Abstract
Selective protein degradation is a key regulator of neuronal development and synaptogenesis. Complexes that target proteins for degradation often contain F-box proteins. Here we characterize MEC-15, an F-box protein with WD repeats, which is required for the development and function of Caenorhabditis elegans touch receptor neurons (TRNs). Mutations in mec-15 produce defects in TRN touch sensitivity, chemical synapse formation, and cell-body morphology. All mec-15 mutant phenotypes are enhanced by mutations in a MAP kinase pathway composed of the MAPKKK DLK-1, the MAPKK MKK-4, and the p38 MAPK PMK-3. A mutation of the rpm-1 gene, which encodes an E3 ubiquitin ligase that negatively regulates this pathway to promote synaptogenesis, suppresses only the mec-15 cell-body defect. Thus, MEC-15 acts in parallel with RPM-1, implicating a second protein degradation pathway in TRN development. In addition, all mec-15 phenotypes can be dominantly suppressed by mutations in mec-7, which encodes a β-tubulin, and dominantly enhanced by mutations in mec-12, which encodes an α-tubulin. Since mec-15 phenotypes depend on the relative levels of these tubulins, MEC-15 may target proteins whose function is affected by these levels.
THE response to touch in animals requires the formation of specialized sensory neurons. In the nematode Caenorhabditis elegans, six touch receptor neurons (TRNs: ALML/R, AVM, PVM, PLML/R) enable the worm to respond to gentle touch to the body. A dozen genes required for TRN function were identified in screens for touch-insensitive mutants (Chalfie and Au 1989). Designated mec for mechanosensory abnormal, most of these genes have been characterized genetically and molecularly (for review, see Bounoutas and Chalfie 2007). Several of these genes encode proteins that contribute to specialized structures required for sensing touch, including large-diameter microtubules, extracellular matrix, and a channel complex that transduces mechanical stimuli into electrical signals.
In the past decade there has been an increasing awareness of the critical role that selective proteolytic degradation plays in regulating a diverse set of steps in neuronal differentiation and function (Segref and Hoppe 2009). Selective protein degradation mediated by ubiquitination is also directly implicated in TRN development. rpm-1, an E3 ubiquitin ligase, regulates the transition from axonal outgrowth to synaptogenesis in both TRNs and other neurons (Schaefer et al. 2000; Zhen et al. 2000). Mutants lacking rpm-1 overextend TRN axons and fail to stabilize synaptic contacts with postsynaptic partners. At least some of the downstream targets of rpm-1 have been identified in genetic screens for suppressor of the GABAergic neuron deficits of rpm-1 mutants (Nakata et al. 2005). RPM-1 in association with the F-box protein FSN-1 and CUL-1 and SKR-1 form an E3 complex that selectively inactivates a p38 MAP kinase pathway consisting of DLK-1, MKK-4, and PMK-3 (Liao et al. 2004; Nakata et al. 2005). Disrupting these kinases completely suppresses the GABAergic synaptic defects associated with RPM-1, suggesting that this pathway is a major substraote of the rpm-1 ubiquitin ligase (Nakata et al. 2005). Thus, modulation of protein levels by proteosome pathways plays a critical role in regulating the development of neurons in C. elegans.
In this article, we describe the molecular and phenotypic characterization of mec-15, which defines an additional ubiquitin ligase pathway that regulates multiple aspects of TRN development. The TRNs in mec-15 mutants do not develop or function properly; they have reduced chemical synapses, enlarged, abnormal cell bodies, and reduced touch sensitivity. The diversity of these phenotypes is consistent with the finding that mec-15 encodes an F-box protein that likely regulates protein degradation. We demonstrate that mec-15 acts in parallel to the p38 MAP kinase pathway because mutations in this pathway enhance mec-15 mutant phenotypes. In addition, modulating the levels of mechanosensory-specific tubulins can suppress or enhance all mec-15 defects, suggesting that MEC-15 may act to regulate levels of cytoskeletal components during the developmental progression of TRNs.
MATERIALS AND METHODS
Nematode strains:
C. elegans strains were cultured at 20° as described by Brenner (1974). Isolation and initial characterization of mec-15 mutants was reported previously (Chalfie and Au 1989; Gu et al. 1996). The putative loss-of-function alleles mec-3(e1338), mec-7(u443), and mec-12(e1607) were also previously described (Chalfie and Sulston 1981; Chalfie and Au 1989; Savage et al. 1994; Fukushige et al. 1999).
mec-15(js414) was identified in a nonclonal compound microscope screen for EMS-induced mutations that disrupt synaptic marker localization in TRNs expressing jsIs37 (Pmec-7snb-1∷gfp;Nonet 1999; Schaefer et al. 2000).
Strain NM2689 expressing jsIs821 was created by ballistic transformation of unc-119 mutants (Praitis et al. 2001) with plasmid NM1234 (see below). jsIs821 maps to the X chromosome near lon-2 and was backcrossed six times into the wild-type strain N2 before being crossed into mutant backgrounds. jsIs821 is likely a single-copy insertion since it expresses at similar levels to known single-copy integrants of the same insert fragment integrated on chromosome II at ttTi5605 using Mos1-mediated excision (Frokjaer-Jensen et al. 2008).
Strain TU3392 expressing uIs62 was generated through germline transformation by injection of plasmid TU#875 (see below) into lin-15(n765ts) animals. Once transformed, the mec-15∷gfp transgene was integrated into the C. elegans genome using γ-radiation (Mello and Fire 1995).
rpm-1(ok364), dlk-1(km12), mkk-4(ok1545), pmk-3(ok169), dpy-21(e428), and dpy-8; mnDp30 mutant strains were provided by the Caenorhabditis Genetics Center (funded by the National Institutes of Health National Center for Research Resources). mec-15(tm2807) was obtained from the National Bioresource Project in Japan. All double, triple, and quadruple mutants generated in this paper were verified by PCR-based sequencing by GeneWiz (North Brunswick, NJ).
