Abstract
Flowering time, a critical adaptive trait, is modulated by several environmental cues. These external signals converge on a small set of genes that in turn mediate the flowering response. Mutant analysis and subsequent molecular studies have revealed that one of these integrator genes, FLOWERING LOCUS T (FT), responds to photoperiod and temperature cues, two environmental parameters that greatly influence flowering time. As the central player in the transition to flowering, the protein coding sequence of FT and its function are highly conserved across species. Using QTL mapping with a new advanced intercross-recombinant inbred line (AI-RIL) population, we show that a QTL tightly linked to FT contributes to natural variation in the flowering response to the combined effects of photoperiod and ambient temperature. Using heterogeneous inbred families (HIF) and introgression lines, we fine map the QTL to a 6.7 kb fragment in the FT promoter. We confirm by quantitative complementation that FT has differential activity in the two parental strains. Further support for FT underlying the QTL comes from a new approach, quantitative knockdown with artificial microRNAs (amiRNAs). Consistent with the causal sequence polymorphism being in the promoter, we find that the QTL affects FT expression. Taken together, these results indicate that allelic variation at pathway integrator genes such as FT can underlie phenotypic variability and that this may be achieved through cis-regulatory changes.
MOLECULAR analysis of the phenotypic variation in life history traits is key to understanding how plants evolve in diverse natural environments. Among such traits, flowering time is critical for the reproductive success of the plant and is highly variable among natural Arabidopsis thaliana strains, providing an attractive paradigm for studying adaptive evolution (Johanson et al. 2000; Hagenblad and Nordborg 2002; Stinchcombe et al. 2004; Lempe et al. 2005; Shindo et al. 2005; Werner et al. 2005a). Two major environmental parameters that modulate flowering time are light and temperature (Koornneef et al. 1998). Temperature and light conditions vary substantially within the geographical range of A. thaliana, and natural populations presumably need to adapt to the local environment to ensure reproductive success. Flowering in A. thaliana is generally accelerated by long photoperiods, vernalization (exposure to winter-like conditions), and elevated ambient temperatures (Bäurle and Dean 2006). All these cues favor flowering of A. thaliana during spring or early summer, although the contribution from each individual cue and the interactions among them vary depending on the local environmental conditions (Wilczek et al. 2009).
Flowering time is controlled through several genetic cascades that converge on a set of integrator genes including FLOWERING LOCUS T (FT), which encodes a protein that is highly conserved in flowering plants (Kardailsky et al. 1999; Kobayashi et al. 1999; Ahn et al. 2006). FT and its homologs are very likely an integral part of the mobile signal (florigen) that is produced in leaves and travels to the shoot apex to induce flowering (Abe et al. 2005; Wigge et al. 2005; Lifschitz et al. 2006; Corbesier et al. 2007; Jaeger and Wigge 2007; Lin et al. 2007; Mathieu et al. 2007; Tamaki et al. 2007; Notaguchi et al. 2008). In A. thaliana, FT expression is controlled by photoperiod, vernalization, and ambient growth temperature. Photoperiod in conjunction with the circadian clock promotes daily oscillations in FT RNA levels, which are greatly elevated at the end of long days. The central role of FT in determining the timing of flowering appears to be conserved in many species, making FT an attractive target for altering flowering time in cereals and other plants of economic importance (recently reviewed by Kobayashi and Weigel 2007; Turck et al. 2008).
Wild strains of A. thaliana show extensive variation in flowering time and much of this is due to variation in the activity of the floral repressor FLOWERING LOCUS C (FLC). While some of this variation maps to FLC itself, much of it is due to differential activity at the epistatically acting FRIGIDA (FRI) locus (Michaels and Amasino 1999; Sheldon et al. 1999; Johanson et al. 2000; Michaels et al. 2003; Lempe et al. 2005; Shindo et al. 2005, 2006). Flowering is typically substantially delayed when the FRI/FLC system is active, unless these plants are first vernalized. However, FRI and FLC do not explain all of the flowering time variation seen in wild strains, and functionally divergent alleles of several additional flowering regulators, including CRYPTOCHROME 2 (CRY2), HUA2, FLOWERING LOCUS M (FLM), PHYTOCHROME C (PHYC), and PHYTOCHROME D (PHYD), have been identified in different strains of A. thaliana (Aukerman et al. 1997; Alonso-Blanco et al. 1998; El-Assal et al. 2001; Werner et al. 2005b; Balasubramanian et al. 2006a; Wang et al. 2007). Finally, there are many genotype-by-environment interactions that dramatically affect the contribution of a specific locus to the overall phenotype.
The study of natural variation in A. thaliana has been greatly facilitated through the use of recombinant inbred line (RIL) populations (Koornneef et al. 2004). We have recently established two advanced intercross (AI)-RIL sets, in which the genetic map is greatly expanded, allowing for high-resolution QTL mapping (Balasubramanian et al. 2009). Here we use one of the new AI-RIL populations along with an independent F2 population to identify the molecular basis of a light and temperature-sensitive flowering time QTL that mapped to the promoter of the FT gene. We show that FT is likely the causal gene for variation in light and temperature-sensitive flowering. Our results, in combination with those from other species, suggest that cis-regulatory variation rather than structural variation at FT contributes to phenotypic variation in natural populations.
MATERIALS AND METHODS
Plant material and growth conditions:
Two early flowering accessions, Est-1 [Estland (Estonia); European Arabidopsis Stock Center N6701] and Col-0 (Columbia; WT-2, Lehle Seeds, Tucson, AZ), were used to create the AI-RIL population for QTL analysis. The population consists of 279 individual lines, genotyped at 221 markers (Balasubramanian et al. 2009). A second F2 population, derived from Dra-1 (Drahonin, Czechoslovakia; N1119), a strain that behaves similarly to photoperiodic mutants (Lempe et al. 2005), and Ler (Landsberg erecta; N8581), consisted of 190 F2 plants that were genotyped at 77 markers. The markers and the ft mutant alleles have been previously described (Koornneef et al. 1991; Yoo et al. 2005; Balasubramanian et al. 2009).
