Abstract
Objective
Calcific aortic stenosis, characterized by excessive fibrosis and deposition of bone-like calcified tissue, affects roughly 2–3% of the US population over 65. Recent studies have suggested that statins have a positive effect on the progression of aoritic stenosis, likely due to their ability to affect the resident cell population, known as valvular interstitial cells (VICs). VICs are fibroblastic cells that can differentiate to form activated myofibroblasts, displaying increased alpha smooth muscle actin (αSMA) expression, contractility, and collagen production.
Methods and Results
In culture, VICs spontaneously form multicellular aggregates that subsequently develop into calcified nodules, providing an in vitro model for aortic stenosis. Using real-time microscopic tracking, we observed that confluent VIC monolayers spontaneously contract into rounded nodules, suggesting that myofibroblastic contractility is a critical step in the process of nodule formation. Over-expression of αSMA increased VIC calcific nodule formation and contractility, while knock-down of αSMA with siRNAs reduced these phenotypes, suggesting that the expression and contractile properties of αSMA are essential to the formation of nodules. Statin treatment of VICs reduced αSMA expression, inhibited contractility, and decreased nodule formation. When statins were used to treat preformed nodules, no decrease in the number of calcified nodules was observed, suggesting that statins may play more of a preventative role in aortic stenosis than a cure.
Conclusions
Our studies provide evidence of a causal relationship between VIC myofibroblastic activity and initial VIC calcific nodule formation. Furthermore we demonstrate that pravastatin inhibition of calcific nodule formation is related to inhibition of myofibroblastic activity.
Keywords: valvular interstitial cells, myofibroblast, calcific nodule, statin, contractility
Introduction
Valvular stenosis is the leading cause of aortic heart valve failure in the United States, a disease that affects roughly 2–3% of the population over the age of 65 1. It has long been thought that aortic stenosis is a degenerative process resulting from passive accumulation of calcium on the aortic valve cusps; however, recent evidence indicates that valvular disease is cell-mediated 2. Valvular interstitial cells (VICs) are the main cell population of the four heart valves and play a critical role in regulating tissue homeostasis. In cases of aortic valve disease, chronic stresses are thought to result in prolonged activation of VICs, which differentiate to become myofibroblasts that express α-smooth muscle actin (αSMA) as well as osteoblastic markers (osteocalcin and bone sialoprotein) 3–5. Over time, chronic activation of VICs likely leads to increased fibrosis of the valve leaflets and the formation of bone-like calcified nodules. These effects have catastrophic effects on the mechanical flexibility of the leaflets and compromise valve function.
Retrospective clinical studies have indicated that patients who are treated with statins for other heart-related issues have a 50% lower incidence of heart valve disease 6–9. This observation has resulted in renewed interest in the effects of statin treatment and the roles that statins play in cell function. Statins, a class of drugs that inhibit HMG-CoA reductase, have long been known for their cholesterol-lowering abilities but are beginning to demonstrate additional beneficial effects including atherosclerotic plaque stabilization 10, enhancement of endothelial cell function 11, and manipulation of cytokine production 12. In the case of valvular stenosis, results have been mixed. While there is agreement that statins can positively impact patients with valvular stenosis, questions remain as to whether statins reverse calcific phenotypes or solely prevent their progression. Regression of valvular stenosis has been reported in retrospective studies where patients who had been treated with statins had lower levels of valvular stenosis than patients not treated with statins 6–9, 13. These studies, however, were limited by their non-randomized, retrospective nature. Prospective clinical studies, on the other hand, have shown little to no effect of statin treatment on slowing or reversing stenotic progression 14–16. Clearly, further investigation into statin-mediated valvular disease prevention and/or regression is required to elucidate these paradoxical clinical results.
In the work presented here, we sought to identify the mechanism of statin action in valvular cells by uncovering the cellular events that lead to calcific nodule formation. Using an in vitro model in which porcine VICs were activated with transforming growth factor-beta1 (TGF-β1) to form calcific nodules in culture, we observed that nodule formation occurs as a result of contractile events that are mediated by increased expression of αSMA. TGF-β1 is a profibrotic growth factor that has been implicated in the development of VIC calcification in vitro and the progression of valvular stenosis in vivo 17, 18. Additionally TGF-β1 has been shown to increase VIC αSMA expression and contractility when added to cultures 19. In valvular stenosis, increased levels of TGF-β1 have been hypothesized to lead to enhanced resident myofibroblast activity resulting in valvular sclerosis and subsequent stenosis 17. In these studies TGF-β1 was utilized to generate enhanced VIC calcific nodule activity. By blocking αSMA expression via a Rho-mediated pathway, statins effectively reduced the contractile activity of VICs and prevented nodule formation. When VIC cultures were treated with statins after the onset of myofibroblast activation and nodule formation, no reversal of calcific nodule numbers was observed. Together, these data provide a mechanism by which statins can prevent the contractile events that initiate nodule formation and thus block the progression of valvular stenosis.
Methods
Cell Culture
VICs were isolated from porcine aortic valve leaflets by sequential collagenase digestion as previously described 20. Cells were expanded in growth media (Medium 199, 15% fetal bovine serum (FBS), 2% penicillin/streptomycin (100 U/mL), 0.4% fungizone (0.5 µg/mL)) and passaged by trypsin digestion. For the experiments described here, VICs from passages two or three were plated at confluence and cultured in low serum media (1% FBS supplemented). TGF-β1 (R & D Systems) was added at 5 ng/mL and pravastatin (EMD Bioscience) at 100 µmol/L. Pravastatin was chosen as a statin inhibitor due to its water-soluble nature, thus eliminating the presence of solvent carriers required by other statins.
Real-Time VIC Nodule Formation
Time lapse images of VIC nodule formation were captured over a 48 hour period using a Nikon TE 2000 PFS epi-fluorescent microscope equipped with a motorized stage and an environmental sample chamber. Images were collected and analyzed using MetaMorph software (Molecular Devices).
