a second site on the sax3 chromosome by generating meiotic recombinants proximal and distal to the mutation and finding that a few sax3/Df(2R)sax-H9 escapers are still generated. While this result does not completely eliminate the possibility of a closely linked second site suppressor, an alternative hypothesis is that the sax3 mutation allows altered splicing or translational read through (Cartegni et al. 2002). Regardless of the molecular explanation, our genetic analysis thus far suggests that sax3 retains some very low level of sax function, and significantly less than saxP. The fact that dpp+ duplications can rescue lethality associated with sax3/sax1rv1 mutants indicates that the defect to BMP signaling in sax3/sax1rv1 animals can be compensated for by an increase in ligand levels and signaling, presumably, through another receptor, such as Tkv.
saxP retains significant function:
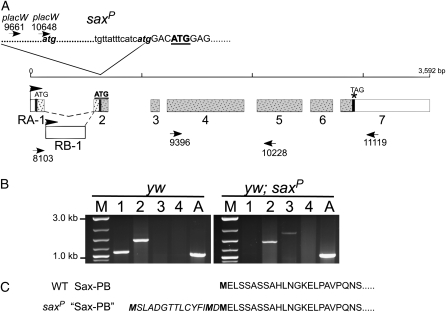
saxP was previously reported to be a null allele, on the basis of the identification of a P-element insertion in the sax locus immediately following codon 36 of the sax PA open reading frame (Nellen et al. 1994). However, given that the saxP allele exhibits traits in common with both sax1 and sax2 (missense mutations that likely produce aberrant proteins), we considered the possibility that the P-element insertion responsible for saxP may not completely disrupt normal sax transcription and may allow for the production of a wild-type protein product or one that is abnormal in some way. We performed RT–PCR on RNA from homozygous saxP animals and detected the clear presence of an mRNA derived from sequences downstream of the proposed placW insertion site (Figure 6B). The sax open reading frame (PA) (Brummel et al. 1994; Nellen et al. 1994) may be generated by one of two possible transcripts from the sax locus, the saxRA transcript (see www.flybase.org). A second transcript, saxRB, can generate a second Sax protein (PB) that initiates at the methionine at codon 36 of the PA open reading frame. We considered the possibility that a transcript from the saxP locus could encode an open reading frame similar to PB. We first determined the orientation of the placW element responsible for saxP by genomic Southerns (data not shown) and then to verify the site of the P-element (placW) insertion, we produced cDNA from saxP homozygotes, generated PCR products, and sequenced the junction between the P3′ end and the sax locus. In contrast to the previous report (Nellen et al. 1994), we found that the placW element is inserted just 5′ to the GAC codon encoding aa 35 of the SaxPA open reading frame and thus, upstream from the ATG encoding methionine aa 36 (underlined in Figure 6A). Thus, the SaxPB open reading frame remains intact in saxP mRNA. Two additional ATG codons are upstream within the P3′ end sequences and could encode methionine codons in frame with SaxPB, providing other possible alternative translational start sites (Figure 6C).
Figure 6.—
P-insertion site of saxP does not disrupt transcription. (A) Genomic structure of sax locus shown with exon (numbered) distribution for the two splice forms of sax mRNAs (RA and RB). The locations of two ATG initiation codons and the TAG termination codon (vertical thick solid lines) indicate the two overlapping open reading frames (speckles) giving rise to the putative protein products PA and PB (shaded speckles). Positions of PCR primers are indicated by arrowheads. The site and junctional sequence of the placW insertion giving rise to saxP is shown at the top. The endogenous second ATG within the sax transcription unit is bold and underlined. (B) RT–PCR products from different primer pairs generated from RNA isolated from control (yw) (left) and saxP homozygous mutant flies (right). Lane 1, primers 8103 + 10228; lane 2, primers 9396 + 11119; lane 3, primers placW10648 + 11119; lane 4, primers placW9661 + 10228. Note the insertion of placW disrupts the wild-type transcription unit initiating at RA-1 (lane 1) but allows transcription to initiate within the placW element between primers placW9661 and placW10648 (presence of PCR product in lane 3 of saxP animals and not in wild type, or in lane 4 of yw or saxP). Transcription in both genotypes extends through the expected translational termination site (PCR product present in lane 2 of both genotypes). M, marker lane. A, actin control. (C) The predicted amino acid sequence of SaxPB produced by open reading frame initiating at second endogenous ATG (bold and underlined in A) is shown in normal type with potential additional amino acids (italics) if translation initiated at an atg within the 3′ P-element of placW (shown in A).
Our identification of lethal, null sax alleles and the genetic analyses indicating that saxP retains some function, led us to conclude that the SaxPB protein (or a SaxPB protein with 2–12 additional amino acids) must have activity and importantly, must contribute in part to BMP signaling during development. saxP homozygotes are viable and display only weak mutant phenotypes, indicating that most requirements for sax function throughout development can be met by the SaxPB protein. While similar to sax1 and sax2 in the maternal enhancement of dpphr/+ lethality and in its ability to be rescued by dpp+ duplications, the reduced eye and mutant wing phenotype of saxP/sax* adults (a loss of the ACV and ectopic vein material near distal L2) set this allele apart and suggest that saxP retains less wild-type sax function than sax1 and sax2.