Flowering time was determined under 23° in long days (LD, 16 hr light/8 hr dark) in growth rooms and under LD conditions in a greenhouse in La Jolla, CA, and under 16° LD in growth rooms in Tübingen, Germany. Short day (SD, 8 hr light/16 hr dark) experiments and flowering time QTL confirmation studies with HIF plants were conducted in a Percival Series 942 growth chamber at 16° or 22° in Madison, WI. For quantitative complementation experiments, F1 populations of Est-1, Col-0, Dra-1, Ler introgression lines, and other arbitrarily chosen strains crossed with various ft mutants were grown in growth rooms, and flowering time was measured as total leaf number at 16° LD for 4–10 plants. Quantitative complementation and knockdown studies with amiR-ft-1 (Schwab et al. 2006) were carried out in Tübingen and Madison in 16° LD or 23° LD.
QTL analyses:
Ten plants per RIL were grown in a completely randomized design and flowering time was measured as days to flowering and as total number of leaves, which were partitioned into juvenile, adult, and cauline leaves. QTL analyses (scanone for simple interval mapping and scantwo for two-dimensional scans) were carried out using the r-qtl package in R (http://www.r-project.org). QTL significance was determined by permutation testing (1000 runs). The scantwo plots are presented as heat maps of additive and epistatic interactions between markers. A color scale for the LOD scores allows comparison to genomewide averages. Thermosensitivity, a measure of the response of a particular RIL line to a change from 16° to 23° compared to the average response of all RILs, was calculated using the slope of the reaction norms as previously described (Lempe et al. 2005). Thermosensitivity, which is a quantifiable measure of temperature response, was then used as a trait in QTL mapping.
HIF experiments:
To confirm the F5I14/FT QTL, two heterogeneous inbred families (HIFs) (Tuinstra et al. 1997; Loudet et al. 2005) segregating only for the QTL region and derived from RIL 110 and RIL 133 were characterized. Initially, 12 seeds from the S8 generation were genotyped to isolate plants homozygous for each parental allele as well as a plant heterozygous at marker F5I14. Seeds were collected from these plants for subsequent experiments, and 200 segregating progeny were analyzed for an association between flowering time and allele status. SD flowering time was measured using only progeny from the two homozygous lines.
Fine mapping with NILs:
To fine map the F5I14/FT QTL, we generated near isogenic lines (NILs). Est-1 was crossed to Col-0 and seeds were collected from the F1 and F2 generations. F2 plants were genotyped at F5I14 for the QTL region and at one marker from each of the other four chromosomes. A single plant was selected that was heterozygous at F5I14, and Col-0 homozygous at the other markers, and backcrossed to Col-0. This was repeated for the BC1 to BC3 generations, selecting for heterozygosity at F5I14 and Col homozygosity for all other regions. From the BC3F2 generation, plants that belonged to the earliest and latest quartile (192 plants) were genotyped at marker F5I14. From this experiment, two lines for each F5I14 allele combination (Est-1/Est-1, Est-1/Col-0, and Col-0/Col-0) were chosen as NILs for subsequent experiments. Progeny testing showed that the flowering behavior of the NILs was stable and that the direction and effect of the alleles agreed with the QTL mapping results. One NIL-Est was genotyped at 94 genomewide loci, and all but 17 of 182 alleles were Col-0.
Second round of fine mapping:
To reduce the QTL region further, 700 NIL plants heterozygous at F5I14 were genotyped at two markers (24.1 Mb and 24.6 Mb), identifying 28 plants with a recombination event between the two markers. It was necessary to phenotypically classify each plant by progeny testing because of the relatively small effect of the QTL (15% difference between NIL-Col and NIL-Est). Therefore, 12 progeny from each of 28 recombinants were used to classify each recombinant as early, intermediate, or late flowering. Heterozygous parents were readily apparent due to a relatively large standard deviation of the flowering time of their progeny. Further SNP genotyping combined with DNA sequencing reduced the QTL region to the final interval of 6.7 kb.
Quantitative complementation and quantitative knockdown:
FT activity was assessed by combining specific natural FT alleles with laboratory-induced ft mutant alleles. ft-1, ft-2, and ft-3 are EMS-induced alleles in the Ler background (Koornneef et al. 1991) and were crossed to Est-1 and Col-0. ft-10 is a T-DNA insertion line in the Col-0 background (Yoo et al. 2005) and was crossed to Ler and Dra-1. Similar experiments were conducted using the two homozygous NILs. Line × cross interaction was determined by the following analysis of variance (ANOVA) model: Total leaf number ∼ Line + Cross + Line × Cross. An artificial miRNA, amiR-ft-1, which specifically reduces FT expression (Schwab et al. 2006), was introduced into Est-1, Col-0, and the homozygous NILs, and flowering time assayed in 20 or more T1 plants for each genotype. The interaction between the genotype and the presence of the amiR-ft-1 transgene was assessed by the following ANOVA model: Total leaf number ∼ Line + Transgene + Line × Transgene.
FT expression studies:
Plants were analyzed over a time course as previously described (Michael et al. 2008). Briefly, seeds from each genotype were vapor sterilized, plated on half strength MS 0.8% agar plates, and stratified for 4 days at 4° in the dark. Plates were then transferred to continuous white light at 70 μmol m−2 s−1 with a daily 12 hr 22°/12 hr 12° temperature regime and grown for 14 days. On the 15th day, plants were harvested into liquid nitrogen every 4 hours starting at the transition from 12° to 22°, for a total of six time points (0, 4, 8, 12, 16, and 20 hr). Frozen tissue was disrupted in 2-ml Eppendorf tubes containing three ball bearings using a Retsch (Hann, Germany) shaker. RNA was extracted with RNeasy (QIAGEN, Valencia, CA) and 5 μg of RNA was used to prepare cDNA (Invitrogen, Carlsbad, CA). cDNA was diluted 1:20, and FT RNA expression was quantified by quantitative real time PCR (qRT-PCR) with SYBRgreen on a MyIQ system (Biorad, Hercules, CA). qRT-PCR protocol and primers have been described (Mockler et al. 2004). The FT primers used were: FT_Q254F, 5′-ATCTCCATTGGTTGGTGACTGATA and FT_Q306R, 5′-GCCAAAGGTTGTTCCAGTTGTAG.
Statistical analysis:
Statistical analysis was carried out using JMP (SAS Institute, Raleigh, NC) or R (http://www.r-project.org). Student's t-tests as implemented in Microsoft Excel were used to determine the significance of the FT expression difference between NILs.
