Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and the relatively non-toxic selective aryl hydrocarbon receptor (AhR) modulator (SAhRM) 6-methyl-1,3,8-trichlorodibenzofuran (MCDF) induced CYP1A1-dependent ethoxyresorufin O-deethylase (EROD) activity and inhibited proliferation of seven estrogen receptor (ER) negative breast cancer cell lines. MCDF, TCDD and structurally related 2,3,7,8-tetrachlorodibenzofuran (TCDF), 1,2,3,7,8-pentachlorodibenzo-p-dioxin (PCDD), 2,3,4,7,8-pentachlorodibenzofuran (PCDF), and 3,3',4,4',5-pentachlorobiphenyl (PCB) induced CYP1A1 and inhibited proliferation of BT474 and MDA-MB-468 cells. In BT474 and MDA-MB-468 cells transfected with a small inhibitory RNA for the AhR (iAhR), the antiproliferative activity of the chlorinated aromatic compounds was reversed, whereas for MCDF, only partial reversal was observed, suggesting that this compound acts through both AhR-dependent and AhR-independent pathways in these two cell lines. MCDF also inhibited tumor growth in athymic nude mice in which MDA-MB-468 cells were injected directly into the mammary fat pad. These results suggests that the AhR is a potential drug target for treatment of ER-negative breast cancer.
Keywords: AhR agonists, chlorinated aromatics, SAhRMs, antiproliferative, ER-negative
INTRODUCTION
The AhR was initially identified as a receptor that bound the environmental toxicant 2,3,7,8-tetrachoroibenzo-p-dioxin (TCDD) with high affinity and studies with AhR knockout mice have confirmed a role for this protein in mediating TCDD-induced toxicity (Poland et al. 1976; Poland & Knutson 1982; Gonzalez & Fernandez-Salguero 1998; Schmidt et al. 1996). The mechanism of AhR action is similar to that described for other ligand-activated receptors and was determined in early studies on AhR-mediated induction of CYP1A1 gene expression [reviewed in (Whitlock et al. 1996; Whitlock, Jr. 1993)]. The unbound cytosolic AhR is associated with heat shock protein 90 (Hsp 90) and other factors and, in the presence of a ligand, the bound receptor forms a heterodimeric nuclear AhR complex containing the AhR and AhR nuclear translocator (Arnt) proteins. This complex binds dioxin response elements (DREs) in target gene promoters to induce transcriptional activation.
TCDD modulates an increasing number of biochemical, toxic and endocrine responses and research in the laboratory has focused on an intriguing AhR-mediated response, namely the tissue-specific inhibition of estrogen-induced genes and pathways (Safe & Wormke 2003; Safe 2005). Kociba and coworkers (Kociba et al. 1978) initially reported that dietary administration of TCDD to female Sprague Dawley rats inhibited age-dependent spontaneous mammary and uterine tumor formation. Subsequent studies in breast cancer cells and other E2-responsive tissues have characterized inhibitory AhR-ER crosstalk at the gene, response and mechanistic level and it is clear that multiple pathways are involved. For example, TCDD induces AhR-dependent degradation of ER via activation of proteasomes and this is due, in part, to the ubiquitin ligase activity of the AhR complex (Wormke et al. 2003; Ohtake et al. 2007).
Studies in several laboratories have demonstrated that the AhR may be a potential drug target for a number of diseases including ER-positive breast cancer, endometrial, prostate and pancreatic cancer and also for some autoimmune diseases (Koliopanus et al. 2002; McDougal et al. 1997; McDougal et al. 2001; Jana et al. 2000; Morrow et al. 2004; Castro-Rivera et al. 1999; Wormke et al. 2000; Quintana et al. 2008; Veldhoen et al. 2008; Kimura et al. 2008; Lawrence et al. 2008). Development of relatively non-toxic selective AhR modulators (SAhRMs) as drugs has been reported (Safe & McDougal 2002; Safe et al. 1999) and 6-methyl-1,3,8-trichlorodibenzofuran (MCDF) and other alternate-substituted dibenzofurans are highly effective agents for inhibiting hormone-responsive breast cancer growth in animal models (McDougal et al. 2001; Safe & McDougal 2002; Safe et al. 1999).
The AhR is also expressed in ER-negative breast cancer cells (Wang et al. 1997; Wang et al. 1995); however, the effectiveness of AhR agonists and SAhRMs against this highly aggressive form of late-stage breast cancer has not been extensively investigated. One report showed that TCDD inhibited ER-negative MDA-MB-468 cell proliferation and this was associated with induction of transforming growth factor-α (TGFα) which exhibits antiproliferative activity in this cell line (Wang et al. 1997). This study investigates the Ah-responsiveness of several different ER-negative breast cancer cell lines including MDA-MB-453, HCC-38, MDA-MB-436, MDA-MB-345, BT-474, MDA-MB-157 and MDA-MB-468 cells using the following AhR agonists: TCDD, 1,2,3,7,8-pentachlorodibenzo-p-dioxin (PCDD), 2,3,7,8-tetrachlorodibenzofuran (TCDF), 2,3,4,7,8-pentachlorodibenzofuran (PCDF), 3,3',4,4',5-pentachlorobiplenyl (PCB), and MCDF. These AhR agonists all induced CYP1A1-dependent activity and decreased proliferation of ER-negative breast cancer cell lines, and RNA interference studies with a small inhibitory RNA for the AhR (iAhR) confirmed that for TCDD and related chlorinated aromatics, their effects on cell growth were AhR-dependent. The effects of MCDF on breast cancer cell proliferation were both AhR-dependent and AhR-independent and this compound also inhibited tumor growth in athymic nude mice in which MDA-MB-468 cells were injected into the mammary fat pad.