sax zygotic lethal phase and phenotypes:
We examined the lethal phase and mutant phenotypes of the new sax alleles (Figure S3, Figure S4). The zygotic lethal phases were determined in crosses of sax4/+ to sax3/+, sax4/+, sax5/+, sax6/+, sax1rv1/+, and Df(2R)H23/+ (collectively sax*) where lethality of sax4/sax* is as expected (∼25% when the lethality of control classes is taken into account). The zygotic lethal phase is primarily larval and to a lesser extent pupal. More than 70% of sax*/Df(2R)H23 third instar larvae are transparent (Figure S4), a phenotype not seen in sax1/Df(2R)H23 and sax2/Df(2R)H23 animals. This transparency reflects in part a qualitative alteration in the morphology of the mutant fat body (S. Ballard, K. Wharton, unpublished data). Despite minimal embryonic lethality, the lethal sax alleles exhibit abnormal embryonic midgut morphology, mainly consisting of a failure in the second midgut constriction, a phenotype not shown by sax1 mutant embryos (C. Savery, K. Wharton, unpublished results). Only a small percentage (≤5%) of hemizygous mutant larvae (sax3/Df(2R)H23, sax4/Df(2R)H23, sax5/Df(2R)H23, and sax6/Df(2R)H23) exhibit other defects, such as developmental delay, lethargy, a reduction in the size of imaginal discs, brain, and midgut structures, as well as tracheal truncations (Figure S4). The pupal lethal phase is variable occurring anywhere from imaginal disc eversion to pharate adults (data not shown).
DISCUSSION
Here we describe a mutational analysis of the saxophone gene encoding the Drosophila ortholog of the human ACVR1/ALK2 and ALK1/ACVRL1 type I receptor. We show that the two defining mutations in the gene, sax1 and sax2, are GOF alleles on the basis of our ability to revert them to alleles that (1) are lethal, (2) do not display maternal effect lethality, and (3) do not enhance dpp mutant phenotypes. The majority of the new lethal alleles represent either the partial or complete loss-of-function state of sax given that in functional assays they primarily behave like deficiencies of the sax locus. Furthermore, we show that saxP, previously reported to be a sax null allele (Nellen et al. 1994), in fact retains significant sax function, which can be eliminated by P-element excision. Our studies demonstrate that sax is an essential gene that is required for multiple developmental processes and the genetic characteristics of different alleles appear to reflect the site of the mutation and the fact that the Sax receptor acts in a multimeric receptor complex in BMP signaling.
Genetic nature of new lethal sax alleles:
Of the 10 lethal alleles, sax3, sax4, sax5, and sax6 arise from point mutations and Df(2R)saxH9, Df(2R)saxH10, and saxPE alleles appear to be deletions of the sax locus on the basis of polytene chromosome in situ hybridizations and Southern blot analysis (data not shown). The molecular lesions associated with revertants sax1rv1, sax1rv2, sax1rv5 have yet to be identified but were not found within the sax transcription unit. Without exception, these alleles are lethal as hemizygotes and distinct from the original sax1 and sax2 GOF alleles. Unlike the GOF alleles, the lethal alleles are not rescued by increased dosage of dpp+ or gbb+ (data not shown) consistent with their designation as LOF alleles; increasing ligand level cannot facilitate signaling through an absent or inactive receptor. The genetic screens used to identify these new alleles each selected mutations according to different criteria: (a) reversion of the maternal effect lethality of sax1 and sax2, (b) reversion of dpp enhancement, or (c) by lethality over regional deficiencies. The frequency at which these new alleles were generated (one per 1400) is comparable with that of other LOF alleles, unlike the rate at which sax1 and sax2 were recovered (one per 4000; Schupbach and Wieschaus 1989).
Lethal sax phenotypes:
The lethal phase of sax LOF alleles is primarily larval with some early pupal lethality (Figure S3). In general the mutant phenotypes observed are consistent with a role for sax in BMP signaling throughout development, including dorsal/ventral patterning, midgut, and tracheal development, imaginal disc patterning, and neuromuscular junction formation and function (reviewed in Immergluck et al. 1990; Panganiban et al. 1990; Affolter et al. 1994; Vincent et al. 1997; Marques et al. 2002; McCabe et al. 2003; O'connor et al. 2006; Rawson et al. 2003). The low frequency, variable expressivity, and general pleiotropy of zygotic sax mutant phenotypes could reflect the substantial maternal contribution of sax, and/or the dual role of Sax in BMP signal transmission (Bangi and Wharton 2006b). Maternal sax mRNA and/or protein is preloaded into the oocyte and is likely to persist through embryogenesis, such that the maternal contribution of wild-type sax can compensate for early functions despite the absence of zygotic sax in complete LOF mutants. Sax requires a second type I receptor Tkv to transduce a BMP signal but alone it influences the availability of ligand. Thus, slight shifts in the balance between receptor and ligand pools can likely result in very different phenotypes (Bangi and Wharton 2006b).
With regard to the maternal contribution, we have previously generated germline clones to eliminate the contribution of wild-type maternal sax mRNA to the egg and found that sax function is required during oogenesis and removal of all sax function from ovaries causes the cessation of egg production within days of inducing clones (Twombly et al. 1996; Xie and Spradling 1998). Of the few sax null embryos produced by germline clones, a dramatic shift in the lethal phase from larval to embryonic was observed, as would be expected from a reduction in sax maternal mRNA due to its clear role in early dorsal/ventral patterning. However, the small number of recovered embryos did not allow a thorough analysis.