Sequencing:
Genomic DNA of two recombinants and the parental accessions, Est-1 (GenBank accession nos. GQ377110 and GQ395499) and Col-0, were sequenced from 24,327,174 to 24,337,983 bp on chromosome 1. Multiple overlapping fragments of 0.6- to 1.0-kb size were amplified by PCR with Advantage cDNA Polymerase Mix (Clontech, Palo Alto, CA), DNA fragments were purified with a spin column (QIAGEN). Sequencing reactions were performed in house with Big Dye terminator (Applied Biosystems, Foster City, CA). Alignments were generated using MegAlign (DNAStar, Madison, WI). To sequence FT from 24 wild strains (GenBank accession nos. GQ395468 to GQ395500), four overlapping fragments covering the coding and upstream region were amplified, purified by gel electrophoresis, and sequenced using nested primers on both strands. Sequences were aligned with the ABI Prism 2.1 Autoassembler (Applied Biosystems), and the alignment was manually verified and edited in SeAl (http://tree.bio.ed.ac.uk/).
RESULTS
QTL analysis of flowering behavior for the Est-1 × Col-0 AI-RIL population:
Flowering time for the Est-1/Col-0 AI-RIL population was measured under inductive LD at two different temperatures (16° and 23°) and in two different light environments (growth room and greenhouse). The distribution of flowering times, measured as days to flowering or total leaf number, was continuous and showed in all conditions transgression well beyond the parental values (Figure 1A, supporting information, Table S1). QTL analysis identified a large-effect locus on chromosome 1 that influenced flowering time under all conditions, with the Est-1 allele causing a delay in flowering (Figure 1B). Single marker association with closely linked markers as factors and flowering time as the response revealed that the QTL effect was stronger at 16°, with the QTL explaining as much as 45% of the total variation in days to flowering, while it accounted for about 25% at 23°.
Figure 1.—
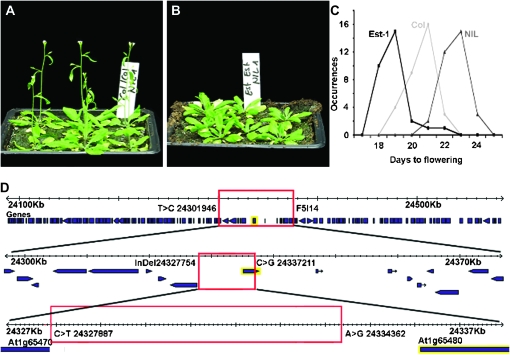
QTL analysis of flowering time in Est-1/Col-0 AI-RIL population. (A) Distribution of flowering time (expressed as leaf number) in the AI-RIL population grown in the greenhouse under 23° LD, including the means for the Est-1 and Col-0 parents. (B) QTL maps of flowering time (measured as days to flower) under three different growth conditions: blue, 16° LD in growth room; red, 23° LD in growth chambers; black, 23° LD in green house. The horizontal line represents the significance threshold for the LOD score. (C and D) Average flowering times of the HIFs based on the genotype of the F5I14 marker. White bars, homozygous for Col-0; gray bars, heterozygous; black bars, homozygous for Est-1. Significant differences (P < 0.0001) between the Est-1 and Col-0 genotypes are indicated by asterisks. Error bars represent standard error of mean (SEM).
The 1.5 LOD confidence interval of this QTL at 16° in LD spanned a 600-kb region that included the well-known floral regulator, FT (At1g65480) (Kardailsky et al. 1999; Kobayashi et al. 1999). Twenty-seven RILs carried recombinant chromosomes in this interval. An ANOVA using these 27 lines with flowering time as the response and allelic state at the markers as factors revealed that the F5I14 marker, which is tightly linked to FT (36 kb away), had the strongest association among the seven surrounding markers (Table S2). To confirm the QTL, we compared the flowering times of individuals from two HIFs (Tuinstra et al. 1997; Loudet et al. 2005) that were segregating for the parental alleles only at this marker. Among 200 segregating progeny for each HIF, plants homozygous for the Est-1 allele flowered 3–5 days later at 23° LD and 16° LD than plants with the Col-0 allele (Figure 1, C and D). The differences in flowering time for the homozygous plants were highly significant (P < 0.0001), while the heterozygous plants had an intermediate phenotype (Figure 1, C and D). In addition, the QTL was specific to LD conditions (Figure 1, C and D).
Flowering time data from 23° LD suggested a second QTL on chromosome 1 at 20.8 Mb (linked to marker nga280), north of F5I14, which is located at 24.3 Mb (Figure 1B). This QTL accounted for ∼25% of variation in flowering time, with the Est-1 allele delaying flowering compared to the Col-0 allele (Table S3). The effect of the nga280 QTL largely disappeared at 16° LD, while the F5I14/FT QTL effect became more pronounced. A two-dimensional genome scan revealed distinct QTL interactions at 16° and 23°. At 23°, a significant additive interaction was detected between the two QTL on chromosome 1 (Figure 2A), while at 16° the F5I14/FT QTL interacted additively with a QTL linked to the marker PLS2 on chromosome 2, for which the Est-1 allele promoted early flowering (Figure 2B, Table S3). This QTL was near the PHYB locus, which is known to affect flowering (Reed et al. 1993). Since there were two interacting QTL that appeared to modulate thermal responses, we calculated the temperature sensitivity in flowering time for each of the RILs (Table S1) and mapped QTL for the same (Figure 2C). This analysis revealed that the F5I14/FT QTL and the PLS2/PHYB QTL on chromosome 2, but not the nga280 QTL on chromosome 1, affected temperature sensitivity.
Figure 2.—
Genetic interactions between flowering time QTL. (A and B) Two-dimensional genome scan in Est-1/Col-0 AI-RIL population in 23° LD (A) and 16° LD (B), with epistatic interactions on top, and additive interactions on the bottom. Interactions between markers on each chromosome are shown. Color scale indicates LOD scores for epistatic (left) and additive interactions (right). (C) QTL analyses of thermosensitivity in Est-1/Col-0 AI-RIL population, contrasting flowering at 16° and 23°, using three different 23° data sets: black, growth room 1; red, growth room 2; blue, green house. The thermosensitivity QTL colocalizes with the QTL in the F5I14/FT region (Figure 1B). (D) Distribution of flowering time in Dra-1 × Ler F2 population at 23° and 16° LD. (E) QTL analysis of flowering time in Dra-1 × Ler F2 population. (F) Two-dimensional genome scan in Dra-1 × Ler F2 population (see A and B for legend).