MATERIALS AND METHODS
Cell lines, constructs, and antibodies
BT474, HCC-38, MDA-MB-453, MDA-MB-435, MDA-MB-436, MDA-MB-157, and MDA-MB-468 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The pDRE3-luciferase reporter plasmid was constructed in this laboratory and contained three tandem consensus dioxin response elements (DRE) (TCT TCT CAC GCA ACT CCG A — a single DRE sequence). Antibodies for CYP1A1, AhR and Arnt proteins were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody for β-actin was obtained from Sigma-Aldrich (St. Louis, MO).
Ethoxyresorufin O-deethylase (EROD) activity
Trypsinized cells were plated into 25 cm2 tissue culture flasks (105 cells/ml), allowed to attained 60% confluency, and treated with 10 nM TCDD for 24 hr. Cells were harvested by manual scraping from the plate, centrifuged at 400 × g for 5 min at 4°C and resuspended in 100 µl Tris-sucrose buffer (38 mM Tris-HCl, 0.2 M sucrose; pH 8.0). Aliquots (50 µM) of the cells were incubated with 1.15 ml cofactor solution (1 mg bovine serum albumin, 0.7 mg NADH, 0.7 mg NADPH, 1.5 mg MgSO4 in 0.1 M HEPES buffer; pH 7.5) in a 37°C water bath for 2 min. The reaction was started by adding 50 µl ethoxyresorufin solution (1 mg ethoxyresorufin/40 ml methanol). After incubation for 15 min, the reaction was stopped by adding 2.5 ml methanol. Samples were centrifuged for 10 min at 1500 × g. The supernatant was analyzed by fluorescence measurement at an excitation wavelength of 550 nm, and an emission wavelength of 595 nm.
Transient transfection assays
Cells were cultured in 12-well plates in 1 ml of DME/F12 medium supplemented with 2.5% fetal bovine serum. After 16–20 hr when cells were 30–50% confluent, the pDRE-luc (0.4 µg) and β-galactosidase (0.1 µg) constructs were transfected using Lipfectamine 2000 Reagent (Invitrogen, Carlsbad, CA) and after 12 hr, cells were treated with DMSO or the AhR agonists. Cells were harvested 36–44 hr after transfection by manual scraping in 1× lysis buffer (Promega, Madison, WI). For whole cell lysates, cells were frozen and thawed in liquid nitrogen, vortexed for 30 s, and centrifuged at 12,000 × g for 1 min. Lysates were assayed for luciferase activity using luciferase assay reagent (Promega). β-Galactosidase activity was measured using Tropix Galacto-Light Plus assay system (Tropix, Bedford, MA) in a Lumicount microwell plate reader (Packard Instrument Co.).
Western immunoblot assay
Cells were seeded into 35-mm six-well tissue culture plates in phenol red-free DME/F12 medium supplemented with 2.5% dextran/charcoal-stripped fetal bovine serum. After 24 hr, cells were treated with the five AhR agonists or DMSO (solvent control) for 24 hr and harvested in ice-cold high salt lysis buffer (50 mM HEPES, 500 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, pH 7.5) supplemented with protease inhibitor cocktail (Sigma). An aliquot of the whole cell lysates containing 30 µg protein was diluted with loading buffer, boiled, and loaded on a 10% SDS-polyacrylamide gel. Samples were electrophoresed at 150–180 V for 3–4 hr and separated proteins were transferred to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). Proteins were detected by incubation with polyclonal primary antibodies against CYP1A1, AhR, Arnt or β-actin (1:1000 dilution), followed by blotting with horseradish peroxidase-conjugated anti-rabbit (for CYP1A1, AhR and Arnt) or anti-mouse (for β-actin) secondary antibody (1:5000 dilution).
Cell proliferation and fluorescence-activated cell sorting (FACS)
Cells were transfected with iAhR or scrambled oligonucleotide. Thirty-six hr after the transfection, cells were trypsinized, syringed and collected by centrifugation. Cells were resuspended in staining solution [50 µg/mL propidium iodide, 30 units/mL RNase, 4 mmol/L sodium citrate, and Triton X-100 (pH 7.8)] and incubated at 37°C for 10 min. Sodium chloride solution was added to a final concentration of 0.15 mol/L. Stained cells were analyzed on a FACS Calibur Flow Cytometer (Becton Dickinson Immunocytometry Systems) using Cell Quest (Becton Dickinson Immunocytometry Systems) acquisition software. For cell proliferation studies, cells were transfected with iAhR or scrambled oligonucleotide using Lipofectamine 2000 reagent (Invitrogen); the medium was changed after 5 hr, and 4 or 7 days, later cells were counted using a Coulter Z1 cell counter (Beckman Coulter).
RNA interference studies
The siRNA targeting AhR was purchased from Dharmacon (Lafayette, CO), with the sequences of: 5'-UAA GGU GUC UGC UGG AUA AUU -3'. The nonspecific siRNA (4613) was purchased from Ambion (Austin, TX) as a negative control. Before the transfection process, cells were seeded in 12 well-plates in DME/F12 medium (Sigma-Aldrich) supplemented with 2.5% dextran/charcoal-stripped fetal bovine serum. After 24 hr, appropriate amounts of plasmids and/or siRNA duplexes were transfected using lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to manufacturer's recommendations. After 6–8 hr, cells were changed to fresh medium and appropriate chemical treatments were added.
In vivo studies with MCDF
Athymic nude Hsd:nu/nu homozygous female virgin mice were purchased from Harlan (Houston, TX) at 3–4 weeks of age and were transported and maintained under sterile conditions. Cancer cells were grown to 90% confluency, trypsinized, centrifuged and resuspended in 200 µl of a 1:1 solution of PBS plus Matrigel (Collaborative Biomed, Bedford, MA) at 4°C. Mice (5 animals per treatment group) were injected subcutaneously in both mammary fat pads, with 0.7 × 107 cells/site in a matrigel suspension. After approximately 7 days, mice were treated with corn oil (vehicle control) or MCDF (25 mg/kg) in corn oil by gavage every second day, and tumors were measured with a micrometer. Tumor area was calculated by the equation: Area = (length/2) × (width/2) × π. Statistical differences were determined as indicated below or by the student's t-test and significant (p < 0.05) differences using these test were consistently observed from days 16 – 22.