Plasmid construction:
Plasmid NM1234 was created in two steps from plasmid NM1023 (Mahoney et al. 2006). First, the rab-3 promoter was replaced with a PstI–NcoI mec-7 promoter fragment derived from pPD52.102 (a gift of Andy Fire) to create plasmid NM1028. Second, a HincII–XbaI fragment containing the unc-119 gene from pMM016 (Praitis et al. 2001) was inserted into SnaBI–XbaI-digested NM1028.
Plasmid TU#875 was generated using the GFP expression vector pPD95.75 (a gift from Andy Fire, Stanford University, Stanford, CA; Fire Laboratory Vector kit; ftp://www.ciwemb.edu/pub/FireLabInfo/FireLabVectors). The MEC-15∷GFP gene fusion was constructed by amplifying the full coding sequence of mec-15 with 3.2-kb upstream DNA using PCR and then cloning it into the SphI and MscI sites of pPD95.75.
Immunochemistry:
Whole-mount immunochemistry was carried out as described previously (Savage et al. 1994). Primary rabbit polyclonal antibody against MEC-18 (S. Zhang and M. Chalfie, unpublished data), MEC-7 (Mitani et al. 1993), and GFP (Invitrogen) as well as T6739 monoclonal anti-acetylated tubulin (Sigma) were used at a dilution of 1:1000; rabbit polyclonal MEC-2 antibody (Zhang et al. 2004) was used at a dilution of 1:200. Secondary antibodies cyanine Cy3 goat anti-rabbit IgG and tetramethyl rhodamine tetramethyl (TRITC) goat anti-mouse IgG (Jackson ImmunoResearch) were used at a dilution of 1:1000.
Cloning and molecular characterization of mec-15:
mec-15(u75) was mapped by three-factor crosses to the region between unc-4 and rol-1 on chromosome II. Deletion mapping with mnDf29 and mnDf59 further narrowed the gene between map positions 2.00158 and 2.4948, a region of ∼20 cosmids. The js414 mutation failed to complement mec-15(u75) and was mapped to an identical position via similar deletion mapping. Using Snip-SNP mapping with the Hawaiian strain CB4856 (Wicks et al. 2001), we narrowed the position of mec-15 to the four cosmids between and including T01E8 and ZK970. Sequencing all 13 predicted genes in this region, we found lesions only in T01E8.4.
Gene rescue:
For initial rescue of mec-15(u75) animals, a PCR fragment spanning the T01E8.4 gene with 3.57 kb of upstream and 1.6 kb downstream DNA were injected at 10 ng/μl and transformed as previously described (Mello and Fire 1995). TRN-specific rescue of mec-15(js414); jsIs821 was performed via injection (10 ng/μl) of a plasmid expressing Pmec-18mec-15; 500 bases of the mec-18 promoter upstream from the start of translation were inserted into the HindIII–BamHI sites in pBSKII(+), and the wild-type mec-15 coding sequence (with 1.06 kb of downstream DNA) was inserted immediately thereafter into SpeI–EagI sites.
Touch sensitivity:
Touch sensitivity of worms was tested by stroking the animals with an eyebrow hair attached to a toothpick (Chalfie and Sulston 1981). Wild-type animals respond to touches to the anterior body by moving backward and to posterior touches by accelerating forward. Each animal was tested 10 times by alternately touching the anterior and posterior.
Microscopy:
For fluorescence microscopy, animals were anesthetized using 0.3 m 2-3 butanedione monoxime in 10 mm HEPES (M. Goodman and M. Chalfie, unpublished data) and were observed using a Zeiss Axiophot microscope. Images for all figures except Figure S2, A, Figure S2, C, and Figure 3C were taken with a Diagnostic Instruments Spot 2 camera at the same settings using a Plan-APOCHROMAT ×63 objective. Images for Figure S2, A and Figure S2, C used a Plan NEOFUAR ×40 objective; images in Figure 3C used a Plan NEOFUAR ×25 objective. To make images clearer for publication, all images in a set were treated equally to enhance contrast and brightness. Images for reported observations were made for at least 30 animals per mutation or condition with similar results.
Figure 3.—
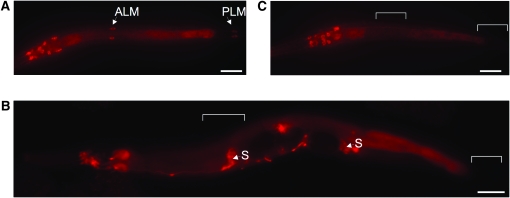
MEC-15∷GFP is expressed in the TRNs in a mec-3-dependent and stage-specific manner. Immunostaining against GFP in (A) L2 larvae, (B) young adult, and (C) mec-3(e1338) L2 larva expressing uIs62(mec-15∷gfp). TRN expression (arrowheads) is absent in adults and mec-3 mutants (positions of TRN cell bodies are designated by brackets; “S” indicates expression in spermathecae). Bars, 20 μm.
Quantification of nerve ring fluorescence:
Nerve rings of young, gravid adults (4 days after hatching if grown at 20°, 3 days if grown at 25°, 6 days if grown at 15°) were photographed at ×63 magnification and the same exposure. JPEG images were cropped identically and rotated to position the nerve rings in the same orientation. We measured the integrated density of intensity of a 250- × 250-pixel square containing the nerve ring using ImageJ 1.40g (http://rsb.info.nih.gov/ij/). To normalize each sample for background, we subtracted the minimum intensity value multiplied by the same pixel area. The remaining integrated density was divided by 100,000 to generate the fluorescence value for one animal. The fluorescence values from 15 animals for each genotype/condition were averaged to produce the score for nerve ring GFP∷RAB-3 accumulation given in Tables 1–3.
TABLE 1.