Colocalizing QTL for flowering time in a Dra-1/Ler F2 population:
We have previously identified Dra-1 as a strain that behaves similarly to photoperiodic mutants in the Col-0 and Ler genetic backgrounds (Lempe et al. 2005). Dra-1 flowers in short days at a similar time as Col-0 and Ler, but flowers later than these strains in long days (although still earlier than in short days). Analysis of an F2 population derived from a cross between Dra-1 and Ler revealed a continuous distribution of flowering times at 23° LD. However, at 16° LD, there was a group of late-flowering plants comprising almost 25% of the population (Figure 2D). Linkage analysis indicated a strong association between flowering time and marker F5I14, with the Dra-1 allele conferring later flowering at 16° LD. QTL analysis at 16° LD with 192 F2 plants identified several significant QTL across the genome (Figure 2E). The most robust QTL was centered on F5I14/FT, with the confidence interval overlapping that of the Est-1/Col-0 QTL. In addition, a QTL that colocalized with the PLS2/PHYB QTL detected in the Est-1/Col-0 RIL population was observed, with the Dra-1 allele conferring late flowering. Two QTL were found on chromosome 5, one linked to marker CA72 near the floral repressor FLC, and one linked to MBK5 near the MAF2-4 cluster of FLC homologs (Figure 2E). The Dra-1 allele conferred late flowering at the CA72/FLC QTL, and early flowering at the MBK5/MAF QTL (Table S3). A two-dimensional genome scan of the Dra-1/Ler population confirmed the epistasis between the F5I14/FT QTL and the PLS2/PHYB QTL on chromosome 2 and revealed additional additive interactions with other QTL (Figure 2F).
Fine mapping of the F5I14 QTL in Est-1 × Col-0:
To fine map the F5I14/FT QTL, we introgressed the QTL interval from Est-1 into Col-0. We classified the progeny of 392 descendants of a single BC3 plant, which had been heterozygous at marker F5I14, for extreme flowering behavior. We found that the QTL in this backcross population roughly segregated in a Mendelian manner, with all but 4 of the 96 latest plants being either homozygous for Est-1 at F5I14 (65 plants) or heterozygous (27 plants). To generate NILs, we propagated a single plant for each of the three possible F5I14 genotypes (Col-0/Col-0, Est-1/Est-1, and Col-0/Est-1). The NIL-Est (homozygous for Est-1 allele at F5I14, with the rest of the genome being mostly Col-0; see materials and methods) was consistently later than Col-0 or the NIL-Col (Figure 3A). In addition, the NIL-Est flowered later than either parent, suggesting that Est-1 contains alleles at other loci that accelerate flowering compared to the Col-0 allele(s) (Figure 3, B and C).
Figure 3.—
Fine mapping of the chromosome 1 Est-1/Col QTL. (A) Four-week-old NIL-Col. (B) NIL-Est of same age and grown in parallel. (C) Distributions of flowering time for Est-1, Col-0, and the Est-NIL. (D) Fine mapping of the QTL. Transcription units are in purple. The FT gene (At1g65480) is highlighted in yellow. The three levels reflect the progressive rounds of fine mapping, with the final 6.7-kb mapping interval in the FT promoter shown on the bottom. The flanking markers used for mapping are shown. FAS1 (At1g65470) is the gene to the left.
For fine mapping, we identified 28 plants with a recombination event within the 600-kb interval surrounding FT and phenotyped 12 progeny each from these recombinants. Combining the flowering time information of these 28 families with additional genotyping with SNP markers around FT reduced the QTL to an interval of 9 kb (Figure 3D). The entire 9-kb interval was sequenced in the two last recombinants, which further reduced the QTL interval to a 6.7-kb region upstream of the FT coding region (Figure 3D).
FT as the causal gene for the F5I14/FT QTL:
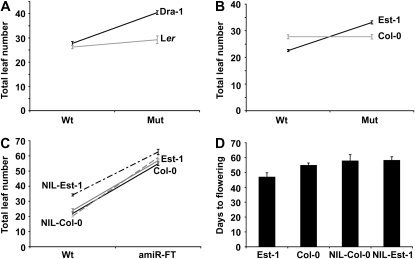
Since fine mapping identified a noncoding fragment upstream of FT as the QTL, we tested whether FT was the causal gene underlying the QTL in both the Est-1 × Col-0 and Dra-1 × Ler populations. First, we performed quantitative complementation experiments (Long et al. 1996) using crosses between strains containing ft mutant alleles and Est-1 or Dra-1. We compared the effect of FT alleles in Est-1 with Col-0, and of Dra-1 with Ler, at 16° LD, the environment where the QTL effect was strongest in both populations. There was a significant line × cross interaction (P < 0.0001), indicating quantitative noncomplementation of the ft mutant alleles by the Est-1 and Dra-1 alleles compared to Col-0 and Ler alleles, respectively (Figure 4, A and B).
Figure 4.—
Genetic evidence for FT being causal for the chromosome 1 QTL. (A and B) Quantitative complementation assays. (A) Flowering time of F1 plants from crosses of Dra-1 and Ler to Col-0 (“wild type”) and the isogenic ft-10 mutant. (B) Flowering time of F1 plants from crosses of Est-1 and Col-0 to Ler (“wild type”) and the isogenic ft-1 mutant. (C) Quantitative knockdown experiment with artificial miRNA against FT (amiR-ft-1) introduced into the two NILs and the two parents. (D) Flowering time of the different genotypes under short days.
Second, we performed a similar set of experiments with the NILs. These experiments again confirmed a significant line × cross interaction (P < 0.01), with the NIL-Est being unable to fully complement the ft mutant at 16° LD. We included a number of additional strains as controls in this analysis and observed significant interactions only with Est-1 and Dra-1, and the respective NILs (Figure 4, A and B; Figure S1, A and B).
Third, we adopted a novel approach, quantitative knockdown, where we tested directly whether inactivating the Est-1 allele of FT was less effective in delaying flowering than inactivation of the Col-0 allele, using an artificial microRNA against FT (amiRNA-ft-1) (Schwab et al. 2006). We transformed the amiR-ft-1 construct into Est-1, Col-0, and the NILs and analyzed the flowering time of at least 20 independent T1 lines in each background. An ANOVA revealed a significant interaction between genetic background and presence of the transgene, with the flowering time of the NIL-Est being the least affected by knocking down FT activity (Figure 4C).
Finally, consistent with the flowering behavior of ft loss-of-function mutants, the effects of allelic variation largely disappeared in SD, with the NILs flowering at the same time as Col-0 (Figure 4D). On the basis of these results, we conclude that FT underlies the detected F5I14/FT QTL.