Statistical analysis
Statistical significance was determined by analysis of variance and Scheffe's test, and the levels of probability are noted. The results are expressed as means ± SE for at least three separate (replicate) experiments for each treatment group in the in vitro studies.
RESULTS
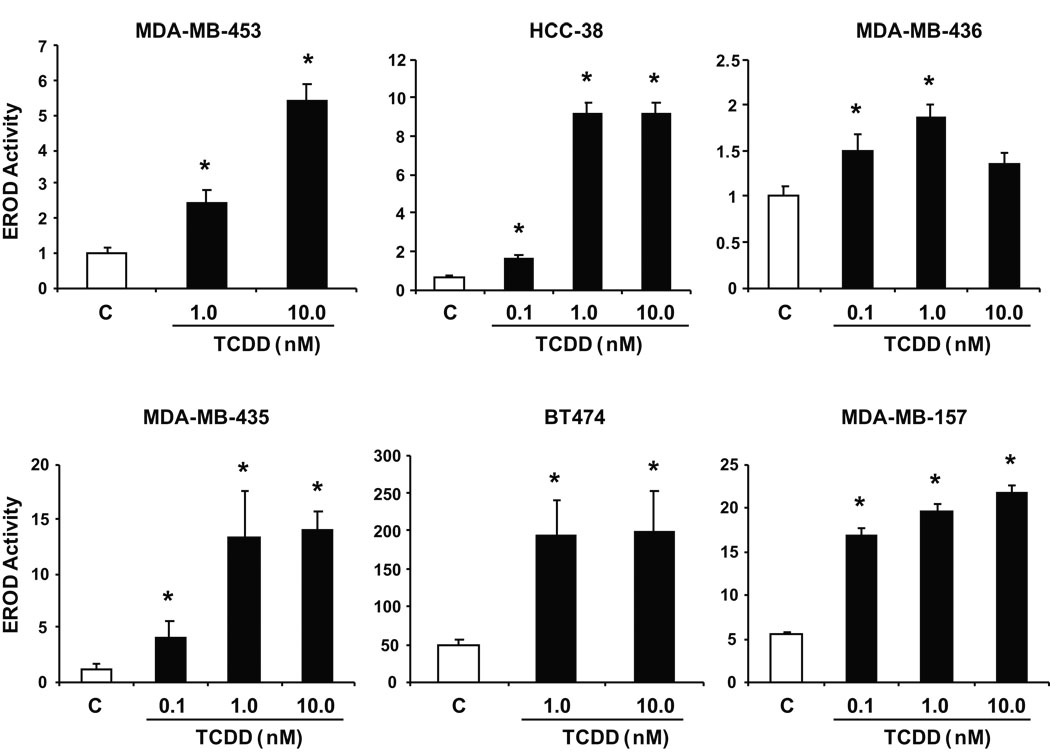
Previous studies show that inhibitory AhR-ERα crosstalk in breast cancer cells results in inhibition of E2-induced growth and gene expression in ER-positive breast cancer cells (Safe & Wormke 2003); however, the Ah-responsiveness and growth inhibitory effects of AhR agonists in ER-negative breast cancer cells is not well defined. Therefore, we initially investigated the Ah-responsiveness of several ER-negative breast cancer cells by determining the effects of TCDD on the induction of CYP1A1-dependent EROD activity. TCDD significantly induced EROD activity in two cell lines that overexpress the oncogene ErbB2 (BT474 and MDA-MB-453 cells) and also significantly induced this response in MDA-MB-435, HCC-38, MDA-MB-157, and MDA-MB-436 cells (Fig. 1). The dose-response curves and fold-inducibility were highly variable; however significant induction of EROD activity was observed in all cell lines. These results coupled with previous studies in MDA-MB-468 cells show that ER-negative breast cancer cells are Ah-responsive (Wang et al. 1997). The BT474 cells used in this study did not express ERα and this is illustrated in supplement Figure 1.
Figure 1.
Ah-responsiveness of ER-negative breast cancer cells. Induction of EROD activity by TCDD. ER-negative breast cancer cells were treated with DMSO or different concentrations of TCDD and EROD activity was determined as described in the Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (p < 0.05) induction is indicated (*).