Temperature sensitivity of mec-15(js414) Sam and cell-body defects
| Nerve ring GFP∷RAB-3 accumulationa |
No. of VNC GFP patchesb |
Average no. of mutant ALM/PLM somasbc |
||||
|---|---|---|---|---|---|---|
| Temperature | Wild type | mec-15 | Wild type | mec-15 | Wild type | mec-15 |
| 15° | 4.2 ± 0.2 | 3.5 ± 0.1 | 2.0 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 2.1 ± 0.2 |
| 20° | 4.6 ± 0.1 | 3.4 ± 0.1 | 1.9 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 | 2.8 ± 0.2 |
| 25° |
5.8 ± 0.2 |
3.1 ± 0.1 |
1.9 ± 0.1 |
0.1 ± 0.1 |
0.0 ± 0.0 |
2.8 ± 0.1 |
Values indicate means ± SEMs.
n = 15; determined as described in materials and methods.
n = 30.
Each animal has two ALM and two PLM neurons; scoring as in Figure S1.
TABLE 2.
Synapse and cell-body defects (at 20°)
| Strain | Nerve ring GFP∷RAB-3 accumulationa | No. of VNC GFP patchesb | Average no. of mutant ALM/PLM somasbc |
|---|---|---|---|
| Wild type | 4.6 ± 0.1 | 1.9 ± 0.1 | 0.0 ± 0.0 |
| mec-15(js414) | 3.4 ± 0.1 | 0.1 ± 0.1 | 2.8 ± 0.2 |
| rpm-1(ok364) | 4.0 ± 0.1 | 0.8 ± 0.1 | 0.0 ± 0.0 |
| dlk-1(km12) | 4.5 ± 0.1 | 1.9 ± 0.1 | 0.0 ± 0.0 |
| pmk-3(ok169) | 4.4 ± 0.1 | 1.9 ± 0.1 | 0.0 ± 0.0 |
| mec-15; rpm-1d | 3.3 ± 0.2 | 0.0 ± 0.0 | 1.6 ± 0.2 |
| dlk-1; mec-15d | 2.6 ± 0.1 | 0.0 ± 0.0 | 3.9 ± 0.1 |
| mec-15; pmk-3d | 2.7 ± 0.1 | 0.0 ± 0.0 | 3.9 ± 0.1 |
| mec-7(u443) | 2.6 ± 0.1 | 0.0 ± 0.0 | 1.2 ± 0.2 |
| mec-15; mec-7e | 2.7 ± 0.1 | 0.0 ± 0.0 | 0.8 ± 0.2 |
| mec-15; mec-7/+f | 4.7 ± 0.2 | 1.9 ± 0.1 | 0.0 ± 0.0 |
| dlk-1; mec-7 | 2.5 ± 0.1 | 0.0 ± 0.0 | 3.9 ± 0.1 |
| dlk-1; mec-7/+ | 4.3 ± 0.1 | 2.0 ± 0.0 | 0.0 ± 0.0 |
| dlk-1; mec-15; mec-7d | 2.2 ± 0.1 | 0.0 ± 0.0 | 3.9 ± 0.1 |
| dlk-1; mec-15; mec-7+d | 4.5 ± 0.2 | 1.9 ± 0.1 | 0.1 ± 0.1 |
| mec-15(ju75) | 3.2 ± 0.1 | 0.0 ± 0.0 | 2.8 ± 0.1 |
| mec-12(e1607) | 2.7 ± 0.1 | 0.0 ± 0.0 | 2.8 ± 0.2 |
| mec-15; mec-12e | 2.6 ± 0.1 | 0.0 ± 0.0 | 3.4 ± 0.1 |
| mec-15; mec-12/+e | 2.8 ± 0.1 | 0.0 ± 0.0 | 3.0 ± 0.2 |
| mec-15/+e | 4.4 ± 0.1 | 1.9 ± 0.1 | 0.1 ± 0.1 |
| mec-12/+ | 3.2 ± 0.1 | 0.6 ± 0.2 | 3.3 ± 0.1 |
|
mec-15/+; mec-12/+e |
3.1 ± 0.1 |
0.0 ± 0.0 |
3.4 ± 0.2 |
Values indicate means ± SEMs.
n = 15; determined as described in materials and methods.
n = 30.
Each animal has two ALM and two PLM neurons; scoring as in Figure S1.
mec-15 allele is js414.
mec-15 allele is u75.
mec-15 allele is js414/u75.
TABLE 3.
Synapse and cell-body defects (at 15°)
| Strain | Nerve ring GFP accumulationa | No. of VNC GFP patchesb | Average no. of mutant ALM/PLM somasbc |
|---|---|---|---|
| Wild type | 4.2 ± 0.2 | 2.0 ± 0.0 | 0.0 ± 0.0 |
| mec-15(js414) | 3.5 ± 0.1 | 0.2 ± 0.1 | 2.1 ± 0.2 |
| rpm-1(ok364) | 3.6 ± 0.1 | 1.5 ± 0.1 | 0.0 ± 0.0 |
|
mec-15, rpm-1d |
3.0 ± 0.1 |
0.0 ± 0.0 |
0.9 ± 0.2 |
Values indicate means ± SEMs.
n = 15; determined as described in materials and methods.
n = 30.
Each animal has two ALM and two PLM neurons; scoring as in Figure S1.
mec-15 allele is js414.
RESULTS
mec-15 is required for TRN mechanosensation, synapse development, and cell-body morphology:
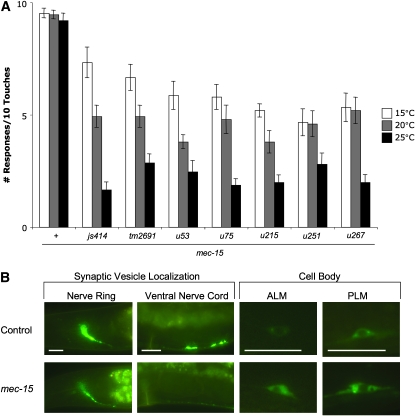
Mutations in mec-15 were originally identified because they caused animals to be insensitive to touch (Chalfie and Au 1989). Five noncomplementing alleles (u53, u75, u215, u251, and u267) were generated, each conferring partial touch insensitivity. An additional allele, js414, arose in a screen for mutants with defective TRN chemical synapses (see materials and methods). All six mutations produce a variable touch insensitivity, with some animals responding to many touches while others fail to respond after a few touches. For all alleles, the severity of the phenotype increased at higher temperatures (Figure 1A).