Molecular lesions and functional consequences of receptor mutations:
The molecular lesions of sax3, sax4, sax5, sax6 and a previously described P-element insertion allele, saxP, were determined. Both sax3 and sax4 are nonsense mutations in the extracellular domain of the Sax receptor and sax5 and sax6 are missense mutations within the intracellular kinase domain (Table 3, Figure 7). The precise location of the placW element inserted in sax was determined for the saxP allele (Figure 6A) and RT–PCR analysis indicates that transcription of this mutant gene must initiate within the 3′ P-element end between the positions of primers placW9661 and placW10648 and extend into the sax locus. The resulting saxP mRNA contains an open reading frame either identical to SaxPB or with a short amino extension (Figure 6, B and C). These results indicate that saxP is not an RNA null. The generation by imprecise P-element excisions of saxP revertants (saxPE*) that are lethal over sax deficiencies and LOF alleles (Table 2) support our conclusion that saxP is not a functional null. Instead, saxP retains partial function, sufficient for viability (Figure 3), but interestingly the resulting protein product produced by saxP in some way interferes with dpp function in the early embryo (Figure 4). It is possible that this difference in function reflects a difference in the role of Sax-PA and Sax-PB proteins in either the early functions of sax or in its interaction with Dpp. The weaker affect of saxP germaria on stem cell loss than that observed in dpp mutant germaria (Xie and Spradling 1998) likely reflects, not a minor role for Sax in the maintenance of the stem cell niche, but rather a mild hypomorphic nature of the saxP allele for this function and a less dramatic difference between the contributions of Sax-PA and Sax-PB to germ cell niche maintenance.
Figure 7.—
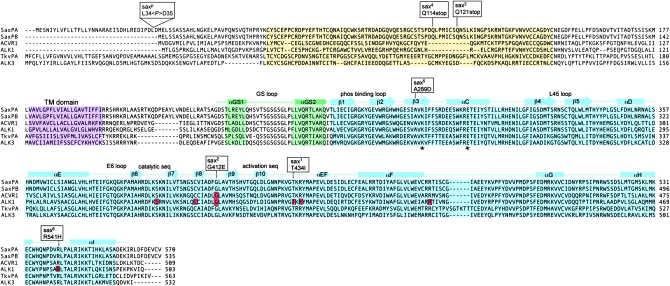
Sequence comparison of Sax (PA and PB isoforms), ACVR1 (ALK2), ALK1 (ACVRL1), Tkv (PA isoform), and ALK3. The extracellular ligand binding domain (yellow), the transmembrane domain (purple), the intracellular GS activation (green), and serine/threonine (blue) kinase domains are shaded. The structural elements of the cytoplasmic GS and kinase domains (based on the TβR1 structure) (Huse et al. 1999) are indicated above the sequence alignment. The positions of mutations associated with the sax alleles discussed are indicated above the sequence alignment. The positions of specific HHT2 and FOP mutations in ALK1 and ACVR1, respectively, are highlighted in red within the sequence. The asterisks mark the invariant Lys and Glu residues critical for stabilization of the catalytic segment with the N and C lobes of the kinase.
The two alleles, sax5 and sax6, arising from missense mutations within the Sax kinase domain, are clearly LOF alleles on the basis of their lethality and inability to maternally enhance dpp lethality (Figure 4). Both mutations are likely to destabilize the kinase domain and the dominant-negative behavior exhibited by sax5 is consistent with this proposal (Figure 5). While the Sax5 protein is null for signaling activity as indicated by multiple genetic assays and a cell-based signaling assay, the dominant-negative behavior of the sax5 mutation appears to stem from the ability of a defective Sax5 receptor to interfere with the function of Tkv, presumably by forming inactive Tkv–Sax5 receptor complexes and effectively reducing the number of functional receptor complexes for BMP signaling. While wild-type Sax possesses a dual function, whereby it can both inhibit and facilitate signaling depending on the relative levels of Sax to Tkv, Sax5 is clearly unable to facilitate signaling as assayed by both phenotypic and cell-based signaling criteria.
Consequences of analogous mutations in human sax orthologs:
Mutations in the two human orthologs of sax, ALK1/ACVRL1 and ACVR1/ALK2, result in hereditary hemorrhagic telangiectasia (HHT2, Osler-Weber-Rendu disease) and fibrodysplasia ossificans progressiva (FOP), respectively (see www.hhtmutation.org; Shore et al. 2006). Interestingly, HHT2, a vascular disorder, and FOP, a devastating heterotopic bone disorder, are both dominant, immediately indicating that the combination of mutant receptors with wild-type receptors is disruptive to normal signaling.
Mutations in the ALK1 gene have been found with an incidence of 1/10,000 in either the extracellular and intracellular domains of the protein (www.hhtmutation.org) and in both cases mutations cause vascular dysplasias, such as mucocutaneous telansiectases and arterovenous malformations, that can result in life-threatening complications (Lesca et al. 2007). The penetrance of these mutations is quite variable even within a family and with an increase in age, leading to considerable controversy as to whether mutations exhibit GOF or LOF behaviors (Massagué et al. 2000; Lesca et al. 2004). ALK1 is expressed in the distal capillaries (Mahmoud et al. 2009) and HHT2 mutations are thought to affect endothelial cell metabolism, angiogenesis, and vascular remodeling with no clear explanation for the variability in expressivity of mutant phenotypes (Lenato et al. 2007).