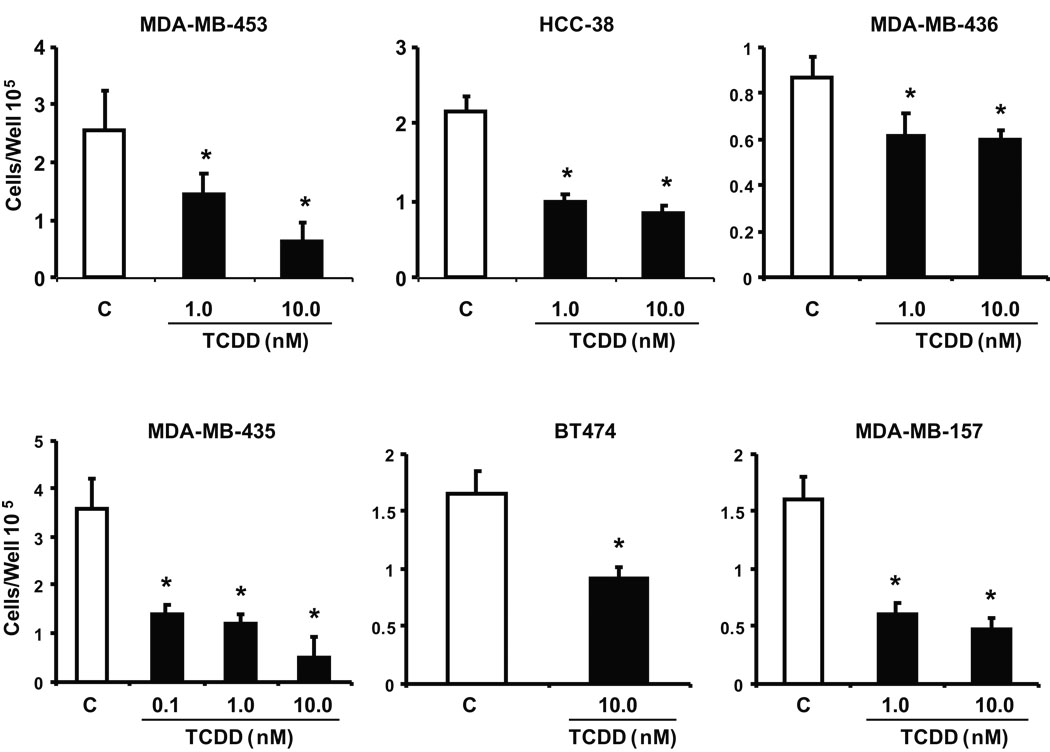
In addition, we also investigated the growth inhibitory effects of TCDD on this same group of ER-negative breast cancer cells (Fig. 2). Incubation of these cells with TCDD significantly decreased cell proliferation after treatment for 4 or 6 days. Two ER-negative lines, BT20 and MDA-MB-134, exhibited minimal Ah-responsiveness (induction of EROD activity by TCDD) and we also observed that TCDD did not appreciably inhibit growth of these cell lines (data not shown).
Figure 2.
Antiproliferative activity of TCDD. Inhibition of ER-negative breast cancer cell growth. ER-negative breast cancer cell lines were treated with DMSO or different concentrations of TCDD for six days and cells were counted as described in the Materials and Methods. Results are expressed as means ± SE for at least 3 replicate determinations for each treatment group and significant (p < 0.05) inhibition is indicated (*).
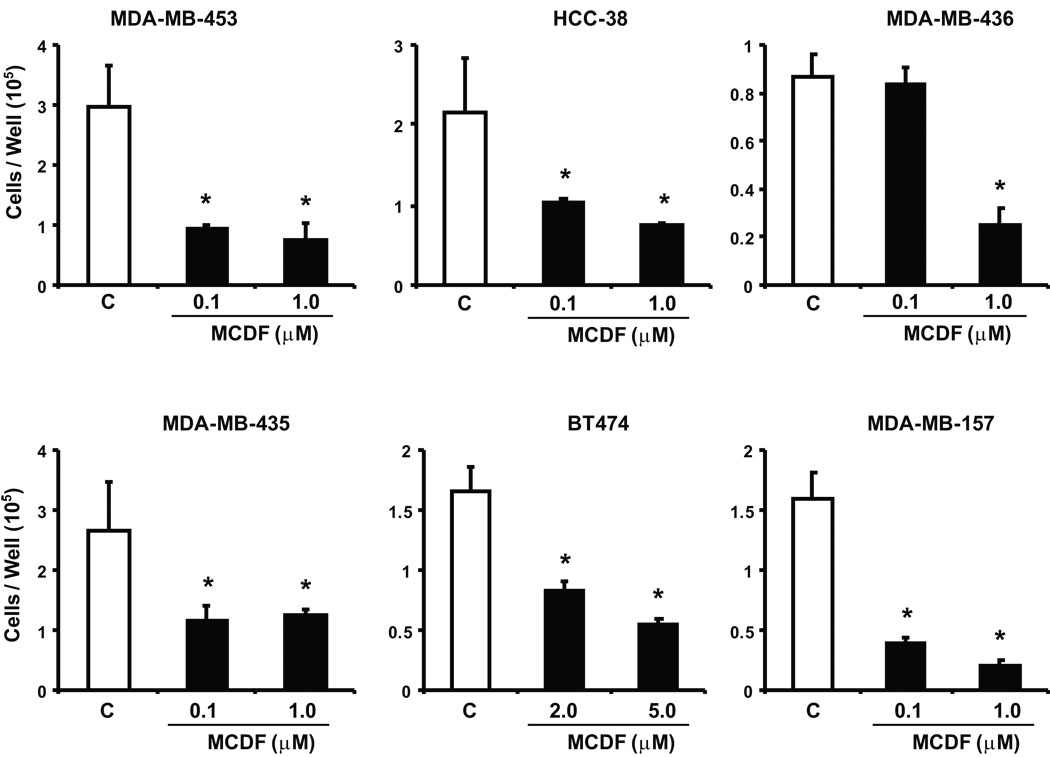
Previous studies indicate that the selective AhR modulator, MCDF, inhibits ER-positive breast cancer cell and tumor growth in vivo (McDougal et al. 2001), and this compound exhibits low toxicity and minimal induction of AhR-mediated toxic responses (Astroff et al. 1988; Harris et al. 1989; Bannister et al. 1989; Yao & Safe 1989). We also investigated the effects of MCDF on proliferation of ER-negative breast cancer cells. Results (Fig. 3) indicate that MCDF also inhibited growth of ER-negative breast cancer cells; however, this was accompanied by variable induction of CYP1A1-dependent EROD activity (data not shown) as previously observed in ER-positive breast cancer cells (Safe & McDougal 2002; Safe et al. 1999).
Figure 3.
Antiproliferative activity of MCDF. Inhibition of ER-negative breast cancer cell growth. ER-negative breast cancer cell lines were treated with DMSO or different concentrations of MCDF for six days and cells were counted as described in the Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (p < 0.05) of cell proliferation is indicated (*).