Allelic variation leads to FT expression differences:
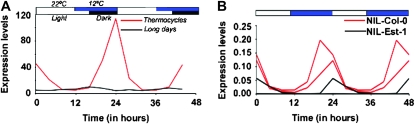
Since the QTL interval did not include any coding sequences, we tested whether the phenotype resulted from a difference in FT RNA levels. FT expression, which is critically correlated with flowering time, is highest at the end of long days (Yanovsky and Kay 2002; Imaizumi et al. 2003). Since the parental strains, Est-1 and Col-0, flowered at similar times, and the effect of the QTL was modest, we reasoned that the differences in FT expression conferred by the two alleles might be small. Therefore, we used conditions where such differences are likely to be most obvious. Temperature is known to affect the circadian clock, which in turn is an important factor in the regulation of FT expression (Blázquez et al. 2003; Balasubramanian et al. 2006b; Michael et al. 2008). The clock can be entrained by both light and temperature (Michael et al. 2003), and thermocycles (which are normally composed of cool nights and warm days) affect >50% of the A. thaliana transcriptome (Michael et al. 2008). Thermocycles have a strong inducing effect on FT expression coupled with causing early flowering (Figure 5A; T. P. Michael, unpublished results). Consistent with the QTL affecting FT expression, the NIL-Est had lower FT RNA levels compared to the NIL-Col (Figure 5B). However, there was little difference in the parents, suggesting that other loci compensate for this allelic variation, which is consistent with the flowering behavior of Est-1 being almost identical to Col-0 at both 16° LD and 23° LD.
Figure 5.—
Allelic variation affects FT expression. (A) Comparison of FT expression in Col-0 under thermocycles (12 hr 22°/12 hr 12°, continuous white light) and light cycles (16 hr light/8 hr dark, constant 23°). (B) Comparison of FT expression levels in NIL-Col and NIL-Est under thermocycles. Two different lines for each NIL are shown. The second NIL-Est line had very low FT expression, and its values are barely visible.
Sequence variation at the FT locus in A. thaliana:
To investigate the basis of the FT QTL, we analyzed in more detail sequence diversity at FT, by sequencing the coding region in 24 strains with variable flowering times in long days, and by sequencing a 4-kb promoter fragment from 12 strains. We found two large overlapping deletions and several other polymorphisms in the upstream region, but few variants in the coding region. We compared the level of polymorphism in our sequence data with published estimates of genomewide sequence polymorphisms. The genomewide average is about five nonsynonymous variants per kilobase coding sequence among 20 divergent strains of A. thaliana (Nordborg et al. 2005; Clark et al. 2007). In the 531-bp FT coding region, we found four synonymous changes, but no nonsynonymous changes. In contrast, the noncoding sequence was more variable. Within the final 6.7-kb QTL interval, there were many polymorphisms that differentiated the Est-1 and Col-0 alleles, including a 29-bp and 17-bp deletion in Est-1 relative to Col-0, and one insertion of 10 bp (Table S4). Partial sequencing of the Dra-1 promoter region revealed a small number of shared polymorphisms that differentiated Est-1 and Dra-1, strains with less active FT alleles, from Col-0 and Ler, strains with more active FT alleles.
DISCUSSION
Complex genetic interactions modulate flowering time variation in A. thaliana:
In the Est-1/Col-0 AI-RIL population, we have detected at least three distinct QTL that interact in an environment-dependent manner. The FT QTL has the largest effect, explaining ∼20–40% of the phenotypic variance depending on the environment, but its impact is modulated by additional loci, as might be expected for a gene that integrates multiple environmental signals. For example, the PLS2/PHYB QTL detected at 16° on chromosome 2 (Figure 1B and Figure 2, B and E) displays significant epistatic interaction with the FT QTL. The effect of the Est-1 allele at the PLS2/PHYB QTL is in the opposite direction of the FT allele of Est-1, providing an explanation for why the two parental strains have very similar flowering times. In addition, our analysis at different temperatures revealed interactions between the two closely linked QTL on chromosome 1. While the FT QTL appeared to be the major factor determining variation at lower temperature, the effect of the linked nga280 QTL increased with higher temperature (M. Todesco, S. Balasubramanian and D. Weigel, unpublished results). A similar picture could also be seen in the Dra-1 × Ler population, in which multiple QTL were mapped and significant interactions between all QTL were detected. While it is conceivable that many of the QTL effects may eventually be mediated through changes in FT expression levels, these results underscore the complexity of the genetic architecture of flowering time variation in A. thaliana.
Some of the complexity might reside within FT itself. The genomic interval surrounding FT is unusual (Figure 3D), with long noncoding regions both upstream and downstream of the FT coding sequence. The FASCIATA1 (FAS1) gene is 7.3 kb upstream of FT and transcribed in the opposite direction of FT, while there are no bona fide open reading frames downstream of FT for >20 kb. Chromatin structure likely plays a prominent role in the regulation of FT, since mutations in LIKE HETEROCHROMATIN PROTEIN1/TERMINAL FLOWER2 (LHP1/TFL2), which is required for epigenetic silencing, cause ectopic FT expression, as do mutations in EARLY BOLTING IN SHORT DAYS (EBS), a gene encoding a putative chromatin remodeling factor (Kotake et al. 2003; Piñeiro et al. 2003; Sung et al. 2006; Turck et al. 2007; Zhang et al. 2007). An analysis of chromatin identified extensive histone modifications in the 3′ region of FT (Turck et al. 2007). These are indicative of epigenetic gene regulation, consistent with a large and complex set of 5′ and 3′ sequences required for proper FT expression. They likely reflect the integrator function of FT, which is the target of many different pathways affecting flowering time (Turck et al. 2008).
Flowering-time QTL in the FT region:
The analysis of the Dra-1 × Ler and the Est-1 × Col-0 populations, and reports of colocalizing QTL in other populations (Werner et al. 2005a; El-Lithy et al. 2006; Shindo et al. 2006; Simon et al. 2008), suggest that a QTL near FT contributes to natural variation in flowering behavior of several A. thaliana strains. In total, seven independent QTL mapping experiments (including this work), with nine different strains, identified the FT region as a flowering time QTL when Col-0 and Ler are the common parental strains, although the directionality of the QTL varied. Thus, it needs to be determined whether these QTL reflect linked genes affecting flowering in natural strains, or whether they are indeed due to functionally divergent alleles at FT.