The most frequent mutation in ACVR1 that gives rise to FOP has been found to result in an R206H alteration near the GS loop, the site of phosphorylation by the type II receptor (Shore et al. 2006). Seven other less common FOP variants have been shown to alter highly conserved residues within the kinase domain (Kaplan et al. 2009). Protein structural homology modeling has suggested that the amino acid substitutions associated with FOP mutations result in an ACVR1 protein conformational change that impacts the activity of the receptor molecule (Groppe et al. 2007; Kaplan et al. 2009). Classic FOP is characterized by great toe malformations and progressive heterotopic ossifications in the medial tibia (osteochondromas), the cervical spine, and femoral neck, often with thumb malformations. Patients with atypical FOP show progressive heterotopic ossifications with abnormalities in other organs, such as the eye and in hair growth (Kaplan et al. 2009). The variability in the expressivity of the clinical features of FOP and its progressive nature is of great interest but at present is not well understood.
Interestingly, we found that the conserved residues altered by sax2, sax1, and sax6 mutations have also been identified as residues altered by various HHT2 and FOP mutations (Figure 7). The Arg near the C-terminal end of the receptor substituted in sax6 is altered in HHT2 mutations (R479L, R479E, R479X) (Lesca et al. 2004; Bayrak-Toydemir et al. 2006). Given its location in the structural scaffold of the kinase domain, these mutations likely destabilize the kinase. The conserved core of all protein kinases consists of an N-terminal lobe and a large C-terminal lobe separated by a cleft where ATP is bound (Huse and Kuriyan 2002). Conformational changes in the αC-helix and its interaction with the activation segment are associated with activation of kinase activity. Furthermore, alterations to residues within the activation segment and/or catalytic segment within the cleft have been shown to modulate kinase activiy (Huse et al. 1999). Both sax1 and sax2 mutations affect conserved residues within the activation segment on either side of the β9 and β10 β-sheets (Figure 7). The identical amino acid substitution of Thr to Ile found in sax1 (T434I) is found in several HHT2 patients (ALK1 T372I) (Wehner et al. 2006; Lenato et al. 2007). Threonine 434 is one of the 12 nearly invariant amino acids found in all kinase domains. The substitution of a nearby Arg in ALK1 R374W has been shown to result in normal expression levels of a mutant protein (Fernandez et al. 2005), suggesting that defects in this region do not necessarily lead to protein destabilization and degradation. In fact, it appears that all individual missense mutations within this region of the ALK1 protein (S333I; C344Y; R374W; R411Q) are expressed and mutant proteins present on the cell surface (Gu et al. 2006; Olivieri et al. 2007). S333I and C344Y display dominant-negative activity when overexpressed in zebrafish embryos while R411Q exhibits lower activity (Gu et al. 2006).
In sax2 mutants, Gly 412 of Sax is substituted (G412E) and mutations of this same residue are found in both HHT2 (ALK1-G350S, ALK1-G350R) (Abdalla et al. 2005; Letteboer et al. 2005; Schulte et al. 2005) and FOP patients (ACVR1-G356D) (Kaplan et al. 2009). Protein modeling has suggested that the ACVR1-G356D substitution could interfere with ion pairing between conserved Lys235 and Glu248 residues that is normally observed in kinases that are in the active state (Kornev et al. 2006; Kaplan et al. 2009).
The functional data we have presented here clearly show that the sax1 and sax2 mutations are not null and that the resulting mutant receptors must retain sufficient function for viability. It is of particular interest that these mutations could affect either substrate recognition and/or the orientation of the αC helix. The orientation of the αC helix is critical for maximal kinase activity depending on stabilization of its interaction with the phosphates of bound ATP and the activation and catalytic segments. In either case, the unique behaviors displayed by sax1 and sax2 that the lethal LOF sax mutations do not possess, such as maternal effect lethality and the maternal enhancement of dpp phenotypes, strongly suggest that Sax1 and Sax2 proteins are not simply dominant negative as would be expected of a fully kinase-defective receptor (such as Sax5). Rather, these proteins appear to be capable of participating in productive signaling complexes that allow for normal zygotic development and viability. However, when the only source of maternal Sax protein is the Sax1 and/or Sax2 mutant form, the resulting signaling output is abnormal such that the developmental process most sensitive to disruptions in BMP signaling, early embryonic dorsal/ventral specification, is affected. The fact that homozygous or transheterozygous sax1 and sax2 females show higher MEL than hemizgous females (Table 1) suggests that the dosage or level of mutant Sax receptor protein is critical in generating the observed phenotypes. Assuming that protein production can be correlated with gene dosage, more maternally loaded aberrant Sax receptor must be more detrimental than less mutant receptor. Given that type I receptors must participate in a heteromeric receptor complex to transduce a signal, it is likely that the ratio of receptor complexes containing only Tkv (Tkv:Tkv) vs. Tkv:Sax1 will change when the dosage of the sax mutant chromosome is reduced. It appears that some developmental processes are more sensitive to a particular Tkv:Sax to Tkv:Tkv ratio than others for optimal signaling. It is not yet clear how Sax1 or Sax2 may affect signaling complex formation but the fact that the mutations that give rise to these aberrant proteins can be reverted is consistent with their assignment as gain of function. It is conceivable that their affect on BMP signaling can be one of partial loss of function, as well as with some antimorphic properties, depending on the presence of other proteins important for complex formation.