Calcific Nodule Formation Assay
VICs were seeded onto 24-well plates at a concentration of 75,000 cells/cm2. The cells were cultured for 4 days before fixing with 10% buffered formalin (Sigma). Fixed cultures were stained with Alizarin Red S dye (Electron Microscopy Science). This histology stain marks hydroxyapatite mineralized matrix orange-red in color. Positively stained nodules were then counted and normalized to culture area.
Collagen Gel Contraction Assay
VICs were encapsulated in fixed collagen gels (Inamed) in 24 well plates at a density of 500,000 cells/mL per manufacturer’s instructions. Cell-gel constructs were cultured fixed to the plate for four days with the indicated supplements before being released. Upon release, images of cell-gel constructs were captured over a 24 hour period to follow gel contraction. Reduction in gel area was measured after 24 hours using ImageJ (NIH) and normalized to initial gel size before release.
Cell Transfections and Luciferase Assays
Transfection of VICs with siRNAs and plasmid DNAs was accomplished using the amaxa Nucleofector system. siRNA pools were obtained from Dharmacon and used at a concentration of 2 µmol/L. The αSMA expression plasmid (pCMV-αSMA) was purchased from Origene and the αSMA-luciferase plasmid (pαSMA-luc) was provided by the laboratory of Dr. Leslie Leinwand 21. Plasmid transfections were performed with 5 µg of plasmid DNA. Luciferase activity was measured using the Promega Bright-Glo Luciferase Assay System per manufacturer’s instructions.
αSMA Cell-Based Fluorometric Enzyme-Linked Immunosorbent Protein Expression Assay
Protein expression of αSMA was analyzed with a cell-based fluorometric enzyme-linked immunosorbent assay as previously described 22. Briefly, VICs seeded onto 96-well plates for 48 hours were fixed with 10% buffered formalin, permeabilized with Tween-20, blocked with 3 wt% BSA, and incubated with mouse anti-αSMA (Abcam) antibody and mouse-anti-goat HRP coupled IgG (Invitrogen). Samples were then incubated with a fluorescent HRP substrate, QunatiBlu (Pierce), and detected on a fluorescent plate reader. Cells were rinsed and lysed with papainase (Worthington) and dsDNA quantified with Quanti-It PicoGreen dsDNA (Invitrogen) fluorescent assay per manufacturers’ instructions for normalization. Concurrently, cells were immunostained with mouse-anti-goat alexa 488 coupled IgG (Invitrogen) to visualize and confirm staining.
Quantifiable Real-Time Polymerase Chain Reaction (qRT-PCR)
Messanger RNA (mRNA) was isolated from treated VIC cultures using the SV total RNA isolation kit (Promega) according to manufacturer’s instructions. After isolation, mRNA purity and amount was confirmed with a NanoDrop (Thermo Fisher) spectrophotometer before reverse transcription was performed using iScript cDNA synthesis kit (BioRad). Amplification of cDNA products was performed using an iCycler (BioRad) and previously reported primers 23 (Integrated DNA Technologies). Data was analyzed according to the Pfaffl method and normalized to GAPDH 24.
Statistical Analysis
All statistical analyses were performed using a standard students t-test for significance. Data was considered significant if p<0.05. Values reported represent data mean, and error bars indicate standard error about the mean. Unless otherwise noted experiments were performed in triplicate.
Results
Nodule Formation Is Initiated By Contractile Events In VIC Cultures
When plated at confluency and treated with TGF-β1, VIC cultures form rounded cell aggregates, or nodules, that stain positive for calcium deposition 17, 18. This process has been likened to the formation of calcific nodules in the heart, providing a means to study some of the basic underlying mechanisms that lead to valvular stenosis.
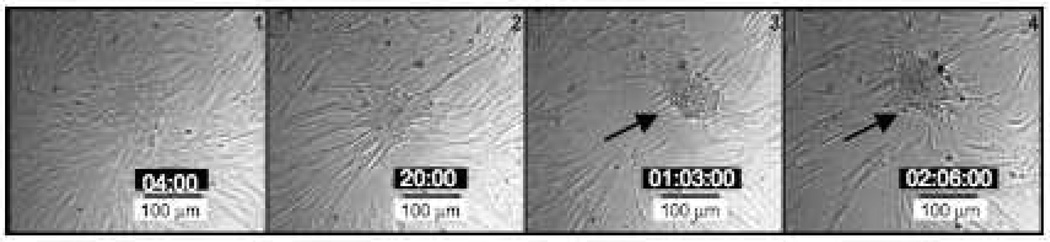
While it is well-documented that VICs form calcific nodules in culture, it is not clear what events initiate this process. Increased cell proliferation, migration, and apoptosis have all been attributed to causing nodules to form in culture, but no consensus has been reached 17, 18, 25. In an effort to better understand how nodules form, we performed real-time tracking microscopy on VIC cultures treated with TGF-β1, allowing us to effectively observe nodules form. Starting as a confluent monolayer, the cells initially showed signs of migration in that some local cell movement was observed. Within 12 hours, however, groups of VICs appeared to pull together to create cell clusters (Fig. 1). Based on the short time period in which the cells went from a monolayer to a nodule, we concluded that contraction, and not cell migration, was likely driving the formation of the nodules. This effect is more clearly observed in time-lapse videos of the cultures (Supp. Videos I and II, please see http://atvb.ahajournals.org.) in which cells furthest from the center of the forming nodules appear to be torn away from the culture plate in unison, leaving bare patches where no cells remain.
Figure 1.
Real-time microscopy of nodule formation in VIC cultures. VICs were seeded at confluency and treated with TGF-β1 to stimulate nodule formation. Over time, these aggregates form nodules that stain positive for calcium deposition (data not shown). Complete videos of this process are presented in supplementary information. The arrow indicates the position of nodule formation. Time-stamps for each image are located in the black boxes as Day:Hour:Minute.