Natural variation at a highly connected gene:
By integrating multiple environmental signals, FT has a central position in the genetic network that controls flowering time. FT is expressed predominantly in leaves and it is thought that the small FT protein moves to the shoot apex, where it induces flowering by interacting with the FD transcription factor (Abe et al. 2005; Wigge et al. 2005; Lifschitz et al. 2006; Corbesier et al. 2007; Jaeger and Wigge 2007; Lin et al. 2007; Mathieu et al. 2007; Tamaki et al. 2007; Li and Dubcovsky 2008; Notaguchi et al. 2008). A major role for FT in flowering time regulation has been shown in many species, including rice, wheat, and poplar (Kojima et al. 2002; Böhlenius et al. 2006; Yan et al. 2006). FT is related in sequence to TERMINAL FLOWER1 (TFL1), which has the opposite effect on flowering (Bradley et al. 1997; Ohshima et al. 1997; Kardailsky et al. 1999; Kobayashi et al. 1999). The 537-bp coding sequence of TFL1 has been analyzed previously in a sample of 15 different strains. In this collection, one synonymous change and three nonsynonymous changes were reported, with one of the nonsynonymous changes surprisingly affecting a residue that appears to be invariant in the entire TFL1/FT gene family across all flowering plants (Olsen et al. 2002). In contrast, among a similar size sample of 22 strains, we did not detect a single nonsynonymous change in the 531-bp FT coding region. These data suggest that FT is highly conserved at the protein level and more constrained than TFL1, consistent with what has been observed at larger phylogenetic distances (Ahn et al. 2006).
The role of cis-regulatory vs. coding sequence variation:
Among genes responsible for natural variation in A. thaliana flowering, variant alleles at six loci, CRY2, HUA2, FLM, FRI, PHYC, and PHYD, are affected in protein activity, which in some cases is absent altogether (Aukerman et al. 1997; Johanson et al. 2000; El-Assal et al. 2001; Gazzani et al. 2003; Werner et al. 2005b; Balasubramanian et al. 2006a; Wang et al. 2007). The exception is the FRI and HUA2 target FLC, where most alleles are affected in expression levels, and only a minority in protein function (Gazzani et al. 2003; Liu et al. 2004; Shindo et al. 2005, 2006; Lempe et al. 2005). FT, which encodes a highly conserved protein (Ahn et al. 2006), is the most downstream component of flowering time control for which natural variants have been identified in A. thaliana. Functional variation at FT is associated with expression differences, and in this study the final QTL interval included only regulatory sequences. The identification of the FT QTL increases the slowly growing number of examples where regulatory sequences contribute to natural phenotypic variation in A. thaliana (Kliebenstein et al. 2001; Lambrix et al. 2001; Koornneef et al. 2004).
Outside of A. thaliana, differences in expression of the FT orthologs Heading date 3a (Hd3a) in rice and VERNALIZATION3 (VRN3) in wheat are responsible for strain-specific flowering differences in these two grasses (Kojima et al. 2002; Yan et al. 2006; Takahashi et al. 2009). In contrast, natural variants at the upstream acting loci Hd1, which encodes the ortholog of CONSTANS (CO) in A. thaliana, and Early heading1 (Ehd1), which has no clear A. thaliana equivalent, are associated with simple loss-of-function mutations in rice (Yano et al. 2000; Doi et al. 2004; Takahashi et al. 2009), as is the case for the upstream acting wheat VRN2 gene (Yan et al. 2004). Finally, further downstream, regulatory variation has also been identified in natural alleles of VRN1, the wheat ortholog of the FT target APETALA1 (AP1) in A. thaliana (Yan et al. 2003). The theme that emerges from these observations is that downstream factors might be more likely to exhibit regulatory sequence variation, while functional diversification at upstream factors might more often involve changes in protein function. These observations might influence the ongoing debate on the importance of regulatory vs. coding sequence variation in adaptation and the evolution of development (Hoekstra and Coyne 2007; Wray 2007; Stern and Orgogozo 2008).
Acknowledgments
We thank Rick Amasino for his support of this work, the National Science Foundation-supported Arabidopsis Biological Resource Centre (ABRC), and the European Arabidopsis Stock Centre (NASC) for seeds; Oliver Bracko for help with growing plants; Marco Todesco for sharing unpublished information; and Richard Clark, Mark Doyle, Johannes Mathieu, Rachel Mooney, and Daniel Ortiz-Barrientos for comments on the manuscript. This work was supported by National Institutes of Health (NIH) National Research Service award fellowship F23-GM65032-1 (to C.S.), a European Molecular Biology Organization long-term fellowship (to S.B.), Human Frontier Science Program and Japan Society for the Promotion of Science postdoctoral fellowships (to Y.K.), NIH grant GM62932 (to J.C. and D.W.), the Howard Hughes Medical Institute (to J.C.), and the Max Planck Society (to D.W.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.104984/DC1.