In conclusion, the isolation and characterization of new sax alleles has clarified the null state of sax and highlighted the consequences of mutations in different domains of this BMP type I receptor. Given the homology between Sax and the human ALK1/ACVRL1 and ACVR1/ALK2 type I receptors, these studies show promise in making use of genetic analyses and unbiased screens to identify mutations with a range of activities. Such functional characterizations of mutations known to cause the human disorders HHT2 and FOP should continue to provide invaluable data on their mechanistic consequences.
Acknowledgments
We thank D. Gubb for deficiency stocks, C. Rushlow for Kr antibody, Mike O'Connor for pAcpA-tkv and pAcpA-sax, and T. Akiyama for discussions. We thank M. Gelbart and G. Krateberg for superb technical assistance, and Diane Duplissa, Lorainne Lucas, and Alex Makovetsky for excellent facilities support. This work was supported in part by National Institutes of Health grants to K.A.W (GM068118) and William M. Gelbart (5 R37 GM28669), a National Research Service Award Trainee Grant to V.T. and to V.L. (T32 GM 007601), a National Research Service Award Postdoctoral Fellowship to M.A.S., a Fundação de Amparo à Pesquisa do Estado de São Paulo award to B.M., and a research grant from The Center for Research in Fibrodysplasia Ossificans Progressiva from The University of Pennsylvania to K.A.W. This work was performed in the laboratories of Bill Gelbart and K.A.W.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.105585/DC1.
References
- Abdalla, S. A., U. Cymerman, D. Rushlow, N. Chen, G. P. Stoeber et al., 2005. Novel mutations and polymorphisms in genes causing hereditary hemorrhagic telangiectasia. Hum. Mutat. 25: 320–321. [DOI] [PubMed] [Google Scholar]
- Abdalla, S. A., and M. Letarte, 2006. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J. Med. Genet. 43: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter, M., D. Nellen, U. Nussbaumer and K. Basler, 1994. Multiple requirements for the receptor serine/threonine kinase thick veins reveal novel functions of TGFβ homologs during Drosophila embryogenesis. Development 120: 3105–3117. [DOI] [PubMed] [Google Scholar]
- Arora, K., M. Levine and M. O'Connor, 1994. The screw gene encodes a ubiquitously expressed member of the TGF-β family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev. 8: 2588–2601. [DOI] [PubMed] [Google Scholar]
- Bangi, E., and K. A. Wharton, 2006. a Dpp and Gbb exhibit different effective ranges in the establsihment of the BMP activity gradient critical for Drosophila wing patterning. Dev. Biol. 295: 178–193. [DOI] [PubMed] [Google Scholar]
- Bangi, E., and K. A. Wharton, 2006. b Dual function of the Drosophila Alk1/Alk2 ortholog, Saxophone shapes the BMP activity gradient in the wing imaginal disc. Development 133: 3295–3303. [DOI] [PubMed] [Google Scholar]
- Bayrak-Toydemir, P., J. McDonald, B. Markewitz, S. Lewin, F. Miller et al., 2006. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am. J. Med. Genet. A 140: 463–470. [DOI] [PubMed] [Google Scholar]
- Brummel, T. J., V. Twombly, G. Marqués, J. L. Wrana, S. J. Newfeld et al., 1994. Characterization and relationship of dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell 78: 251–261. [DOI] [PubMed] [Google Scholar]
- Brummel, T., S. Abdollah, T. E. Haerry, M. J. Shimell, J. Merriam et al., 1999. The Drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 13: 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, R., and K. Basler, 1996. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development 122: 2261–2269. [DOI] [PubMed] [Google Scholar]
- Calvi, B. R., and W. M. Gelbart, 1994. The basis for germline specificity of the hobo transposable element in Drosophila melanogaster. EMBO J. 13: 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcamo, J., F. Weis, F. Ventura, R. Wieser, J. Wrana et al., 1994. Type I receptors specify growth-inhibitory and transcriptional responses to transforming growth factor β and activin. Mol. Cell. Biol. 14: 3810–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartegni, L., S. L. Chew and A. R. Krainer, 2002. Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat. Rev. Genet. 3: 285–298. [DOI] [PubMed] [Google Scholar]
- Chen, Y., M. J. Riese, M. A. Killinger and F. M. Hoffmann, 1998. a A genetic screen for modifiers of Drosophila decapentaplegic signaling identifies mutations in punt, Mothers against dpp and the BMP-7 homologue, 60A. Development 125: 1759–1768. [DOI] [PubMed] [Google Scholar]
- Chen, Y. G., A. Hata, R. S. Lo, D. Wotton, Y. Shi et al., 1998. b Determinants of specificity in TGF-beta signal transduction. Genes Dev. 12: 2144–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. G., and J. Massague, 1999. Smad1 recognition and activation by the ALK1 group of transforming growth factor-beta family receptors. J. Biol. Chem. 274: 3672–3677. [DOI] [PubMed] [Google Scholar]