Figure 1.—
mec-15 mutations produce temperature-sensitive defects in TRN mechanosensation, synapse formation, and cell-body morphology. (A) mec-15 mutants show a reduction in touch sensitivity that increases with temperature. Error brackets indicate SEM; n ≥ 15. (B) GFP∷RAB-3 expression in nerve ring, VNC, ALM, and PLM neurons in young adult wild type and mec-15(js414) animals grown at 20°. Bars, 20 μm.
All these mutants share two other phenotypes: they have defects in the localization of presynaptic markers and distorted cell bodies. To visualize TRN chemical synapses, we generated an integrated transgene (jsIs821) that expresses a fusion of RAB-3, a Rab GTPase that regulates synaptic transmission (Mahoney et al. 2006), with GFP under the mec-7 promoter (Pmec-7gfp∷rab-3). In wild-type animals, the GFP fusion accumulates in the nerve ring and in two visible patches midway along the ventral nerve cord (VNC; Figure 1B). These areas are where the anterior TRNs (ALML, ALMR, and AVM) and the posterior TRNs (PLML and PLMR) form chemical synapses, respectively (Chalfie et al. 1985; White et al. 1986). This pattern was disrupted in all mec-15 mutant backgrounds; nerve ring fluorescence was often significantly reduced and visible GFP patches in the VNC were abolished (this phenotype is referred to as Sam for synaptic vesicle tag abnormal in mechanosensory neurons). Additionally, mec-15 mutations distorted cell-body morphology, producing somas that were generally larger and misshapen and accumulated more GFP (a representative example of Sam and cell-body phenotypes is given in Figure 1B). As with the mec-15 mechanosensory defect, the Sam and cell-body phenotypes were greater at higher temperatures (Table 1; scoring criteria for mutant cell bodies is presented in Figure S1).
Despite the general effects of mec-15 mutations on the cell bodies and synapses of the TRNs, other features of these cells appear normal. MEC-2, a mechanosensory channel subunit, is distributed as regular puncta throughout the TRN process (Huang et al. 1995; Zhang et al. 2004) that relies on intact microtubules (A. Bounoutas et al., unpublished results). This distribution was unchanged in mec-15 animals, indicating no obvious defects in axonal transport (Figure S2, A). MEC-18 is a TRN-specific enzyme that is diffusely expressed in the cytoplasm (Gu 1998); its levels were unchanged in mec-15 mutants (Figure S2, B). Finally, we looked at expression of MEC-7 β-tubulin and acetylated α-tubulin, both of which comprise the large, 15-protofilament microtubules uniquely found in these cells. Both markers appeared normal in mec-15 mutants (Figure S2, C and Figure S2, D), suggesting that density of TRN microtubules was unaffected.
mec-15 encodes an F-Box-containing protein with WD repeats:
mec-15 is the previously uncharacterized gene T01E8.4 (see materials and methods). T01E8.4 encodes a protein with an F-box domain and WD repeats. Both motifs are involved in protein–protein interactions and predict a role for MEC-15 in SCF (Skp1, Cullin, F-box containing) ubiquitin ligase complexes that target proteins for proteasomal degradation (Kipreos and Pagano 2000). The F-box domain binds the Skp subunit, while the WD domains provide interactions that determine substrate specificity.
All six mec-15 mutations affect the coding sequence. The u53 and u267 alleles have missense mutations in the WD repeats domain; the u267 allele contains an additional missense mutation in the F-box domain (Figure 2A). The remaining mutations result in early nonsense codons that lead to a protein that is truncated after the F-box domain. We believe that all six mec-15 lesions produce the complete loss-of-function phenotype because all alleles produced the same phenotype and placing alleles (u75 and u215) over deletions of the region (e.g., mnDf29) did not enhance the mutant defects (data not shown). In addition, mec-15(tm2691), an allele containing a 352-bp deletion that results in a frameshift after the first 28 amino acids [generated by the National BioResource Project through random TMP/UV mutagenesis (Gengyo-Ando and Mitani 2000)] produced the same defects as the other mutant alleles (the Mec phenotype is shown in Figure 1A), including their temperature sensitivity. Moreover, animals doubly mutant for a mec-15 allele (u75, u251, u267, js414) and smg-4(ma116), which prevents nonsense-mediated mRNA decay (Pulak and Anderson 1993), exhibited no additional phenotypes (data not shown). These results suggest that all mutations, even u251, which truncates the gene after the F-box and WD domains, are probably null alleles.
Figure 2.—
mec-15 mutations and rescue. (A) Schematic of MEC-15 protein with notable domains and location of mutant lesions. tm2691 produces a deletion after the first 28 amino acids (black line), resulting in a frameshifted protein sequence that terminates shortly thereafter (red dashed line). (B) Touch sensitivity and (C) vulva Sam phenotype of mec-15(js414) animals are rescued with Pmec-18mec-15(+). Experiments were performed at 25°. Error brackets indicate SEM; n = 20.
We verified the identity of mec-15 as T01E8.4 by transgene rescue. mec-15(u75) mutants transformed with the wild-type T01E8.4 gene with 3 kb of upstream DNA were fully touch sensitive (data not shown). This rescue required expression only in the TRNs because transforming mec-15(js414); jsIs821 animals with the wild-type mec-15 gene expressed from the TRN-specific mec-18 promoter (Zhang et al. 2002) yielded wild-type, i.e., nonMec and nonSam, animals (Figure 2, B and C).
mec-15 is expressed in the TRNs in a stage and mec-3-dependent manner:
We generated animals expressing an integrated mec-15∷gfp transgene (uIs62) to characterize MEC-15 expression and distribution (Figure 3). As predicted, MEC-15 is expressed in the TRNs as well as several neurons in the head, tail, and ventral cord (MEC-15 is also found in other tissues such as the spermathecae). MEC-15 localization in neurons was diffuse and non-nuclear. MEC-15 expression in TRNs was larva specific; GFP fluorescence was reduced in the last (L4) larval stage and absent in adults (Figure 3, A and B; MEC-15 expression in other neurons persisted in adults). Larval expression in TRNs is absent in mec-3 mutants (Figure 3C), indicating that its transcription in these neurons requires the MEC-3 transcription factor required for TRN differentiation (Duggan et al. 1998).