αSMA Is Necessary and Sufficient For VIC Calcific Nodule Formation
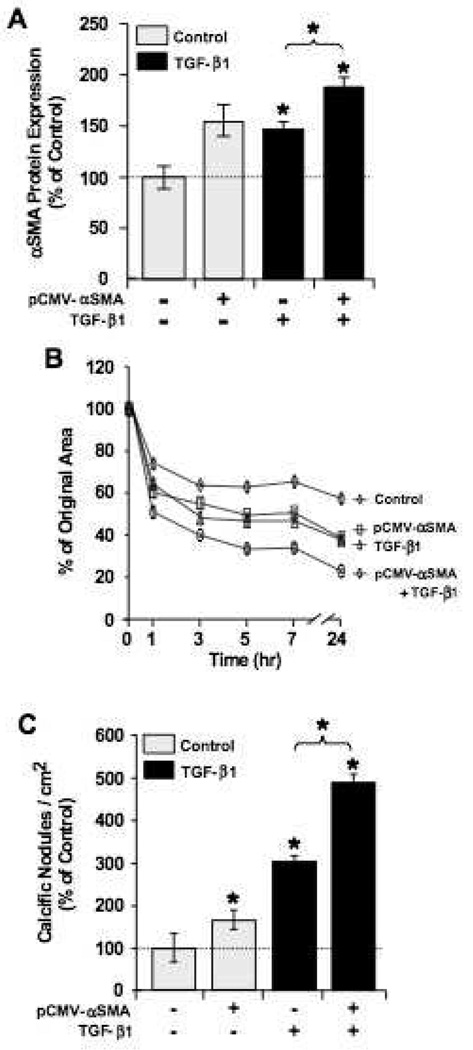
These data suggest that αSMA expression, a hallmark of myofibroblast activation, has a causal effect on nodule formation. Because αSMA is a central component of myofibroblast-associated stress fibers, we hypothesized that the increased expression of αSMA in these cells would cause an increase in cellular contraction and, hence, nodule formation. Previous studies have confirmed the link between αSMA and contractile activity in fibroblasts 26, 27, however the role of contractility in calcific nodule formation has not been elucidated. To test this hypothesis, we transfected VICs with an αSMA over-expression plasmid (pCMV-αSMA) to specifically increases the expression of only αSMA. While the addition of TGF-β1 also increases the expression of αSMA, it also upregulates many other genes in a complex signaling cascade. Following αSMA over-expression, the effect of increased αSMA expression on cellular contraction and nodule formation was measured. In gel contraction assays, transfected VICs were embedded in 3-dimensional collagen matrices and cultured for 48 hours before being released from the plate surface. After release, gel diameter was measured at specific time points, providing an estimate of the total contractile force exerted by the encapsulated cells. As hypothesized, VICs transfected with the pCMV-αSMA plasmid showed increased αSMA protein expression (Fig. 2A) and increased contractility (Fig. 2B). The levels of αSMA expression were further increased by treatment with TGF-β1 (Fig. 2A) with a corresponding increase in collagen gel contraction (Fig. 2B). Increasing αSMA expression also led to increased numbers of calcific nodules in subsequent 2D experiments (Fig. 2C).
Figure 2.
Overexpression of αSMA enhances VIC contractility and calcific nodule formation. A. Relative expression of αSMA protein in VIC cultures was measured by cell-based fluorometric enzyme-linked immunosorbent assay. Expression was normalized to the untransfected, untreated controls and represent two independent experiments, each with n = 24. B. Transfected VICs were encapsulated in collagen gels, treated ± 5 ng/mL TGF-β1, and then released to allow gel contraction. Results are shown as reduction from original gel area with time and represent two separate experiments, each with four repeats per condition. C. Calcific nodule formation per area of cell culture with indicated treatment and transfection conditions. Nodule numbers were normalized to control conditions (no transfection and no TGF-β1), n = 12. *p<0.01 over control and between indicated groups.
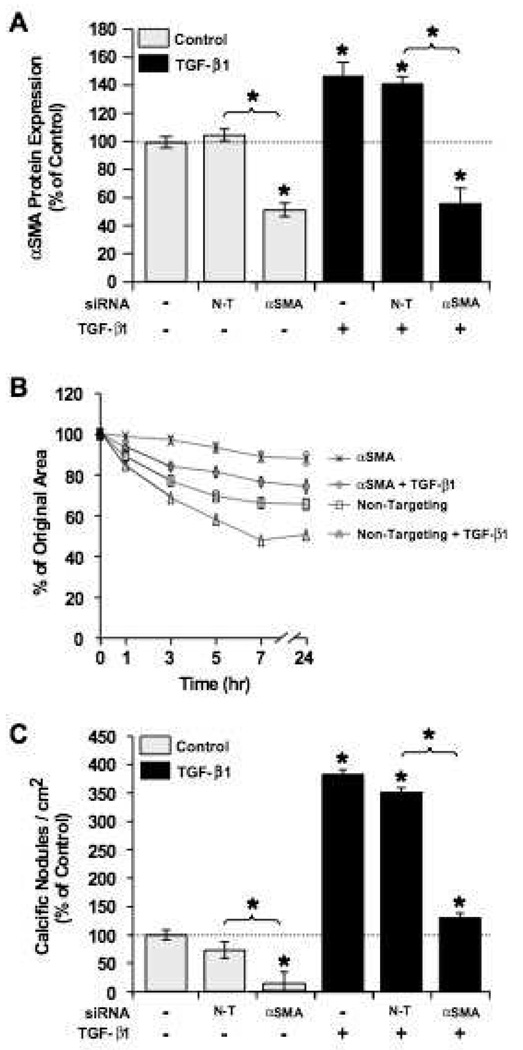
Given the apparent effects of increasing αSMA expression on contractility and calcific nodule formation of VIC cultures, we hypothesized that the opposite would be seen if αSMA expression was specifically decreased. To test this hypothesis, siRNAs were used to knock down αSMA expression. As shown in Figure 3A, protein levels of αSMA were significantly reduced in VICs treated with αSMA-targeting siRNAs when compared to cells treated with non-targeting siRNA controls. As expected, collagen gels containing VICs treated with αSMA-siRNAs showed decreased contraction (Fig. 3B). While the addition of TGF-β1 effectively increased contractility when compared to untreated control samples, a comparison of αSMA expression levels and contractility showed a strong correlation. In addition, reduced αSMA expression resulted in significantly reduced calcific nodules (Fig. 3C). Together, these data suggest that increased αSMA expression is both necessary and sufficient for contraction-initiated nodule formation in VIC cultures.