- Childs, S. R., J. L. Wrana, K. Arora, L. Attisano, M. B. O'Connor et al., 1993. Identification of a Drosophila activin receptor. Proc. Natl. Acad. Sci. USA 90: 9475–9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doctor, J. S., D. Jackson, K. E. Rashka, M. Visalli and F. M. Hoffmann, 1992. Sequence, biochemical characterization, and developmental expression of a new member of the TGF-β superfamily in Drosophila melanogaster. Dev. Biol. 151: 491–505. [DOI] [PubMed] [Google Scholar]
- Dorfman, R., and B. Z. Shilo, 2001. Biphasic activation of the BMP pathway patterns the Drosophila embryonic dorsal region. Development 128: 965–972. [DOI] [PubMed] [Google Scholar]
- Fernandez, L. A., F. Sanz-Rodriguez, R. Zarrabeitia, A. Perez-Molino, R. P. Hebbel et al., 2005. Blood outgrowth endothelial cells from hereditary haemorrhagic telangiectasia patients reveal abnormalities compatible with vascular lesions. Cardiovasc. Res. 68: 235–248. [DOI] [PubMed] [Google Scholar]
- Finnson, K. W., W. L. Parker, P. ten Dijke, M. Thorikay and A. Philip, 2008. ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J. Bone Miner. Res. 23: 896–906. [DOI] [PubMed] [Google Scholar]
- FlyBase, 2003. The FlyBase database of the Drosophila genome projects and community literature. Nucleic Acids Res. 31: 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen, P., C. H. Heldin and K. Miyazono, 1995. The GS domain of the transforming growth factor-beta type I receptor is important in signal transduction. Biochem. Biophys. Res. Commun. 207: 682–689. [DOI] [PubMed] [Google Scholar]
- Goumans, M. J., G. Valdimarsdottir, S. Itoh, F. Lebrin, J. Larsson et al., 2003. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol. Cell 12: 817–828. [DOI] [PubMed] [Google Scholar]
- Groppe, J. C., E. M. Shore and F. S. Kaplan, 2007. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin. Orthop. Relat. Res. 462: 87–92. [DOI] [PubMed] [Google Scholar]
- Gu, Y., P. Jin, L. Zhang, X. Zhao, X. Gao et al., 2006. Functional analysis of mutations in the kinase domain of the TGF-beta receptor ALK1 reveals different mechanisms for induction of hereditary hemorrhagic telangiectasia. Blood 107: 1951–1954. [DOI] [PubMed] [Google Scholar]
- Haerry, T. E., O. Khalsa, M. B. O'Connor and K. A. Wharton, 1998. Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development 125: 3977–3987. [DOI] [PubMed] [Google Scholar]
- Howe, J. R., J. L. Bair, M. G. Sayed, M. E. Anderson, F. A. Mitros et al., 2001. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat. Genet. 28: 184–187. [DOI] [PubMed] [Google Scholar]
- Huse, M., Y. G. Chen, J. Massague and J. Kuriyan, 1999. Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell 96: 425–436. [DOI] [PubMed] [Google Scholar]
- Huse, M., and J. Kuriyan, 2002. The conformational plasticity of protein kinases. Cell 109: 275–282. [DOI] [PubMed] [Google Scholar]
- Immergluck, K., P. A. Lawrence and M. Bienz, 1990. Induction across germ layers in Drosophila mediated by a genetic cascade. Cell 62: 261–268. [DOI] [PubMed] [Google Scholar]
- Kaplan, F. S., M. Xu, P. Seemann, J. M. Connor, D. L. Glaser et al., 2009. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum. Mutat. 30: 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa, O., J.-w. Yoon, S. Schumann-Torres and K. Wharton, 1998. TGF-b/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and establish cell identity in the Drosophila wing. Development 125: 2723–2734. [DOI] [PubMed] [Google Scholar]
- Kim, I. J., J. H. Park, H. C. Kang, K. H. Kim, J. H. Kim et al., 2003. Identification of a novel BMPR1A germline mutation in a Korean juvenile polyposis patient without SMAD4 mutation. Clin. Genet. 63: 126–130. [DOI] [PubMed] [Google Scholar]
- Kirsch, T., J. Nickel and W. Sebald, 2000. a Isolation of recombinant BMP receptor IA ectodomain and its 2:1 complex with BMP-2. FEBS Lett. 468: 215–219. [DOI] [PubMed] [Google Scholar]
- Kirsch, T., W. Sebald and M. K. Dreyer, 2000. b Crystal structure of the BMP2-BRIA ectodomain complex. Nat. Struc. Biol. 7: 492–496. [DOI] [PubMed] [Google Scholar]
- Kornev, A. P., N. M. Haste, S. S. Taylor and L. F. Eyck, 2006. Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc. Natl. Acad. Sci. USA 103: 17783–17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, K., P. Seemann, S. Stricker, M. Sammar, B. Meyer et al., 2003. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc. Natl. Acad. Sci. USA 100: 12277–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, K., P. Seemann, J. Boergermann, G. Morin, S. Reif et al., 2006. A novel R486Q mutation in BMPR1B resulting in either a brachydactyly type C/symphalangism-like phenotype or brachydactyly type A2. Eur. J. Hum. Genet. 14: 1248–1254. [DOI] [PubMed] [Google Scholar]
- Lenato, G. M., P. Suppressa, P. Giordano, G. Guanti, E. Guastamacchia et al., 2007. Hereditary haemorrhagic telangiectasia: a rare disease as a model for the study of human atherosclerosis. Curr. Pharm. Des. 13: 3656–3664. [DOI] [PubMed] [Google Scholar]