Mutations in a p38 MAPK pathway enhance mec-15 phenotypes:
In C. elegans motor neurons, another F-box protein, FSN-1, forms an SCF complex with the Skp and Cullin homologs SKR-1 and CUL-1, respectively, to regulate levels of proteins involved in presynaptic differentiation (Liao et al. 2004). This SCF complex associates with a large RING-finger protein, RPM-1, to negatively regulate targets including SCD-2 and a p38 MAPK pathway consisting of the MAPKKK DLK-1, the MAPKK MKK-4, and the p38 MAPK PMK-3 (Liao et al. 2004; Nakata et al. 2005).
rpm-1 mutants produce a temperature-sensitive Sam phenotype similar to mec-15 mutations, although TRNs in rpm-1 mutants have normal cell bodies and the animals are touch sensitive (Schaefer et al. 2000). fsn-1 mutants do not produce a Sam phenotype (data not shown), suggesting that MEC-15 may be the F-box protein that associates with RPM-1 to regulate synaptogenesis in the TRNs. To test this hypothesis, we generated mec-15(js414) double mutants with rpm-1(ok364) as well as dlk-1(km12), mkk-4(ok1545), and pmk-3(ok169), which suppress the rpm-1 phenotypes.
The genetic interactions of rpm-1 and mec-15 are complex. At 20°, the rpm-1(ok364) mutation had no significant effect on mec-15 Mec or Sam defects and partially suppressed the cell-body phenotype (Figure 4 and Table 2). At 15°, rpm-1 slightly enhanced the Sam phenotype while further suppressing the cell-body phenotype (Table 3). These results suggest that, while both mec-15 and rpm-1 promote chemical synaptogenesis, they function antagonistically to each other in determining cell shape.
Figure 4.—
Mutations in a p38 MAPK pathway enhance all mec-15 phenotypes. (A) Touch sensitivity experiments of animals grown at 20°. Error brackets indicate SEM; n = 40. Asterisks (*) indicate significant (P < 0.00001) decrease of touch sensitivity compared to mec-15(js414) animals. (B) Synapse formation and cell bodies visualized by GFP∷RAB-3. All animals for these experiments were grown at 20°. Bars, 20 μm.
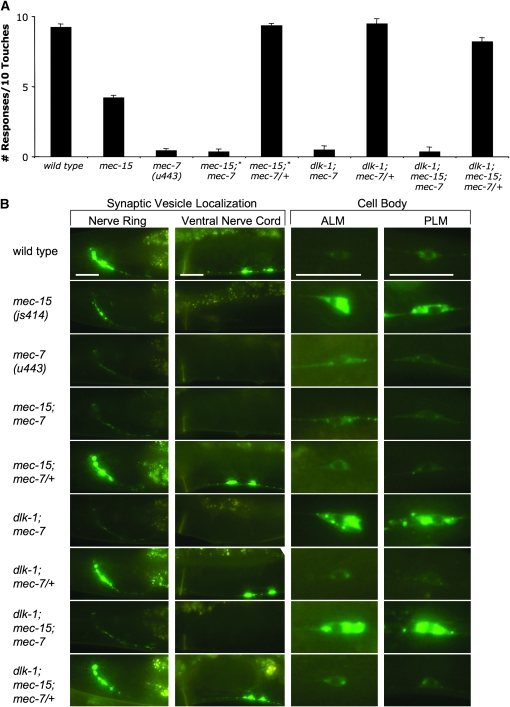
Instead of suppressing mec-15 mutations (as predicted if mec-15 acted through the p38 MAPK pathway), mutations in dlk-1, mkk-4, and pmk-3 enhanced all of the mec-15 TRN phenotypes. Double mutants responded significantly less to touch (Figure 4A) and had a more severe Sam phenotype, with further reduction of GFP∷RAB-3 fluorescence in the nerve ring and complete absence of fluorescence in the ventral nerve cord (Figure 4B and Table 2; mkk-4 doubles were not examined). Furthermore, the double mutants displayed a markedly enhanced penetrance and severity of the cell-body defect, with TRN somas even larger and more misshapen (Figure 4B and Table 2). dlk-1, mkk-4, and pmk-3 mutants without a mec-15 mutation do not have any of these defects. Thus, the increased severity of defects in the mec-15 double mutants suggests a redundant function. We believe that MEC-15 functions in parallel to this p38 MAPK pathway to regulate TRN development and synaptogenesis; the absence of both sets of genes drastically disrupts these processes.
mec-15 phenotypes are dominantly suppressed by mutations in mec-7:
Another gene required for touch sensitivity, mec-7, encodes a β-tubulin that is needed for the formation of the large-diameter (15-protofilament) microtubules found only in the TRNs (Savage et al. 1989). Previous characterization of the mec-15(u75) mutation revealed that loss of a single copy of mec-7(+) suppressed the mec-15 Mec phenotype (Gu et al. 1996). We confirmed this result by generating mec-15(u75, u251, u267, and js414); mec-7(u443)/+ doubles [the u443 deletes the mec-7 gene (Savage et al. 1994)]; all of these animals were touch sensitive (Figure 5A).
Figure 5.—
mec-7 mutations dominantly suppress all mec-15 phenotypes. (A) Touch sensitivity experiments of animals grown at 20°. Error brackets indicate SEM; n = 40. (B) Synapse formation and cell bodies visualized by GFP∷RAB-3. The mec-15 allele in all experiments is js414, with the exception of mec-15(u75); mec-7 and mec-15(u75/js414); mec-7/+. All animals for these experiments were grown at 20°. Bars, 20 μm.