References
- Abe, M., Y. Kobayashi, S. Yamamoto, Y. Daimon, A. Yamaguchi et al., 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056. [DOI] [PubMed] [Google Scholar]
- Ahn, J. H., D. Miller, V. J. Winter, M. J. Banfield, J. H. Lee et al., 2006. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 25: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco, C., S. E. El-Assal, G. Coupland and M. Koornneef, 1998. Analysis of natural allelic variation at flowering time loci in the Landsberg erecta and Cape Verde Islands ecotypes of Arabidopsis thaliana. Genetics 149: 749–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman, M. J., M. Hirschfeld, L. Wester, M. Weaver, T. Clack et al., 1997. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9: 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, S., S. Sureshkumar, M. Agrawal, T. P. Michael, C. Wessinger et al., 2006. a The PHYTOCHROME C photoreceptor gene mediates natural variation in flowering and growth responses of Arabidopsis thaliana. Nat. Genet. 38: 711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, S., S. Sureshkumar, J. Lempe and D. Weigel, 2006. b Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet. 2: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, S., C. Schwartz, A. Singh, N. Warthmann, M. C. Kim et al., 2009. QTL mapping in new Arabidopsis thaliana advanced intercross-recombinant inbred lines. PLoS ONE 4: e4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäurle, I., and C. Dean, 2006. The timing of developmental transitions in plants. Cell 125: 655–664. [DOI] [PubMed] [Google Scholar]
- Blázquez, M. A., J. H. Ahn and D. Weigel, 2003. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat. Genet. 33: 168–171. [DOI] [PubMed] [Google Scholar]
- Böhlenius, H., T. Huang, L. Charbonnel-Campaa, A. M. Brunner, S. Jansson et al., 2006. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043. [DOI] [PubMed] [Google Scholar]
- Bradley, D., O. Ratcliffe, C. Vincent, R. Carpenter and E. Coen, 1997. Inflorescence commitment and architecture in Arabidopsis. Science 275: 80–83. [DOI] [PubMed] [Google Scholar]
- Clark, R. M., G. Schweikert, C. Toomajian, S. Ossowski, G. Zeller et al., 2007. Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science 317: 338–342. [DOI] [PubMed] [Google Scholar]
- Corbesier, L., C. Vincent, S. Jang, F. Fornara, Q. Fan et al., 2007. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033. [DOI] [PubMed] [Google Scholar]
- Doi, K., T. Izawa, T. Fuse, U. Yamanouchi, T. Kubo et al., 2004. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev. 18: 926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Assal, S. E.-D., C. Alonso-Blanco, A. J. Peeters, V. Raz and M. Koornneef, 2001. A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat. Genet. 29: 435–440. [DOI] [PubMed] [Google Scholar]
- El-Lithy, M. E., L. Bentsink, C. J. Hanhart, G. J. Ruys, D. Rovito et al., 2006. New Arabidopsis recombinant inbred line populations genotyped using SNPWave and their use for mapping flowering-time quantitative trait loci. Genetics 172: 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzani, S., A. R. Gendall, C. Lister and C. Dean, 2003. Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol. 132: 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenblad, J., and M. Nordborg, 2002. Sequence variation and haplotype structure surrounding the flowering time locus FRI in Arabidopsis thaliana. Genetics 161: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra, H. E., and J. A. Coyne, 2007. The locus of evolution: evo devo and the genetics of adaptation. Evolution 61: 995–1016. [DOI] [PubMed] [Google Scholar]
- Imaizumi, T., H. G. Tran, T. E. Swartz, W. R. Briggs and S. A. Kay, 2003. FKF1 is essential for photoperiodic-specific light signalling in Arabidopsis. Nature 426: 302–306. [DOI] [PubMed] [Google Scholar]
- Jaeger, K. E., and P. A. Wigge, 2007. FT protein acts as a long-range signal in Arabidopsis. Curr. Biol. 17: 1050–1054. [DOI] [PubMed] [Google Scholar]
- Johanson, U., J. West, C. Lister, S. Michaels, R. Amasino et al., 2000. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290: 344–347. [DOI] [PubMed] [Google Scholar]
- Kardailsky, I., V. Shukla, J. H. Ahn, N. Dagenais, S. K. Christensen et al., 1999. Activation tagging of the floral inducer FT. Science 286: 1962–1965. [DOI] [PubMed] [Google Scholar]
- Kliebenstein, D. J., V. M. Lambrix, M. Reichelt, J. Gershenzon and T. Mitchell-Olds, 2001. Gene duplication in the diversification of secondary metabolism: tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 13: 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, Y., H. Kaya, K. Goto, M. Iwabuchi and T. Araki, 1999. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y., and D. Weigel, 2007. Move on up, it's time for change–mobile signals controlling photoperiod-dependent flowering. Genes Dev. 21: 2371–2384. [DOI] [PubMed] [Google Scholar]
- Kojima, S., Y. Takahashi, Y. Kobayashi, L. Monna, T. Sasaki et al., 2002. Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 43: 1096–1105. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., C. J. Hanhart and J. H. van der Veen, 1991. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229: 57–66. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., C. Alonso-Blanco, A. J. Peeters and W. Soppe, 1998. Genetic control of flowering time in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 345–370. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., C. Alonso-Blanco and D. Vreugdenhil, 2004. Naturally occurring genetic variation in Arabidopsis thaliana. Annu. Rev. Plant Biol. 55: 141–172. [DOI] [PubMed] [Google Scholar]
- Kotake, T., S. Takada, K. Nakahigashi, M. Ohto and K. Goto, 2003. Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol. 44: 555–564. [DOI] [PubMed] [Google Scholar]
- Lambrix, V., M. Reichelt, T. Mitchell-Olds, D. J. Kliebenstein and J. Gershenzon, 2001. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 13: 2793–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempe, J., S. Balasubramanian, S. Sureshkumar, A. Singh, M. Schmid et al., 2005. Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genet. 1: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., and J. Dubcovsky, 2008. Wheat FT protein regulates VRN1 transcription through interactions with FDL2. Plant J. 55: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz, E., T. Eviatar, A. Rozman, A. Shalit, A. Goldshmidt et al., 2006. The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc. Natl. Acad. Sci. USA 103: 6398–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. K., H. Belanger, Y. J. Lee, E. Varkonyi-Gasic, K. Taoka et al., 2007. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell 19: 1488–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J., Y. He, R. Amasino and X. Chen, 2004. siRNAs targeting an intronic transposon in the regulation of natural flowering behavior in Arabidopsis. Genes Dev. 18: 2873–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, A. D., S. L. Mullaney, T. F. Mackay and C. H. Langley, 1996. Genetic interactions between naturally occurring alleles at quantitative trait loci and mutant alleles at candidate loci affecting bristle number in Drosophila melanogaster. Genetics 144: 1497–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudet, O., V. Gaudon, A. Trubuil and F. Daniel-Vedele, 2005. Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theor. Appl. Genet. 110: 742–753. [DOI] [PubMed] [Google Scholar]