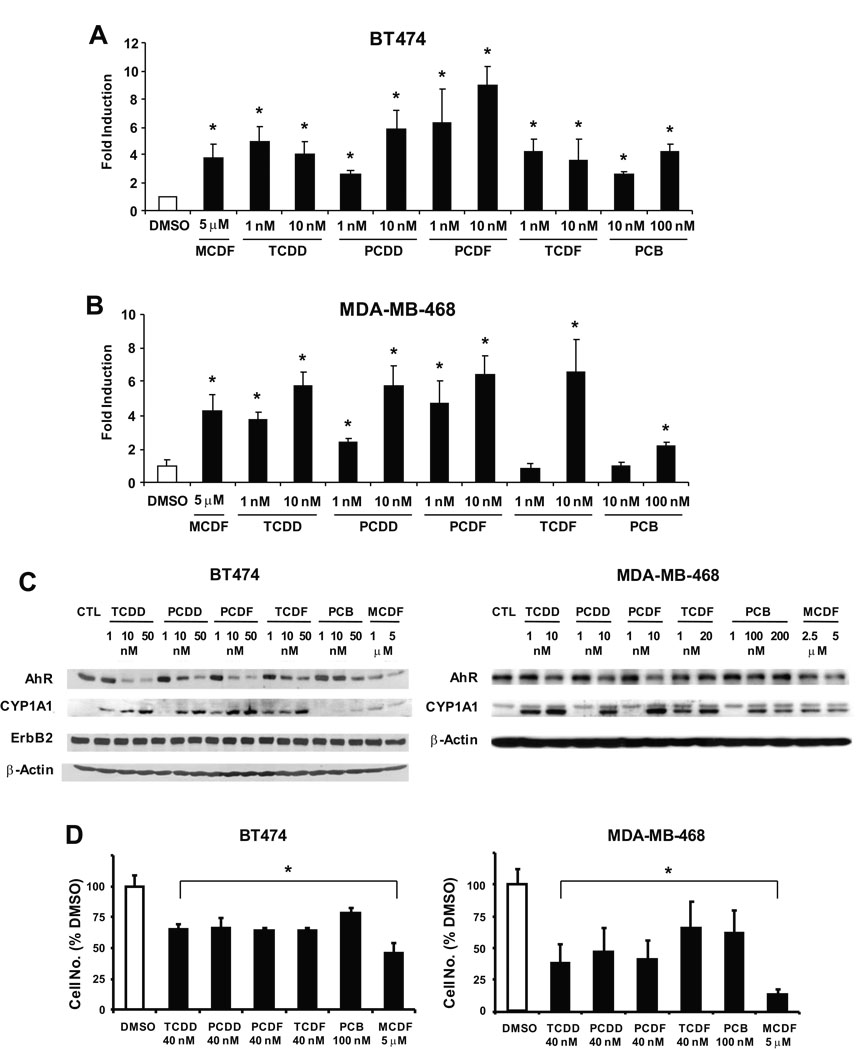
These data suggest that AhR ligands such as TCDD and MCDF decrease proliferation of ER-negative breast cancer cell lines; however, with the exception of previous studies with TCDD in MDA-MB-468 cells (Wang et al. 1997), the expression and role of the AhR in mediating the growth inhibitory effects of AhR agonists in ER-negative breast cancer cells has not been determined. In this study, we used TCDD and related chlorinated aromatics with known differences in their potencies as AhR agonists (Van den Berg et al. 2006). Figures 4A and 4B show that TCDD and related chlorinated aromatics and MCDF induced luciferase activity in BT474 and MDA-MB-468 cells transfected with an Ah-responsive DRE-luc construct, and treatment of these cells with the same compounds also resulted in the induction of CYP1A1 protein; in addition, the AhR was also expressed in both cell lines (Fig. 4C) and ErbB2 was highly expressed in BT474 cells but only minimal expression was observed in MDA-MB-468 cells (data not shown). The effects of these compounds on cell growth were also investigated in BT474 and MDA-MB-468 cells and the results (Fig. 4D) indicate that at the concentrations used in this study, all of the congeners significantly decreased proliferation of BT474 and MDA-MB-468 cells. Higher concentrations of AhR agonists were used in this 96 hr cell proliferation study compared to the 6 day experiments (Fig. 2 and Fig. 3) to ensure significant growth inhibition.
Figure 4.
Structure-dependent activation of AhR-dependent responses by chlorinated aromatics in BT474 and MDA-MB-468 cells. Activation of DRE-luc in BT474 (A) and MDA-MB-468 cells (B). Cells were transfected with the DRE-luc construct and treated with DMSO or different concentrations of TCDD, PCDD, TCDF, PCDF and PCB, and luciferase activity determined as described in the Materials and Methods. Results are expressed as means ± SE for 3 replicate determinations for each treatment group and significant (p < 0.05) induction is indicated (*). Structure-dependent induction of CYP1A1 protein and AhR expression (C) and growth inhibition (D) by AhR agonists. Cells were treated for either 24 (C) or 96 hr (D) and whole cell lysates were analyzed by western blots (C) or cells were counted (D) as described in the Materials and Methods. Results in (D) are presented as means ± SE for at least 3 replicate determinations for each treatment group and significant (p < 0.05) growth inhibition is indicated (*).