Using the jsIs821 transgene, we characterized the Sam and cell-body phenotypes of mec-7(u443), mec-15(u75); mec-7(u443) double mutants and mec-15 mutants with one copy of mec-7(u443) (Figure 5B and Table 2). mec-7 mutations alone produced a severe Sam phenotype. Loss of large-diameter microtubules in mec-7 mutants produces general defects in axonal transport and TRN gene expression (Bounoutas et al. 2009; A. Bounoutas, L. Emtage and M. Chalfie, unpublished data), so reduction of visible GFP∷RAB-3 at synapses may be attributable to these defects. The cell bodies of mec-7 TRNs sometimes had irregular, elongated shapes, but they generally did not have both the atypical morphology and increased fluorescence observed in mec-15 animals (Figure 5B and Figure S1). mec-15; mec-7 mutants appeared identical to mec-7 animals; the mec-15 cell-body phenotype was suppressed (Figure 5B and Table 2), indicating that mec-7 is epistatic to mec-15. When only one copy of wild-type mec-7 was present, all mec-15 phenotypes were fully suppressed (Figure 5 and Table 2). These results indicate that mec-15 mutations require a suitable level of MEC-7 to produce their mutant phenotypes.
Because loss of dlk-1 and other genes of the p38 MAPK pathway enhances the mec-15 phenotype, we tested whether the dominant suppression by mec-7 required these genes. If these genes were required, we would expect that dlk-1; mec-15; mec-7/+ animals would have the same phenotypes as mec-15 mutants. The Mec, Sam, and cell-body phenotypes of dlk-1; mec-15; mec-7/+ animals, however, were significantly suppressed (Figure 5 and Table 2), suggesting that functional dlk-1 and the MAPK pathway are not required for mec-7 dominant suppression of mec-15. [In preparing these multiply mutant strains, we also found that dlk-1;mec-7 and dlk-1; mec-15; mec-7 mutants looked indistinguishable from dlk-1; mec-15 animals (Figure 4B, Figure 5B, Table 2)].
Because mec-15 animals require a suitable amount of MEC-7 to produce mutant phenotypes, we examined whether increasing mec-7 gene expression could phenocopy mec-15 defects. We looked at touch sensitivity and GFP∷RAB-3 in animals containing the free duplication mnDp30, which covers mec-7, as well as dpy-21 mutants, which increase expression of genes on the X chromosome (where mec-7 is located). Both sets of animals responded normally to touch and produced wild-type synapse and cell bodies (data not shown), suggesting that increasing MEC-7 mRNA production is not enough to produce mec-15 phenotypes. However, because levels of free MEC-7 tubulin autoregulate the stability of its transcript (Savage et al. 1994), we cannot rule out that these genetic backgrounds fail to produce more MEC-7 tubulin protein, especially if MEC-15 provides additional negative regulation at the protein level.
mec-15 phenotypes are dominantly enhanced by mec-12:
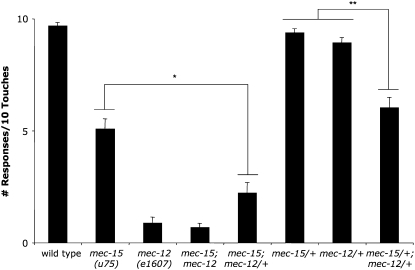
Since one copy of the mec-7 gene suppressed mec-15 phenotypes, we examined whether one copy of mec-12, which encodes the α-tubulin of large-diameter TRN microtubules, would produce a similar effect. Unlike mec-7, no true mec-12 nulls exist, although one allele, e1607, is a recessive missense mutation that fails to produce immuno-detectable acetylated α-tubulin (Fukushige et al. 1999). Interestingly, mec-12(e1607) dominantly enhanced the Mec phenotype of mec-15(u75) (Figure 6). Moreover, double heterozygote mec-15/+; mec-12/+ mutants produced a pronounced touch insensitivity defect not observed in either singly heterozygous animal (Figure 6). Therefore, loss of mec-12 produces the opposite effect on mec-15 touch sensitivity compared to mec-7.
Figure 6.—
mec-12(e1607) dominantly enhances mec-15 touch insensitivity. The mean number of responses resulting from 10 touches to 20 animals grown at 20°. Error brackets indicate SEM. A single asterisk (*) indicates a significant decrease (P < 0.0001) of touch sensitivity compared to mec-15(u75) animals; double asterisks (**) indicate a significant decrease (P < 0.00001) of touch sensitivity compared to mec-15(u75)/+ and mec-12(e1607)/+ animals.
We also analyzed the Sam and cell-body phenotypes of animals carrying the mec-12(e1607) allele using the jsIs821 transgene (Table 2). Homozygous mec-12(e1607) animals had a Sam phenotype nearly indistinguishable from mec-7(u443) animals. However, the cell bodies in these mutants were often larger and more intensely fluorescent than those observed in mec-15 mutants (Figure S1). In addition, unlike mec-7(u443) heterozygotes, mec-12(e1607) heterozygous animals had Sam and cell-body defects (Table 2). These results indicate that mec-7 and mec-12 mutations do not produce identical defects with respect to synapse formation and cell-body morphology.
mec-12 mutations also enhanced mec-15 synaptic and cell-body defects. One copy of mec-12 significantly enhanced the Sam phenotype of mec-15 (Table 2). mec-15; mec-12 double mutants had a Sam phenotype similar to that of mec-12 mutants with enhanced penetrance of cell-body phenotype (Table 2). Finally, double heterozygotes (mec-15/+; mec-12/+) enhanced the Sam phenotype compared to mec-15/+ and mec-12/+ animals (Table 2).