- Mathieu, J., N. Warthmann, F. Küttner and M. Schmid, 2007. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr. Biol. 17: 1055–1060. [DOI] [PubMed] [Google Scholar]
- Michael, T. P., P. A. Salome and C. R. McClung, 2003. Two Arabidopsis circadian oscillators can be distinguished by differential temperature sensitivity. Proc. Natl. Acad. Sci. USA 100: 6878–6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael, T. P., T. C. Mockler, G. Breton, C. McEntee, A. Byer et al., 2008. Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet. 4: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S. D., and R. M. Amasino, 1999. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels, S. D., Y. He, K. C. Scortecci and R. M. Amasino, 2003. Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proc. Natl. Acad. Sci. USA 100: 10102–10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler, T. C., X. Yu, D. Shalitin, D. Parikh, T. P. Michael et al., 2004. Regulation of flowering time in Arabidopsis by K homology domain proteins. Proc. Natl. Acad. Sci. USA 101: 12759–12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg, M., T. T. Hu, Y. Ishino, J. Jhaveri, C. Toomajian et al., 2005. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 3: e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaguchi, M., M. Abe, T. Kimura, Y. Daimon, T. Kobayashi et al., 2008. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol. 49: 1645–1658. [DOI] [PubMed] [Google Scholar]
- Ohshima, S., M. Murata, W. Sakamoto, Y. Ogura and F. Motoyoshi, 1997. Cloning and molecular analysis of the Arabidopsis gene TERMINAL FLOWER 1. Mol. Gen. Genet. 254: 186–194. [DOI] [PubMed] [Google Scholar]
- Olsen, K. M., A. Womack, A. R. Garrett, J. I. Suddith and M. D. Purugganan, 2002. Contrasting evolutionary forces in the Arabidopsis thaliana floral developmental pathway. Genetics 160: 1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeiro, M., C. Gómez-Mena, R. Schaffer, J. M. Martínez-Zapater and G. Coupland, 2003. EARLY BOLTING IN SHORT DAYS is related to chromatin remodeling factors and regulates flowering in Arabidopsis by repressing FT. Plant Cell 15: 1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, J. W., P. Nagpal, D. S. Poole, M. Furuya and J. Chory, 1993. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab, R., S. Ossowski, M. Riester, N. Warthmann and D. Weigel, 2006. Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, C. C., J. E. Burn, P. P. Perez, J. Metzger, J. A. Edwards et al., 1999. The FLF MADS box gene: a repressor of flowering in Arabidopsis regulated by vernalization and methylation. Plant Cell 11: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo, C., M. J. Aranzana, C. Lister, C. Baxter, C. Nicholls et al., 2005. Role of FRIGIDA and FLOWERING LOCUS C in determining variation in flowering time of Arabidopsis. Plant Physiol. 138: 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo, C., C. Lister, P. Crevillen, M. Nordborg and C. Dean, 2006. Variation in the epigenetic silencing of FLC contributes to natural variation in Arabidopsis vernalization response. Genes Dev. 20: 3079–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, M., O. Loudet, S. Durand, A. Bérard, D. Brunel et al., 2008. Quantitative trait loci mapping in five new large recombinant inbred line populations of Arabidopsis thaliana genotyped with consensus single-nucleotide polymorphism markers. Genetics 178: 2253–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, D. L., and V. Orgogozo, 2008. The loci of evolution: How predictable is genetic evolution? Evolution 62: 2155–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe, J. R., C. Weinig, M. Ungerer, K. M. Olsen, C. Mays et al., 2004. A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc. Natl. Acad. Sci. USA 101: 4712–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, S., R. J. Schmitz and R. M. Amasino, 2006. A PHD finger protein involved in both the vernalization and photoperiod pathways in Arabidopsis. Genes Dev. 20: 3244–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y., K. M. Teshima, S. Yokoi, H. Innan and K. Shimamoto, 2009. Variations in Hd1 proteins, Hd3a promoters, and Ehd1 expression levels contribute to diversity of flowering time in cultivated rice. Proc. Natl. Acad. Sci. USA 106: 4555–4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki, S., S. Matsuo, H. L. Wong, S. Yokoi and K. Shimamoto, 2007. Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036. [DOI] [PubMed] [Google Scholar]
- Tuinstra, M., G. Ejeta and P. Goldsbrough, 1997. Heterogenous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor. Appl. Genet. 95: 1005–1011. [Google Scholar]
- Turck, F., F. Roudier, S. Farrona, M. L. Martin-Magniette, E. Guillaume et al., 2007. Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet. 3: e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck, F., F. Fornara and G. Coupland, 2008. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu. Rev. Plant Biol. 59: 573–594. [DOI] [PubMed] [Google Scholar]
- Wang, Q., U. Sajja, S. Rosloski, T. Humphrey, M. C. Kim et al., 2007. HUA2 caused natural variation in shoot morphology of A. thaliana. Curr. Biol. 17: 1513–1519. [DOI] [PubMed] [Google Scholar]
- Werner, J. D., J. O. Borevitz, N. H. Uhlenhaut, J. R. Ecker, J. Chory et al., 2005. a FRIGIDA-independent variation in flowering time of natural Arabidopsis thaliana accessions. Genetics 170: 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, J. D., J. O. Borevitz, N. Warthmann, G. T. Trainer, J. R. Ecker et al., 2005. b Quantitative trait locus mapping and DNA array hybridization identify an FLM deletion as a cause for natural flowering-time variation. Proc. Natl. Acad. Sci. USA 102: 2460–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge, P. A., M. C. Kim, K. E. Jaeger, W. Busch, M. Schmid et al., 2005. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059. [DOI] [PubMed] [Google Scholar]
- Wilczek, A. M., J. L. Roe, M. C. Knapp, M. D. Cooper, C. Lopez-Gallego et al., 2009. Effects of genetic perturbation on seasonal life history plasticity. Science 323: 930–934. [DOI] [PubMed] [Google Scholar]
- Wray, G. A., 2007. The evolutionary significance of cis-regulatory mutations. Nat. Rev. Genet. 8: 206–216. [DOI] [PubMed] [Google Scholar]
- Yan, L., A. Loukoianov, G. Tranquilli, M. Helguera, T. Fahima et al., 2003. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 100: 6263–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L., A. Loukoianov, A. Blechl, G. Tranquilli, W. Ramakrishna et al., 2004. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L., D. Fu, C. Li, A. Blechl, G. Tranquilli et al., 2006. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 103: 19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano, M., Y. Katayose, M. Ashikari, U. Yamanouchi, L. Monna et al., 2000. Hd1, a major photoperiod sensitivity Quantitative Trait Locus in rice, is closely related to the Arabidopsis flowering-time gene CONSTANS. Plant Cell 12: 2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky, M. J., and S. A. Kay, 2002. Molecular basis of seasonal time measurement in Arabidopsis. Nature 419: 308–312. [DOI] [PubMed] [Google Scholar]
- Yoo, S. K., K. S. Chung, J. Kim, J. H. Lee, S. M. Hong et al., 2005. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol. 139: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., E. J. Richards and J. O. Borevitz, 2007. Genetic and epigenetic dissection of cis regulatory variation. Curr. Opin. Plant Biol. 10: 142–148. [DOI] [PubMed] [Google Scholar]