Figure 3.
siRNA knockdown of αSMA expression reduces contraction and calcific nodule formation. A. Relative expression of αSMA after transfection with Non-Targeting siRNAs (N-T, non specific siRNAs), αSMA-targeting siRNAs, and no transfection (control) after three days of culture (n = 24). B. VICs were encapsulated in collagen gels, supplemented with 5 ng/mL TGF-β1, and released to allow gel contraction. Results are shown as reduction from original gel area with time. Results represent two independent experiments each with four replicates per condition. C. Calcific nodule formation per area of cell culture with indicated treatment and transfection conditions. Nodule numbers have been normalized to control conditions (no transfection and no TGF-β1), n = 12. *p<0.01 over control and between indicated groups.
Pravastatin Inhibits Nodule Formation
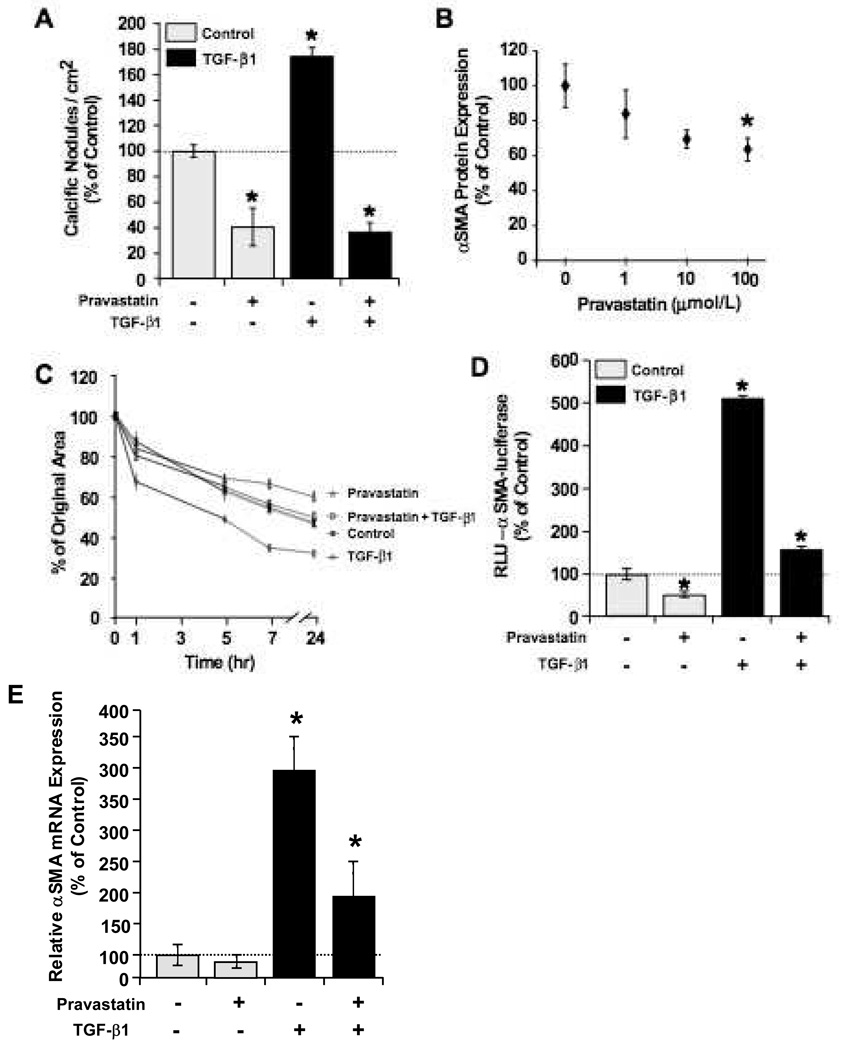
Due to the heightened interest in statins for their possible role in abrogating valvular stenosis, we tested the ability of pravastatin to block nodule formation in VIC cultures. As shown in Figure 4A, pravastatin treatment significantly reduced the number of calcific nodules formed. Treatment of VICs with TGF-β1 doubled the number of calcific nodules, an effect that was blocked when TGF-β1 was co-delivered with pravastatin. Nodule formation in VIC cultures treated with both TGF-β1 and pravastatin showed similar nodule numbers as cultures treated with pravastatin alone.
Figure 4.
Pravastatin treatment reduces calcific nodule formation. A. VIC cultures treated with pravastatin (100 µmol/L) showed a significant reduction in the number of calcific nodules formed per cm2. Nodule numbers have been normalized to control conditions (no transfection and no TGF-β1), n = 12. B. Relative protein expression of αSMA in VICs after 3 days of treatment with pravastatin (100 µmol/L) is shown (n = 24). C. VICs were encapsulated in collagen gels, treated with 100 µmol/L pravastatin and/or 5 ng/mL TGF-β1, and released to allow gel contraction. Results are shown as reduction from original gel area with time (n = 8). D. VICs transfected with αSMA-luciferase reporter plasmid were treated for 48 hours with 100 mmol/L pravastatin and/or 5 ng/mL TGF-β1. Relative luciferase activity is shown (n = 18). E. qRT-PCR analysis of mRNA expression of αSMA after cells were treated for four days with the indicated supplements. Expression was normalized to GAPDH (reference gene) and control (no treatment) (n = 4). *p<0.01 over control and between indicated groups.
In order to ensure that pravastatin-mediated effects on VIC activity was specific to HMG-CoA reductase inhibition and not a non-specific effect of the treatment conditions, we supplemented our VIC cultures with activated mevalonate, the immediate downstream metabolic product of HMG-CoA reductase activity. As shown in Supplemental Figure I (please see http://atvb.ahajournals.org.), the addition of mevalonate to the cultures abrogated pravastatin-mediated effects on VIC activity. Furthermore, analysis of the downstream signaling pathways involved in pravastatin action confirm that pravastatin inhibits HMG-CoA-mediated prenylation pathways (Supp. Fig. I, please see http://atvb.ahajournals.org.) that lead to the activation of RhoA (Supp. Fig. II, please see ttp://atvb.ahajournals.org.), Rho kinase (ROCK), and myosin light chain kinase (MLCK) (Supp. Fig. III, please see ttp://atvb.ahajournals.org.).