DISCUSSION
A model for MEC-15 function:
We envision that MEC-15 functions, as do many F-box proteins, in an SCF complex to regulate the levels of proteins required for TRN shape and synaptogenesis. Indeed, MEC-15 interacts with the Skp homolog SKR-1 in yeast two-hybrid experiments (Yamanaka et al. 2002), predicting a role in SCF ubiquitin ligases. Although the target substrate (or substrates) of MEC-15-binding remains unknown, the genetic interactions with mec-7 suggest that this β-tubulin or the microtubules containing it are important for the mutant phenotypes. With only one copy of functional mec-7, all of the mec-15 mutant phenotypes are absent. When MEC-7 is absent, the cell-body phenotype of mec-15 TRNs is suppressed, although touch insensitivity and the Sam phenotype persist. These latter phenotypes are likely due solely to the mec-7 mutation and the lack of specialized microtubules necessary for mechanosensation and for protein expression and transport (Bounoutas et al. 2009).
MEC-7 β-tubulin may itself be targeted for proteasomal degradation by MEC-15. In this scenario, elevated levels of free MEC-7 could underlie mec-15 phenotypes. This hypothesis is consistent with the observation that a recessive mec-12 mutation enhances mec-15 phenotypes, since reducing the available pool of MEC-12 α-tubulin may increase levels of free MEC-7 β-tubulin. Unfortunately, because touch receptor neurons are densely packed with the large-diameter microtubules, free tubulin probably accounts for a very small percentage of total tubulin in the neuron, making measurement of free β-tubulin levels using the MEC-7 antibody difficult. Future work will focus on testing this hypothesis through other biochemical means.
mec-15 and MAP kinases:
MEC-15 and the p38 MAPK pathway probably function in parallel in TRN development. Mutations of dlk-1, mkk-4, or pmk-3 alone do not cause touch insensitivity, loss of synaptic markers, or aberrant cell-body morphology. However, placing these mutations in a mec-15 background enhances all of these mec-15 mutant phenotypes, exposing redundancies in MAPK signaling.
The relationship between targets of MEC-15 and the p38 MAPK pathways in regulating synaptogenesis appears complex. Mutations in the p38 MAPK pathway have been reported to increase synaptic vesicle docking (Nakata et al. 2005), while mutations in rpm-1, which negatively regulates this pathway, reduce synaptic marker localization in the TRNs (Schaefer et al. 2000). mec-15 mutations produce Sam phenotypes similar to rpm-1, suggesting that MEC-15 also targets proteins required for synapse formation. Since mec-15 synaptic defects are enhanced by rpm-1 mutations, both ubiquitin ligase pathways are likely to be needed for proper synaptogenesis.
Interestingly, mutations in the p38 MAPK pathway also enhance mec-15 synaptic phenotypes. The fact that both rpm-1 and MAPK mutations enhance mec-15 suggests that rpm-1 has targets separate from the MAPK cascade that regulate TRN synaptogensis. A similar situation has been found in motor neurons (Liao et al. 2004). For their part, DLK-1, MKK-4, and PMK-3 appear to work antagonistically with the targets of MEC-15 in promoting TRN synapse formation.
MEC-15 targets and the p38 MAPK pathway also appear to function antagonistically to regulate cell-body shape. Loss of MEC-15 results in larger, irregularly shaped TRNs, suggesting that its target(s) promotes growth and morphogenesis. Mutations in the p38 MAP kinase pathway enhance these defects, indicating that they normally function to suppress cell growth. As predicted by this model, increasing levels of DLK-1 partially compensate for the lack of MEC-15 in morphogenesis, since mutations in rpm-1, which negatively regulate DLK-1 levels, significantly suppress the mec-15 cell-body phenotypes.
We also found that dlk-1 and mec-7 mutations act synthetically to produce abnormal TRN cell-body morphology that mimics the defect seen in dlk-1; mec-15 animals. A connection between a p38 MAPK pathway and the microtubules was first observed in mouse neurons, where elevated levels of DLK activate a p38 MAPK pathway to regulate cytoskeletal organization in growth cones (Lewcock et al. 2007). In C. elegans, the p38 MAPK pathway has been implicated in axon termination and neuronal regeneration, processes that depend on regulating microtubule dynamics (Grill et al. 2007; Hammarlund et al. 2009). The morphological defects in dlk-1; mec-7 TRNs suggest that without an active p38 MAPK pathway to respond to the loss of the MEC-7 β-tubulin, the remaining cytoskeleton is in disarray, resulting in abnormal cell-body growth.
Why mec-15 and mec-7 produce identical morphology defects with dlk-1 remains unclear. One possibility is that MEC-15 negatively regulates a protein that also functions in cytoskeletal organization, which is not unlikely given its dependence on MEC-7 levels. Overexpression of this protein could disrupt microtubule function, producing cell-body defects; without active DLK to compensate, the severity of morphological abnormalities increases.
MEC-15 and mechanosensation:
We do not believe that the touch insensitivity phenotype of mec-15 is due to the observed synaptic defects. Chemical synapses, unlike gap junctions, are inhibitory in nature and not directly required for the touch response (Chalfie et al. 1985; Wicks and Rankin 1995), and rpm-1 mutants produce a similar Sam phenotype without significant touch insensitivity. Loss of touch sensitivity in mec-15 mutants may be attributable to defects in TRN development or to effects on the formation of gap junction connections. While general gene expression and transport of mechanosensory channel subunits appear to be wild type in mec-15 TRNs, their cell bodies are clearly deformed. If loss of MEC-15 causes an overexpression of a target protein involved in cytoskeletal organization, the structure and/or orientation of TRN microtubules required for mechanosensation may be disrupted. Alternately, elevated levels of this protein may be toxic to the cell. Either condition would suppress the optimal response to touch.
Acknowledgments
We thank Oliver Hobert for suggestions for the mapping of mec-15. This work was supported by National Institutes of Health grants GM30997 to M.C. and NS40094 to M.L.N.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.105726/DC1.