- Lesca, G., H. Plauchu, F. Coulet, S. Lefebvre, G. Plessis et al., 2004. Molecular screening of ALK1/ACVRL1 and ENG genes in hereditary hemorrhagic telangiectasia in France. Hum. Mutat. 23: 289–299. [DOI] [PubMed] [Google Scholar]
- Lesca, G., C. Olivieri, N. Burnichon, F. Pagella, M. F. Carette et al., 2007. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet. Med. 9: 14–22. [DOI] [PubMed] [Google Scholar]
- Letteboer, T. G., R. A. Zewald, E. J. Kamping, G. de Haas, J. J. Mager et al., 2005. Hereditary hemorrhagic telangiectasia: ENG and ALK-1 mutations in Dutch patients. Hum. Genet. 116: 8–16. [DOI] [PubMed] [Google Scholar]
- Lewis, E. B., and F. Bacher, 1968. Method of feeding ethyl methane sulfonate (EMS) to Drosophila males. Dros. Inf. Serv. 43: 193. [Google Scholar]
- Loeys, B. L., J. Chen, E. R. Neptune, D. P. Judge, M. Podowski et al., 2005. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 37: 275–281. [DOI] [PubMed] [Google Scholar]
- Macias-Silva, M., P. A. Hoodless, S. J. Tang, M. Buchwald and J. L. Wrana, 1998. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J. Biol. Chem. 273: 25628–25636. [DOI] [PubMed] [Google Scholar]
- Mahmoud, M., G. M. Borthwick, A. A. Hislop and H. M. Arthur, 2009. Endoglin and activin receptor-like-kinase 1 are co-expressed in the distal vessels of the lung: implications for two familial vascular dysplasias, HHT and PAH. Lab. Invest. 89: 15–25. [DOI] [PubMed] [Google Scholar]
- Marques, G., H. Bao, T. E. Haerry, M. J. Shimell, P. Duchek et al., 2002. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33: 529–543. [DOI] [PubMed] [Google Scholar]
- Massagué, J., S. Blain and R. Lo, 2000. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103: 295–309. [DOI] [PubMed] [Google Scholar]
- McCabe, B. D., G. Marques, A. P. Haghighi, R. D. Fetter, M. L. Crotty et al., 2003. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39: 241–254. [DOI] [PubMed] [Google Scholar]
- Muller, B., B. Hartmann, G. Pyrowolakis, M. Affolter and K. Basler, 2003. Conversion of an extracellular Dpp/BMP morphogen gradient into an inverse transcriptional gradient. Cell 113: 221–233. [DOI] [PubMed] [Google Scholar]
- Nellen, D., M. Affolter and K. Basler, 1994. Receptor serine/threonine kinase implicated in the control of Drosophila body pattern by decapentaplegic. Cell 78: 225–237. [DOI] [PubMed] [Google Scholar]
- Neul, J., and E. L. Ferguson, 1998. Spatially restricted activation of the SAX receptor by SCW modulates DPP/TKV signaling in Drosophila dorsal-ventral patterning. Cell 95: 483–494. [DOI] [PubMed] [Google Scholar]
- Nguyen, M., S. Park, G. Marques and K. Arora, 1998. Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell 95: 495–506. [DOI] [PubMed] [Google Scholar]
- Nicholls, R. E., and W. M. Gelbart, 1998. Identification of Chromosomal Regions Involved in decapentaplegic Function in Drosophila. Genetics 149: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, M. B., D. Umulis, H. G. Othmer and S. S. Blair, 2006. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, S. P., T. Seki, K. A. Goss, T. Imamura, Y. Yi et al., 2000. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. USA 97: 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri, C., F. Pagella, L. Semino, L. Lanzarini, C. Valacca et al., 2007. Analysis of ENG and ACVRL1 genes in 137 HHT Italian families identifies 76 different mutations (24 novel). Comparison with other European studies. J. Hum. Genet. 52: 820–829. [DOI] [PubMed] [Google Scholar]
- Padgett, R., R. St. Johnston and W. Gelbart, 1987. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-β family. Nature 325: 81–84. [DOI] [PubMed] [Google Scholar]
- Panganiban, G. E. F., R. Reuter, M. P. Scott and F. M. Hoffmann, 1990. A Drosophila growth factor homolog, decapentaplegic regulates homeotic gene expression within and across germ layers during midgut morphogenesis. Development 110: 1041–1050. [DOI] [PubMed] [Google Scholar]
- Penton, A., Y. Chen, K. Staehling-Hampton, J. L. Wrana, L. Attisano et al., 1994. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell 78: 239–250. [DOI] [PubMed] [Google Scholar]
- Raftery, L., V. Twombly, K. Wharton and W. Gelbart, 1995. Genetic screens to identify elements of the decapentaplegic signaling pathway in Drosophila. Genetics 139: 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery, L. A., and D. J. Sutherland, 2003. Gradients and thresholds: BMP response gradients unveiled in Drosophila embryos. Trends Genet. 19: 701–708. [DOI] [PubMed] [Google Scholar]
- Rawson, J. M., M. Lee, E. L. Kennedy and S. B. Selleck, 2003. Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J. Neurobiol. 55: 134–150. [DOI] [PubMed] [Google Scholar]
- Ray, R., and K. Wharton, 2001. Context-dependent relationships between the BMPs gbb and dpp during development of the Drosophila wing imaginal disc. Development 128: 3913–3925. [DOI] [PubMed] [Google Scholar]
- Schulte, C., U. Geisthoff, A. Lux, S. Kupka, H. P. Zenner et al., 2005. High frequency of ENG and ALK1/ACVRL1 mutations in German HHT patients. Hum. Mutat. 25: 595. [DOI] [PubMed] [Google Scholar]