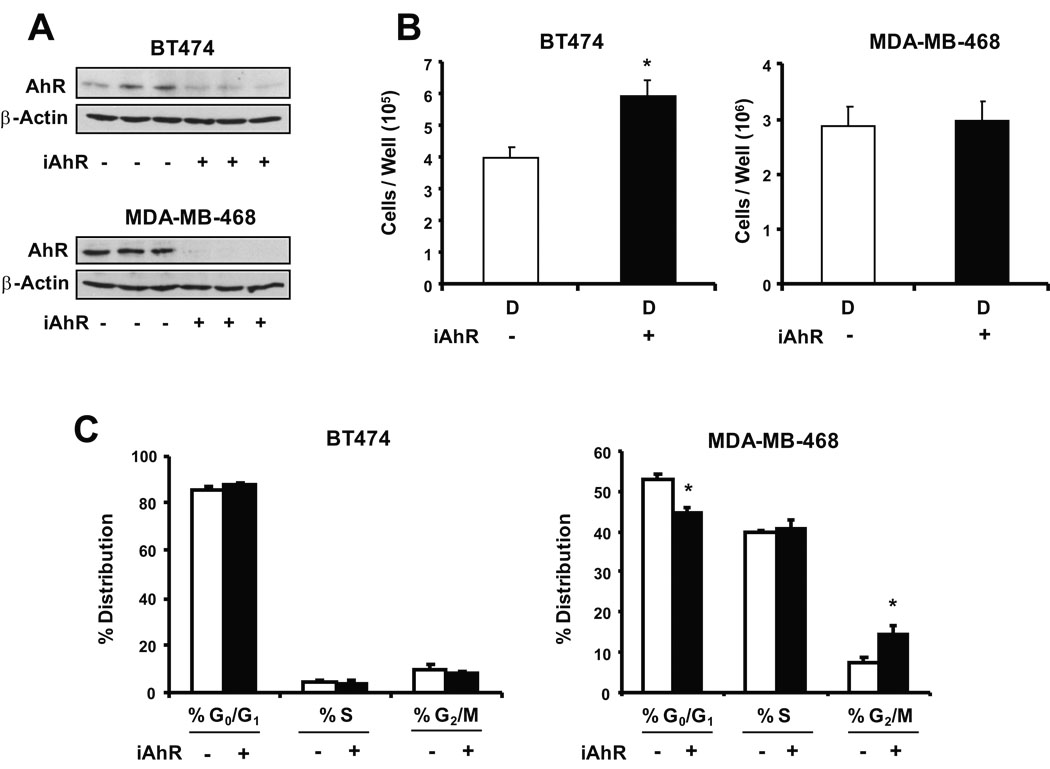
The role of the AhR in mediating the effects of the AhR agonists on ER-negative breast cancer cell survival was also investigated in BT474 and MDA-MB-468 cells transfected with a non-specific oligonucleotide (iCtr) and a small inhibitory RNA for the AhR (iAhR). Data in Figure 5A show that transfection with iAhR resulted in a > 80% decrease in AhR expression in BT474 and MDA-MB-468 cells. Knockdown of the AhR in BT474 cells resulted in a significant increase in cell proliferation compared to cells transfected with iCtr (Fig. 5B). This indicated that in BT474 cells, basal expression of the AhR inhibited cell proliferation, and similar results were previously reported in ER-positive MCF-7 breast cancer cells (Abdelrahim et al. 2003). In contrast, a comparison of cell numbers in MDA-MB-468 cells transfected with iCtr or iAhR treated with DMSO indicated that basal expression of the AhR did not affect proliferation of this cell line (Fig. 5B). The effects of AhR knockdown on distribution of BT474 and MDA-MB-468 cells in G0/G1, S and G2/M phases of the cell cycle were also determined (Fig. 5C). No significant effects were observed in BT474 cells, whereas AhR knockdown in MDA-MB-468 cells decreased cells in G0/G1 and induced a G2/M arrest.
Figure 5.
RNA interference and FACS analysis. Effects of iAhR on AhR protein (A) and cell proliferation (B). Cells were transfected with iAhR or non-specific oligonucleotide and the effects on AhR protein and proliferation of BT474 and MDA-MB-468 cells were determined as described in the Materials and Methods. Replicate (3) experiments were carried out for each treatment and, for the cell proliferation studies, results are expressed as means ± SE (after treatment for 96 hr) and significant (p < 0.05) effects of iAhR on cell proliferation are indicated (*). (C) FACS analysis. The effects of iAhR on distribution of BT474 and MDA-MB-468 cells in G0/G1, S and G2/M phases of the cell cycle were determined by FACS analysis as described in the Materials and Methods. Results obtained in cells transfected with iAhR are compared to cells transfected with a non-specific oligonucleotide as indicated above in (A) and (B). Results are expressed as means ± SE for three replicate experiments and significant changes after transfection with iAhR are indicated (*).