References
- Bounoutas, A., and M. Chalfie, 2007. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 454 691–702. [DOI] [PubMed] [Google Scholar]
- Bounoutas, A., R. O'Hagan, and M. Chalfie, 2009. The multipurpose 15-protofilament microtubules in C. elegans have specific roles in mechanosensation. Curr. Biol. 19 1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie, M., and M. Au, 1989. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243 1027–1033. [DOI] [PubMed] [Google Scholar]
- Chalfie, M., and J. Sulston, 1981. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82 358–370. [DOI] [PubMed] [Google Scholar]
- Chalfie, M., J. E. Sulston, J. G. White, E. Southgate, J. N. Thomson et al., 1985. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan, A., C. Ma and M. Chalfie, 1998. Regulation of touch receptor differentiation by the Caenorhabditis elegans mec-3 and unc-86 genes. Development 125 4107–4119. [DOI] [PubMed] [Google Scholar]
- Frokjaer-Jensen, C., M. W. Davis, C. E. Hopkins, B. J. Newman, J. M. Thummel et al., 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige, T., Z. K. Siddiqui, M. Chou, J. G. Culotti, C. B. Gogonea et al., 1999. MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. J. Cell Sci. 112(Pt. 3): 395–403. [DOI] [PubMed] [Google Scholar]
- Gengyo-Ando, K., and S. Mitani, 2000. Characterization of mutations induced by ethyl methanesulfonate, UV, and trimethylpsoralen in the nematode Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 269 64–69. [DOI] [PubMed] [Google Scholar]
- Grill, B., W. V. Bienvenut, H. M. Brown, B. D. Ackley, M. Quadroni et al., 2007. C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1. Neuron 55 587–601. [DOI] [PubMed] [Google Scholar]
- Gu, G., 1998. A molecular model of mechanosensory transduction in Caenorhabditis elegans. Ph.D. Thesis, Columbia University, New York.
- Gu, G., G. A. Caldwell and M. Chalfie, 1996. Genetic interactions affecting touch sensitivity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 93 6577–6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund, M., P. Nix, L. Hauth, E. M. Jorgensen and M. Bastiani, 2009. Axon regeneration requires a conserved MAP kinase pathway. Science 323 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M., G. Gu, E. L. Ferguson and M. Chalfie, 1995. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature 378 292–295. [DOI] [PubMed] [Google Scholar]
- Kipreos, E. T., and M. Pagano, 2000. The F-box protein family. Genome Biol. 1: REVIEWS3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewcock, J. W., N. Genoud, K. Lettieri and S. L. Pfaff, 2007. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron 56 604–620. [DOI] [PubMed] [Google Scholar]
- Liao, E. H., W. Hung, B. Abrams and M. Zhen, 2004. An SCF-like ubiquitin ligase complex that controls presynaptic differentiation. Nature 430 345–350. [DOI] [PubMed] [Google Scholar]
- Mahoney, T. R., Q. Liu, T. Itoh, S. Luo, G. Hadwiger et al., 2006. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol. Biol. Cell 17 2617–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello, C., and A. Fire, 1995. DNA transformation. Methods Cell Biol. 48 451–482. [PubMed] [Google Scholar]
- Mitani, S., H. Du, D. H. Hall, M. Driscoll and M. Chalfie, 1993. Combinatorial control of touch receptor neuron expression in Caenorhabditis elegans. Development 119 773–783. [DOI] [PubMed] [Google Scholar]
- Nakata, K., B. Abrams, B. Grill, A. Goncharov, X. Huang et al., 2005. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 120 407–420. [DOI] [PubMed] [Google Scholar]
- Nonet, M. L., 1999. Visualization of synaptic specializations in live C. elegans using synaptic vesicle-GFP protein fusions. J. Neurosci. Methods 89 33–40. [DOI] [PubMed] [Google Scholar]
- Praitis, V., E. Casey, D. Collar and J. Austin, 2001. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics 157 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulak, R., and P. Anderson, 1993. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 7 1885–1897. [DOI] [PubMed] [Google Scholar]
- Savage, C., M. Hamelin, J. G. Culotti, A. Coulson, D. G. Albertson et al., 1989. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev. 3 870–881. [DOI] [PubMed] [Google Scholar]
- Savage, C., Y. Xue, S. Mitani, D. Hall, R. Zakhary et al., 1994. Mutations in the Caenorhabditis elegans beta-tubulin gene mec-7: effects on microtubule assembly and stability and on tubulin autoregulation. J. Cell Sci. 107(Pt. 8): 2165–2175. [DOI] [PubMed] [Google Scholar]
- Schaefer, A. M., G. D. Hadwiger and M. L. Nonet, 2000. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 26 345–356. [DOI] [PubMed] [Google Scholar]
- Segref, A., and T. Hoppe, 2009. Think locally: control of ubiquitin-dependent protein degradation in neurons. EMBO Rep. 10 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. G., E. Southgate, J. H. Thomas and S. Brenner, 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B 314 1–340. [DOI] [PubMed] [Google Scholar]
- Wicks, S. R., and C. H. Rankin, 1995. Integration of mechanosensory stimuli in Caenorhabditis elegans. J. Neurosci. 15 2434–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterston and R. H. Plasterk, 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28 160–164. [DOI] [PubMed] [Google Scholar]
- Yamanaka, A., M. Yada, H. Imaki, M. Koga, Y. Ohshima et al., 2002. Multiple Skp1-related proteins in Caenorhabditis elegans: diverse patterns of interaction with Cullins and F-box proteins. Curr. Biol. 12 267–275. [DOI] [PubMed] [Google Scholar]
- Zhang, S., J. Arnadottir, C. Keller, G. A. Caldwell, C. A. Yao et al., 2004. MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr. Biol. 14 1888–1896. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., C. Ma, T. Delohery, B. Nasipak, B.C. Foat et al., 2002. Identification of genes expressed in C. elegans touch receptor neurons. Nature 418 331–335. [DOI] [PubMed] [Google Scholar]
- Zhen, M., X. Huang, B. Bamber and Y. Jin, 2000. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron 26 331–343. [DOI] [PubMed] [Google Scholar]