- Schupbach, T., and E. Wieschaus, 1989. Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics 121: 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y., and J. Massague, 2003. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113: 685–700. [DOI] [PubMed] [Google Scholar]
- Shimmi, O., D. Umulis, H. Othmer and M. B. O'Connor, 2005. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore, E. M., M. Xu, G. J. Feldman, D. A. Fenstermacher, M. A. Brown et al., 2006. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 38: 525–527. [DOI] [PubMed] [Google Scholar]
- Singer, M. A., A. Penton, V. Twombly, F. M. Hoffmann and W. M. Gelbart, 1997. Signaling through both type I DPP receptors is required for anterior-posterior patterning of the entire Drosophila wing. Development 124: 79–89. [DOI] [PubMed] [Google Scholar]
- Smith, D., J. Wohlgemuth, B. R. Calvi, I. Franklin and W. M. Gelbart, 1993. hobo enhancer trapping mutagenesis in Drosophila reveals an insertion specificity different from P elements. Genetics 135: 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, F. A., F. M. Hoffmann and W. M. Gelbart, 1982. decapentaplegic: a gene complex affecting morphogenesis in Drosophila melanogaster. Cell 28: 451–461. [DOI] [PubMed] [Google Scholar]
- Su, G. H., R. Bansal, K. M. Murphy, E. Montgomery, C. J. Yeo et al., 2001. ACVR1B (ALK4, activin receptor type 1B) gene mutations in pancreatic carcinoma. Proc. Natl. Acad. Sci. USA 98: 3254–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke, P., and C. S. Hill, 2004. New insights into TGF-beta-Smad signalling. Trends Biochem. Sci. 29: 265–273. [DOI] [PubMed] [Google Scholar]
- Terracol, R., and J. A. Lengyel, 1994. The thick veins gene of Drosophila is required for dorsoventral polarity of the embryo. Genetics 138: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török, T., G. Tick, M. Alvarado and I. Kiss, 1993. P-lacW insertional mutagenesis on the second chromosome of Drosophila melenogaster: isolation of lethals with different overgrowth phenotypes. Genetics 135: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twombly, V., R. K. Blackman, H. Jin, J. M. Graff, R. Padgett et al., 1996. The TGF-β signaling pathway is essential for Drosophila oogenesis. Development 122: 1555–1565. [DOI] [PubMed] [Google Scholar]
- Vincent, S., E. Ruberte, N. C. Grieder, C. K. Chen, T. Haerry et al., 1997. Dpp controls tracheal cell migration along the dorsoventral body axis of the Drosophila embryo. Development 124: 2741–2750. [DOI] [PubMed] [Google Scholar]
- Wehner, L. E., B. J. Folz, L. Argyriou, S. Twelkemeyer, U. Teske et al., 2006. Mutation analysis in hereditary haemorrhagic telangiectasia in Germany reveals 11 novel ENG and 12 novel ACVRL1/ALK1 mutations. Clin. Genet. 69: 239–245. [DOI] [PubMed] [Google Scholar]
- Weis-Garcia, F., and J. Massague, 1996. Complementation between kinase-defective and activation-defective TGF-beta receptors reveals a novel form of receptor cooperativity essential for signaling. EMBO J. 15: 276–289. [PMC free article] [PubMed] [Google Scholar]
- Wharton, K., R. Ray, S. Findley, H. Duncan and W. Gelbart, 1996. Molecular lesions associated with alleles of decapentaplegic identify residues necessary for TGF-β/BMP cell signaling in Drosophila melanogaster. Genetics 142: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton, K. A., R. P. Ray and W. M. Gelbart, 1993. An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development 117: 807–822. [DOI] [PubMed] [Google Scholar]
- Wharton, K. A., G. H. Thomsen and W. M. Gelbart, 1991. Drosophila 60A gene, another transforming growth factor β family member, is closely related to human bone morphogenetic proteins. Proc. Natl. Acad. Sci. USA 88: 9214–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana, J. L., L. Attisano, R. Wieser, F. Ventura and J. Massagué, 1994. Mechanism of activation of the TGF-β receptor. Nature 370: 341–347. [DOI] [PubMed] [Google Scholar]
- Xie, T., A. L. Finelli and R. W. Padgett, 1994. The Drosophila saxophone gene: a serine-threonine kinase receptor of the TGF-β superfamily. Science 263: 1756–1759. [DOI] [PubMed] [Google Scholar]
- Xie, T., and A. C. Spradling, 1998. decapentaplegic is essential for the maintenance and division of germline stem cells in Drosophila ovary. Cell 94: 251–260. [DOI] [PubMed] [Google Scholar]
- Yamashita, H., P. ten Dijke, P. Franzen, K. Miyazono and C. H. Heldin, 1994. Formation of hetero-oligomeric complexes of type I and type II receptors for transforming growth factor-β. J. Biol. Chem. 269: 20172–20178. [PubMed] [Google Scholar]
- Zecca, M., K. Basler and G. Struhl, 1996. Direct and long-range action of a wingless morphogen gradient. Cell 87: 833–844. [DOI] [PubMed] [Google Scholar]
- Zhou, X. P., K. Woodford-Richens, R. Lehtonen, K. Kurose, M. Aldred et al., 2001. Germline mutations in BMPR1A/ALK3 cause a subset of cases of juvenile polyposis syndrome and of Cowden and Bannayan-Riley-Ruvalcaba syndromes. Am. J. Hum. Genet. 69: 704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]