Pravastatin Represses αSMA Transcription
Because αSMA expression appears to play a central role in myofibroblast contraction and subsequent nodule formation, we hypothesized that pravastatin inhibits αSMA expression. As shown in Figure 4B, increasing pravastatin concentrations reduced αSMA protein levels in a dose dependent manner. As expected, pravastatin also reduced VIC contractile activity in collagen gel assays (Fig. 4C).
In order to investigate whether statins repress αSMA transcription, VICs were transfected with an αSMA-luciferase reporter (pαSMA-luc) followed by treatment with TGF-β1 and/or pravastatin. After 48 hours, the luciferase activity of VICs treated with statins was significantly reduced when compared to controls (Fig. 4D), indicating that pravastatin acts to block αSMA expression at the transcriptional level. As would be expected, TGF-β1 significantly increased luciferase activity, an effect that was abrogated by co-delivery with pravastatin. Pravastatin treatment of VICs also reduced αSMA expression at the transcriptional level as shown by qRT-PCR analysis (Fig. 4E). Furthermore, this effect appears to be mediated by Smad transcription factors as pravastatin treatment also caused a decrease in luciferase activity in VICs transfected with a pSmad-luc reporter plasmid (Supp. Fig. IV, please see http://atvb.ahajournals.org).
Pravastatin Does Not Reverse Calcific Nodule Phenotype
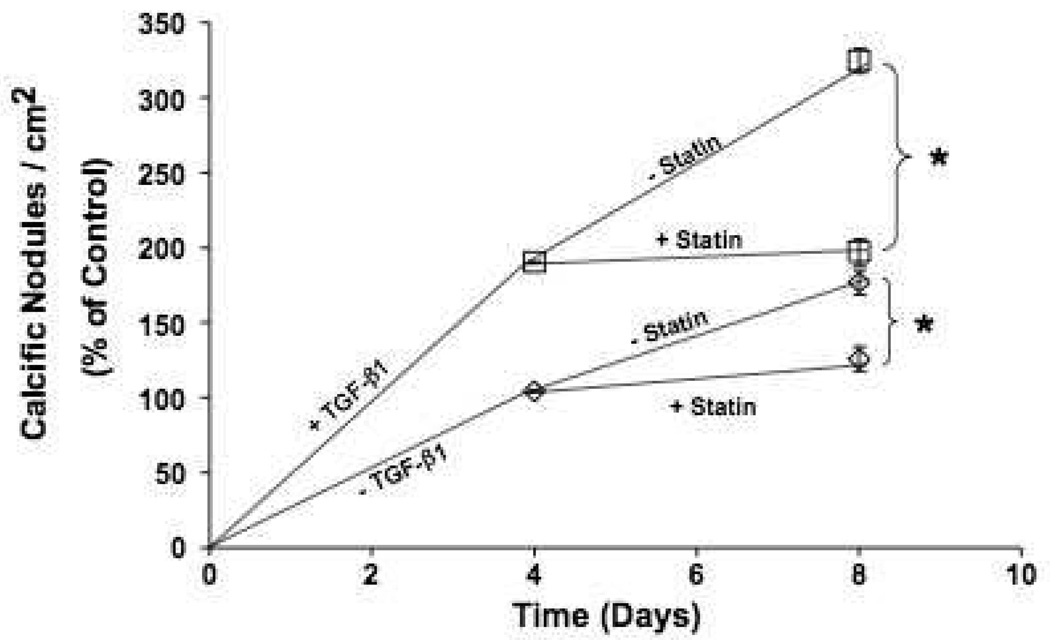
While it clear that statins are capable of inhibiting nodule formation, the ability of statins to cause a reversal of calcific nodules remains controversial. To test this, confluent VIC cultures were allowed to form nodules for four days before treatment with pravastatin. As shown in Figure 5, pravastatin blocked an increase in calcific nodules but did not appear to reduce the number of calcified nodules that were observed. Interestingly, while the number of calcium positive nodules was unaffected by pravastatin treatment, the nodules that were present in these samples appeared to have a flattened profile even though they stained positive for calcium, likely due to a decrease in cellular contractility (Supp. Fig. V, please see http://atvb.ahajournals.org.).
Figure 5.
Pravastatin does not revert preformed calcific nodules. VIC cultures were treated with or without 5 ng/mL TGF-β1 for four days before treatment with 100 µmol/L pravastatin. Calcium-positive nodules were counted at Days 4 and 8, and the number of nodules was normalized to the Day 4 untreated controls (n=24). *p<0.001 over control and between indicated groups.