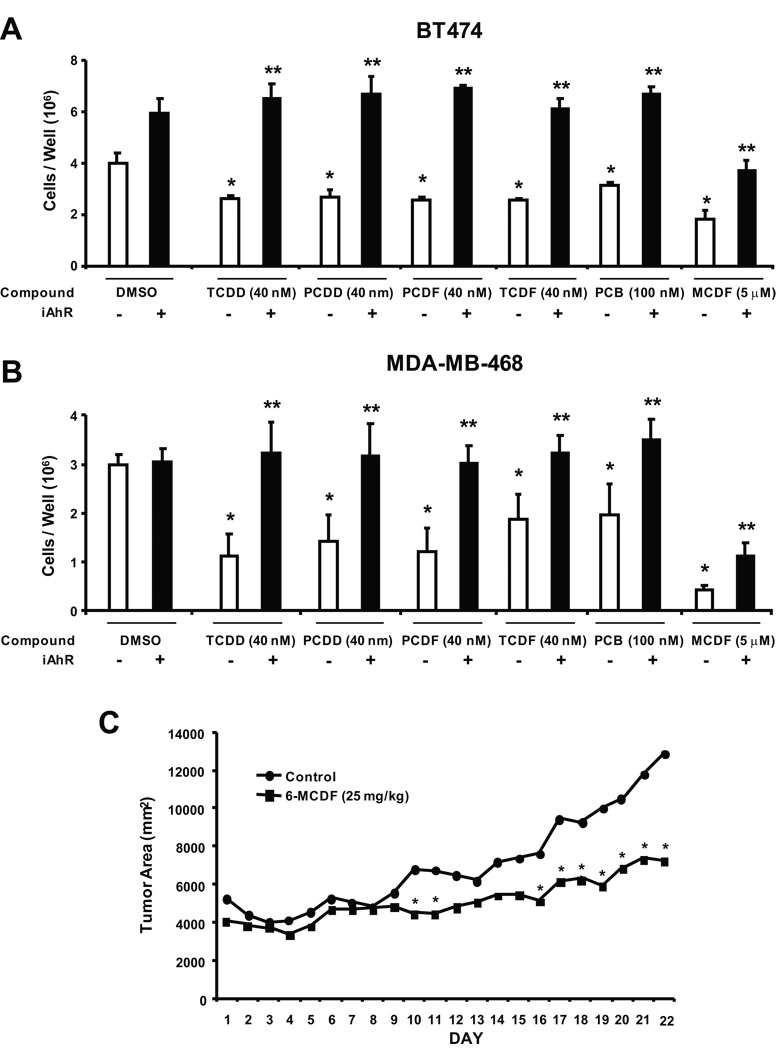
Due to the temporal limitations in AhR knockdown by RNA interference, we used higher concentrations of AhR agonists in the short term inhibition of cell proliferation study summarized in Figures 6A and 6B. Treatment of BT474 cells with 5 µM MCDF, 40 µM TCDD, 40 µM PCDD, 40 µM PCDF, 40 µM TCDF and 100 µM PCB all significantly decreased BT474 cell proliferation. In contrast, after transfection with iAhR, the antiproliferative effects of the AhR agonists were significantly inhibited and the chlorinated aromatics (TCDD, PCDD, TCDF, PCDF and PCB) did not significantly inhibit growth of BT474 cells compared to the solvent (DMSO) control. MCDF partially inhibited BT474 cell growth, even after AhR knockdown, suggesting that the growth inhibitory effects of this compound were both AhR-dependent and AhR-independent. The effects of iAhR on MDA-MBA-468 cell proliferation after treatment with the same set of compounds showed that the AhR agonist-dependent inhibition of growth was blocked after AhR knockdown by RNA interference (Fig. 6B). Moreover, the results obtained for MCDF were similar in MDA-MB-468 and BT474 cells, indicating an AhR-dependent and AhR-independent mechanism of action for this compound in both cell lines. Results in Figure 6C demonstrate that MCDF (25 mg/kg every second day) also inhibited growth of tumors in athymic nude mice bearing MDA-MB-468 cells injected directly into the mammary fat pad. Tumors derived from MDA-MB-468 cells grew slowly and consistent differences in tumor area between control and MCDF treatment groups were not observed until day 16 and significant (p < 0.05) differences were observed from days 16 – 22. Treatment with MCDF did not significantly affect body, liver, uterine, heart, spleen or kidney weight or expression of hepatic CYP1A1 (data not shown). These results demonstrate the potential clinical applications of SAhRMs for treatment of ER-negative breast cancer.
Figure 6.
Antiproliferative and antitumorigenic activity of AhR agonists. Role of the AhR in mediating the antiproliferative effects of AhR agonists in BT474 (A) and MDA-MB-468 (B) cells. Cells were transfected with non-specific scrambled oligonucleotide (iCtr) or iAhR and treated with DMSO or different AhR agonists for 4 days, and the number of cells were counted as described in the Materials and Methods. Results are expressed as means ± SE for at least 3 replicate determinations for each treatment group and significant (p < 0.05) inhibition of cell growth by the AhR agonists (*) and reversal of this effect by iAhR (**) are indicated. (C) Tumor growth inhibition. MDA-MB-468 cells were injected into the mammary fat pad of athymic nude mice and after palpable tumors were detected (7 – 10 days), mice were treated with corn oil (vehicle control) or MCDF (25 mg/kg) every 48 hr. Tumor volumes were determined as described in the Materials and Methods. Significant (p < 0.05) inhibition of tumor growth is indicated by an asterisk. Body weights and liver, uterine, heart, spleen and kidney weights as % body weight in control/MCDF-treated mice were 26±1/25±1, 5.2±0.3/5.3±0.1, 0.35±0.1/0.35±0.04, 0.46±0.01/0.49±0.01, 0.72±0.1/0.66±0.01 and 0.68±0.03/0.63±0.02, respectively.
DISCUSSION
Breast cancer is a highly complex disease in which treatment options depend on the staging of the tumor, localization or spreading of the tumor, and the molecular characteristics of the tumor including its estrogen receptor status or expression of other genes such as the ErbB2 (HER2/neu) oncogene (Moulder & Hortobagyi 2008; Buzdar 2003; Macaskill & Dixon 2007). Many early stage mammary tumors are ER-positive and have been successfully treated with antiestrogens such as tamoxifen, raloxifene, fulvestrant or aromatase inhibitors (Fisher et al. 2005; Vogel et al. 2006; Semiglazov et al. 2007; Howell et al. 2005). Prolonged use of tamoxifen can result in development of drug-resistant tumors and there is evidence that long term use of tamoxifen increases the risk for endometrial cancer (Vogel et al. 2006; Clarke et al. 2001). Some early stage and most later stage mammary tumors are ER-negative and patients with ER-negative breast cancer do not respond well to endocrine therapy and successful adjuvant chemotherapy requires the use of more highly cytotoxic drugs commonly used to treat other endocrine-independent tumors (Semiglazov et al. 2007; Moulder & Hortobagyi 2008). These agents generally target some aspect of nuclear function or modulate microtubule formation/breakdown and include compounds such as adriamycin, cyclophosphamide, gemcitabine, taxanes (taxol and taxotere), and capecitabine, a precursor of 5-FU (Moulder & Hortobagyi 2008). More recently, there has been an increase in the applications and development of more targeted therapies that include antibodies that interact with the angiogenic factor vascular endothelial growth factor (VEGF). In addition, tyrosine kinase inhibitors that target VEGF receptor and growth factor receptors have also been developed for clinical treatment of breast cancer (Moulder & Hortobagyi 2008; Buzdar 2003; Macaskill & Dixon 2007; Hobday & Perez 2005; Demonty et al. 2007). Another important advance for breast cancer treatment has been the increased use of combined agents which often target different pathways responsible for tumor survival, growth, angiogenesis and metastasis. Herceptin or trastuzumab is a monoclonal antibody directed against the extracellular domain of ErbB2 and objective response rates of 25–40% are observed with this antibody in patients that overexpress ErbB2 (Demonty et al. 2007).