Discussion
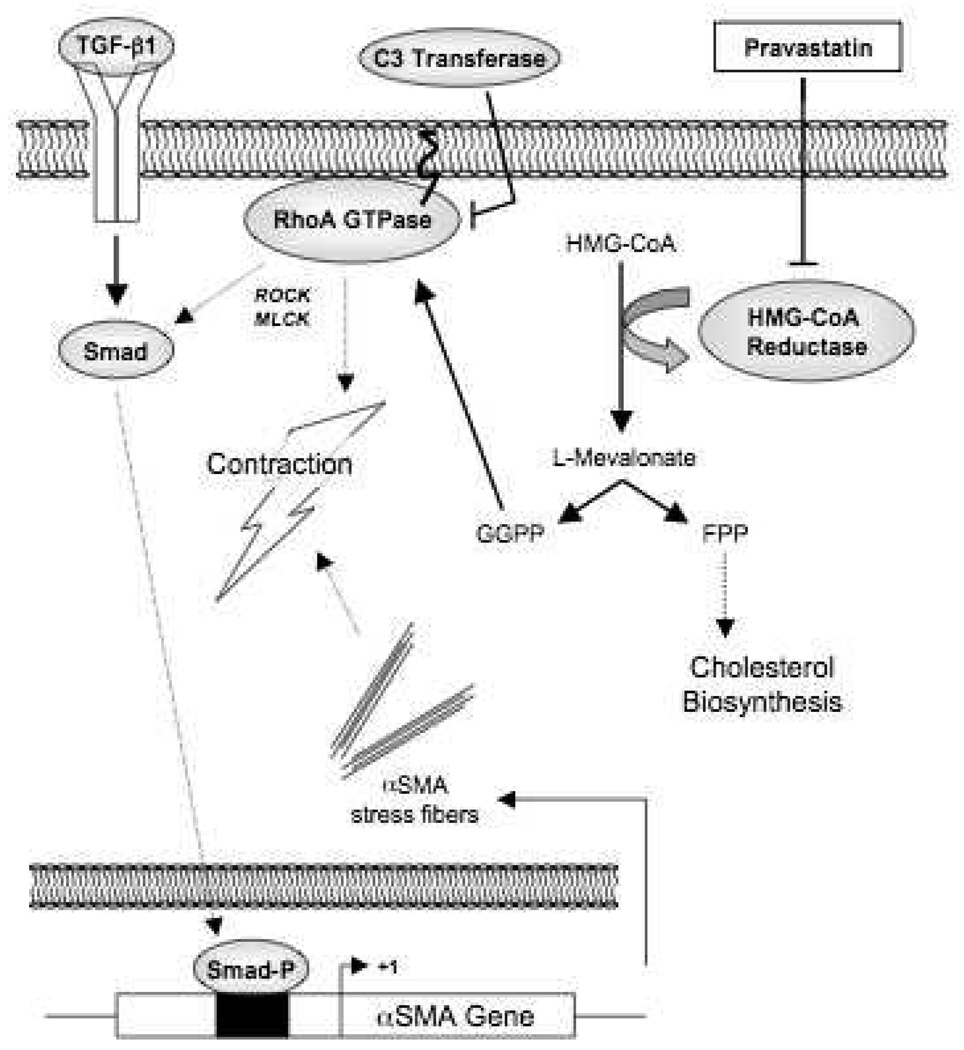
In the work presented here, we identify the underlying mechanism of initial calcific nodule formation in VIC cultures and explore the signaling pathways involved in statin-mediated inhibition of this phenotype. By artificially altering the expression levels of αSMA, we demonstrate for the first time that this protein, a marker of myofibroblast activation, is both necessary and sufficient for initiation of calcific nodule formation in VIC cultures. A central component of stress fibers, αSMA appears to mediate contractile activity in these cells, which has a profound effect on initiating cell-cell aggregation that ultimately leads to calcific nodules. αSMA expression is repressed by treatment of VICs with pravastatin, leading to decreased contractility and nodule formation. Figure 6 provides an overview of the signaling pathways examined.
Figure 6.
Proposed model for the inhibition of contraction-mediated nodule formation by pravastatin. Pravastatin blocks the production of isoprenoid intermediates through inhibition of HMG-CoA reductase activity. Prenylation of the downstream target RhoA leads to increased αSMA expression and VIC contractility through a Smad-dependent pathway.
Although widely accepted as a model of valvular calcification, the processes leading to the appearance of calcific nodules in VIC cultures have not been well understood. The first study examining VIC calcific nodules described dense round nodules positive for hydroxyapatite and collagen with mostly dead cells in the core, surrounded by a ring of living cells with osteoblast-like features 18. It was also shown that TGF-β1, as well as oxidized lipids and BMP-2, accelerated this ‘spontaneous’ process 17, 18. The mechanism that initiates nodule formation remained unclear, however. To test the hypothesis that cell migrated into nodules, Jian et. al. treated VIC cultures with cytochalasin D as a migration inhibitor 17. While cells were unable to move or form nodules due to a de-polymerized cytoskeleton, viability was greatly reduced and calcium content of the culture was increased. Follow-up studies have focused on the role of VIC apoptosis in the calcification process 17, 25. In apoptosis inhibition studies performed by Jian et. al., no correlation was found with nodule formation and apoptosis inhibition. Rather, apoptosis inhibition lead with subsequent calcium content reduction and concluded that apoptosis leads to mineralization of nodules but is not causal in initiation events 17, 25. In our time-lapse observations was also saw no visual change in cell number (Supp. Videos I and II, please see http://atvb.ahajournals.org.) and did not measure any significant change in cell dsDNA content with treatments of either TGF-β1 or pravastatin, preliminarily indicating that proliferation is not causal in the formation of VIC calcific nodules (Supp. Fig. VI, please see http://atvb.ahajournals.org.). In addition, VIC cultures may contain a subpopulation of osteoprogenitor cells that are maintained only through a limited number of population doublings and would likely not be present in cultures described herein 28. Furthermore, from our time-lapse images, it is apparent that a large number of VICs are participating in the initial formation of nodules rather than a small population. While all of these studies provided valuable insight for understanding to process of calcification, none addressed the mechanism initiating nodule formation. Further, it should be noted the VIC calcific nodule formation model is inherently limited in its ability to recapitulate the exact in vivo stenotic environment, it is a useful model for understanding basic VIC mediated calcification in a controlled and straightforward manner. Future in vivo studies will be required to understand the complete pathological situation.
In the work presented here, we sought to address this issue by first tracking nodule formation in real-time to identify the initial events that cause nodule formation without the use of cytotoxic agents that would adversely affect VIC function. Time-lapse images of VIC cultures suggested that contractility of confluent monolayers leads to the initial formation of cell aggregates that subsequently can be identified as calcific nodules.
Contractility in VICs and most fibroblastic cells is mediated through myofibroblast differentiation wherein contractile stress fibers positive for αSMA are formed de novo 29. This acquisition of contractility allows fibroblastic cells to close wounds at sites of injury. In vivo, repeated mechanical loading is believed to lead to initial events triggering valvular fibrosis and calcification 4. Additionally, increased deposition of lipids occurs, causing injury to the endothelial cell layer leading to the presence of inflammatory cells that are the sources of inflammatory cytokines such as TGF-β1 30. Each of these events can stimulate myofibroblast differentiation in the resident fibroblast population, and it has been shown that healthy valves consist mostly of quiescent fibroblast VICs with a small population of myofibroblastic VICs. In diseased valves, however, the VIC population shifts to be mainly activated myofibroblasts 31. This shift toward an activated phenotype has a profound effect on αSMA expression and contractility of the resident VICs.
Statins, long used to inhibit HMG-CoA reductase and lower cholesterol, have recently been recognized for having beneficial effects on valvular stenosis. In the work presented here, treatment of VIC cultures with pravastatin decreased cellular contractility and nodule formation, likely a result of decreased αSMA expression. Previous studies also show reduced no effect of apoptosis levels 32, alkaline phosphatase (ALP) activity, downregulation of cytokine production 33, and reduced contractility 32 with statin treatment. Wu et. al. also observed mevalonate rescue of calcification in VIC cultures treated with pravastatin, but not with Manumycin, an inhibitor of farnesyl transferase and suggest a non-prenylation pathway 34. Monzack et. al. however, showed opposite mevalonate effects and did not investigate downstream prenylation intermediates 32. To more thoroughly investigate the mechanism of pravastatin action in these cells, we used a series of supplements and specific enzyme inhibitors to selectively analyze the isoprenylation pathways downstream of HMG-CoA reductase (Supp. Fig. I and II, please see http://atvb.ahajournals.org.). Our data indicate that signaling kinases RhoA and ROCK act downstream of HMG-CoA reductase because the inhibition of either ROCK or MLCK reduces nodule formation (Supp. Fig. III, please see http://atvb.ahajournals.org.) in agreement with previous studies 32. Furthermore, as shown in Supplemental Figure IV (please see http://atvb.ahajournals.org.), pravastatin treatment also caused a decrease in luciferase activity in VICs transfected with a pSmad-luc reporter plasmid, suggesting that statins inhibit αSMA expression by regulating Smad activity. These data also indicate that a link exists between RhoA activation and Smad activity, though more investigation is required to uncover the nature of this interaction. The involvement of mitogen activated kinases (MAPKs), or p38 activation are possible candidates 35–37 linking RhoA and Smad interactions.
Lastly, we were interested in investigating if statins had the ability to reverse calcification in VIC cultures to attempt to address some of the mixed results from clinical studies. We observed that pravastatin was able to prevent additional increases in calcific nodule numbers but did not reduce or remove calcific nodules present. This data has potential ramifications in the use of statins to treat valvular fibrosis and stenosis, and may help to explain some of the paradoxical clinical study results. While the prospective studies showed no benefit of statin treatment for valvular disease, retrospective studies showed those taking statins for a long period of time for other conditions had reduced incidence of valvular fibrosis and calcification. This may be related to longer-term inhibition of VIC activation resulting in reduced fibrosis and calcification, whereas prospective study patients were treated with statins and observed for a shorter time period limiting impacts of fibrotic processes. However, mechanisms that are not present in porcine VIC cultures may exist in vivo and act to remove calcium deposits in statin-relaxed valve nodules. If so, a reduction in nodule number may be observed in human patients as a result of statin treatment.
In summary, we show for the first time that the formation of VIC calcific nodules is initiated by VIC myofibroblast activation and contractility. Moreover, statin inhibition of VIC activation is a result of reduced αSMA. Critical to possible clinical applications, pravastatin inhibits, but does not reverse, the formation of VIC calcific nodules. This work suggests that statins should be considered as potential therapeutics for those at risk of acquiring valvular fibrosis and stenosis and may have wider uses in the regulation of myofibroblast activity.
Supplementary Material
Acknowledgements
Sources of Funding
This work was supported by the Howard Hughes Medical Institute and NIH (HL089260). We also thank the National Science Foundation Graduate Research Fellowship Program (NSF-GRFP) and the Department of Education Graduate Assistantships in Areas of National Need (DoEd GAANN) for fellowships to JAB.
Footnotes
Disclosure
None.
References
- 1.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence Of Aortic-Valve Abnormalities In The Elderly - An Echocardiographic Study Of A Random-Population Sample. Journal Of The American College Of Cardiology. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 2.Rajamannan NM, Otto CM. Targeted therapy to prevent progression of calcific aortic stenosis. Circulation. 2004;110:1180–1182. doi: 10.1161/01.CIR.0000140722.85490.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaden JJ, Bickelhaupt S, Grobholz R, Vahl CE, Hagl S, Brueckmann M, Haase KK, Dempfle CE, Borggrefe M. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. Journal Of Heart Valve Disease. 2004;13:560–566. [PubMed] [Google Scholar]
- 4.Thubrikar MJ, Aouad J, Nolan SP. Patterns Of Calcific Deposits In Operatively Excised Stenotic Or Purely Regurgitant Aortic Valves And Their Relation To Mechanical-Stress. American Journal Of Cardiology. 1986;58:304–308. doi: 10.1016/0002-9149(86)90067-6. [DOI] [PubMed] [Google Scholar]
- 5.Rajamannan NM. Calcific Aortic Stenosis Lessons Learned From Experimental and Clinical Studies. Arteriosclerosis Thrombosis And Vascular Biology. 2009;29:162–168. doi: 10.1161/ATVBAHA.107.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. American Journal Of Cardiology. 2001;88:693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 7.Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. Journal Of The American College Of Cardiology. 2002;40:1723–1730. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 8.Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme A reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–2209. doi: 10.1161/hc4301.098249. [DOI] [PubMed] [Google Scholar]
- 9.Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensin-converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. doi: 10.1161/01.CIR.0000140723.15274.53. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: New mechanisms and clinical targets. Nature Medicine. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 11.Egashira K. Clinical importance of endothelial function in arteriosclerosis and ischemic heart disease. Circulation Journal. 2002;66:529–533. doi: 10.1253/circj.66.529. [DOI] [PubMed] [Google Scholar]
- 12.Rezaie-Majd A, Maca T, Bucek RA, Valent P, Muller MR, Husslein P, Kashanipour A, Minar E, Baghestanian M. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arteriosclerosis Thrombosis And Vascular Biology. 2002;22:1194–1199. doi: 10.1161/01.atv.0000022694.16328.cc. [DOI] [PubMed] [Google Scholar]
- 13.Shavelle DM, Takasu J, Budoff MJ, Mao SS, Zhao XQ, O'Brien KD. HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet. 2002;359:1125–1126. doi: 10.1016/S0140-6736(02)08161-8. [DOI] [PubMed] [Google Scholar]
- 14.Chan KL, Teo K, Tam J, Dumesnil JG. Rationale, design, and baseline characteristics of a randomized trial to assess the effect of cholesterol lowering on the progression of aortic stenosis: The Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) trial. American Heart Journal. 2007;153:925–931. doi: 10.1016/j.ahj.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. New England Journal Of Medicine. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 16.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. New England Journal Of Medicine. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 17.Jian B, Narula N, Li QY, Mohler ER, Levy RJ. Progression of aortic valve stenosis: TGF-beta 1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Annals of Thoracic Surgery. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. [DOI] [PubMed] [Google Scholar]
- 18.Mohler ER, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH. Identification and characterization of calcifying valve cells from human and canine aortic valves. Journal Of Heart Valve Disease. 1999;8:254–260. [PubMed] [Google Scholar]
- 19.Walker GA, Masters KS, Shah DN, Anseth KS, Leinwand LA. Valvular myofibroblast activation by transforming growth factor-beta - Implications for pathological extracellular matrix remodeling in heart valve disease. Circulation Research. 2004;95:253–260. doi: 10.1161/01.RES.0000136520.07995.aa. [DOI] [PubMed] [Google Scholar]
- 20.Johnson CM, Hanson MN, Helgeson SC. Porcine Cardiac Valvular Subendothelial Cells In Culture - Cell Isolation And Growth-Characteristics. Journal Of Molecular And Cellular Cardiology. 1987;19:1185–1193. doi: 10.1016/s0022-2828(87)80529-1. [DOI] [PubMed] [Google Scholar]
- 21.Rice NA, Leinwand LA. Skeletal myosin heavy chain function in cultured lung myofibroblasts. Journal of Cell Biology. 2003;163:119–129. doi: 10.1083/jcb.200303194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushing MC, Mariner PD, Liao JT, Sims EA, Anseth KS. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. Faseb Journal. 2008;22:1769–1777. doi: 10.1096/fj.07-087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benton JA, Kern HB, Anseth KS. Substrate Properties Influence Calcification in Valvular Interstitial Cell Culture. Journal Of Heart Valve Disease. 2008;17:689–699. [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29 doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark-Greuel JN, Connolly JM, Sorichillo E, Narula NR, Rapoport HS, Mohler ER, Gorman JH, Gorman RC, Levy RJ. Transforming growth factor-beta 1 mechanisms in aortic valve calcification: Increased alkaline phosphatase and related events. Annals Of Thoracic Surgery. 2007;83:946–953. doi: 10.1016/j.athoracsur.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-Ter-Vehn T, Sieprath S, Katzenberger B, Gebhardt S, Grehn F, Schlunck G. Contractility as a prerequisite for TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Investigative Ophthalmology & Visual Science. 2006;47:4895–4904. doi: 10.1167/iovs.06-0118. [DOI] [PubMed] [Google Scholar]
- 27.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Molecular Biology Of The Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JH, Yip CYY, Sone ED, Simmons CA. Identification and Characterization of Aortic Valve Mesenchymal Progenitor Cells with Robust Osteogenic Calcification Potential. American Journal Of Pathology. 2009;174:1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming Growth-Factor-Beta-1 Induces Alpha-Smooth Muscle Actin Expression in Granulation-Tissue Myofibroblasts and in Quiescent and Growing Cultured Fibroblasts. Journal of Cell Biology. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arteriosclerosis Thrombosis And Vascular Biology. 1999;19:1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 31.Olsson M, Rosenqvist M, Nilsson J. Expression Of Hla-Dr Antigen And Smooth-Muscle Cell-Differentiation Markers By Valvular Fibroblasts In Degenerative Aortic-Stenosis. Journal Of The American College Of Cardiology. 1994;24:1664–1671. doi: 10.1016/0735-1097(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 32.Monzack EL, Gu XX, Masters KS. Efficacy of Simvastatin Treatment of Valvular Interstitial Cells Varies With the Extracellular Environment. Arteriosclerosis Thrombosis And Vascular Biology. 2009;29:246–253. doi: 10.1161/ATVBAHA.108.179218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I547–I552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 34.Wu B, Elmariah S, Kaplan FS, Cheng GJ, Mohler ER. Paradoxical effects of statins on aortic valve myofibroblasts and osteoblasts - Implications for end-stage valvular heart disease. Arteriosclerosis Thrombosis And Vascular Biology. 2005;25:592–597. doi: 10.1161/01.ATV.0000154278.01871.64. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Liu YL, Dutt P, Fanburg BL, Toksoz D. Inhibition of serotonin-induced mitogenesis, migration, and ERK MAPK nuclear translocation in vascular smooth muscle cells by atorvastatin. American Journal Of Physiology-Lung Cellular And Molecular Physiology. 2007;293:L463–L471. doi: 10.1152/ajplung.00133.2007. [DOI] [PubMed] [Google Scholar]
- 36.Meyer-Ter-Vehn T, Katzenberger B, Han H, Grehn F, Schlunck G. Lovastatin inhibits TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Investigative Ophthalmology & Visual Science. 2008;49:3955–3960. doi: 10.1167/iovs.07-1610. [DOI] [PubMed] [Google Scholar]
- 37.Yamashita M, Otsuka F, Mukai T, Otani H, Inagaki K, Miyoshi T, Goto J, Yamamura M, Makino H. Simvastatin antagonizes tumor necrosis factor-a inhibition of bone morphogenetic proteins-2-induced osteoblast differentiation by regulating Smad signaling and Ras/Rho-mitogen-activated protein kinase pathway. Journal Of Endocrinology. 2008;196:601–613. doi: 10.1677/JOE-07-0532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.