Drugs such as MCDF that target the AhR are highly effective for inhibition of E2-responsive tumor growth in carcinogen-induced female Sprague Dawley rats, and MCDF and tamoxifen in combination synergistically blocked tumor formation and growth (McDougal et al. 2001). Although the AhR is widely expressed in ER-negative and ER-positive breast cancer cell lines (Wang et al. 1997; Wang et al. 1995), the potential applications of AhR agonists for treatment of ER-negative breast cancer is not well defined. One study in ER-negative MDA-MB-468 cells showed that TCDD inhibited survival of these cells through the induction of TGFα which exhibits antiproliferative activity in this cell line (Wang et al. 1997). Figure 1 shows that in addition to MDA-MB-468 cells, at least six other ER-negative breast cancer cell lines including two that overexpress ErbB2 (BT474 and MDA-MB-468 cells) were Ah-responsive, and TCDD and five structurally related chlorinated aromatics induced CYP1A1-dependent EROD activity. In this study, TCDD did not induce EROD activity in BT20 and MDA-MB-134 cells, and the reasons for the lack of Ah-responsiveness in these cells lines are currently being investigated. We also examined the comparative effects of TCDD and MCDF on survival of this panel of ER-negative breast cancer cells (Fig. 2 and Fig. 3) and both compounds significantly decreased growth of the six Ah-responsive cell lines.
The AhR interacts with structurally diverse ligands including synthetic aromatics, phytochemicals such as flavonoids and indole derivatives, drugs, pesticides, endogenous biochemicals including bilirubin, and other polyaromatics (Denison & Nagy 2003). The structure-dependent potencies of chlorinated aromatics such as TCDD, TCDF, PCDF, PCDD and PCBs as AhR agonists has been extensively investigated (Van den Berg et al. 2006) and for some responses such as induction of CYP1A1, there is a rank order correlation between their structure-AhR binding versus structure-activity relationships. Results in Figures 4A and 4B show that the chlorinated aromatics and MCDF induced luciferase activity in BT474 and MDA-MB-468 cells transfected with an Ah-responsive DRE-luciferase construct. Moreover, treatment of the two cell lines with the same set of compounds also induced CYP1A1 protein and western blot analysis of whole cell lysates also showed that the AhR was expressed in BT474 and MDA-MB-468 cells (Fig. 4C). In previous studies with ErbB2-overexpressing BT474 and MDA-MB-453 cells, we also showed that TCDD and MCDF inhibited cell proliferation but did not affect ErbB2 or its phosphorylation, and downstream kinases were also unchanged (unpublished results). However, results of the CYP1A1 induction studies coupled with the structure-dependent effects of the chlorinated aromatics and MCDF on decreased BT474 and MDA-MB-468 cell proliferation (Fig. 4D) are consistent with a role for the AhR in mediating the effects of these compounds.
Endogenous expression of the AhR in cancer cell lines can affect cell growth (Abdelrahim et al. 2003). Knockdown of the AhR in ER-positive MCF-7 breast cancer cells enhanced cell proliferation, whereas in HepG2 liver cancer cells, AhR knockdown decreased the rate of cell growth (Abdelrahim et al. 2003). In this study, iAhR transfection in BT474 cells resulted in enhanced growth; however, this was not accompanied by changes in the % distribution of cells in G0/G1, S or G2/M phases (Fig. 5C). Moreover, AhR agonists did not affect expression of ErbB2, phospho-ErbB2 or downstream kinases (data not shown), and we are currently investigating how the AhR and AhR agonists inhibit BT474 cell proliferation without changing the distribution of cells in G0/G1, S and G2/M phases of the cell cycle. In contrast to BT474 cells, no significant changes in proliferation were observed in MDA-MB-468 cells transfected with iAhR (Fig. 5B) but these cells exhibited a decrease in G0/G1 and an arrest at G2/M. Thus, the AhR differentially affects proliferation and % distribution of ER-negative breast cancer cells in G0/G1, S or G2/M phases of the cycle, and current studies are investigating the cell context-dependent modulation of Ah-responsive genes, proteins and microRNAs that determine these responses. The growth inhibitory effects of the chlorinated aromatic compounds in BT474 and MDA-MB-468 cells (Fig. 4 and Fig. 6) were reversed in both cell lines after transfection with iAhR (Figs. 6A and 6B) and this was consistent with the role of the ligand-activated AhR in mediating the decreased proliferation of ER-negative breast cancer cell.
MCDF also decreased breast cancer cell survival and inhibited tumor growth in athymic nude mice bearing MDA-MB-468 cells as xenografts (Fig. 6). These data complement previous studies showing the effectiveness of this compound as a mammary tumor growth inhibitor in carcinogen-induced female Sprague-Dawley rats (McDougal et al. 2001). MCDF was initially characterized as an AhR antagonist (McDougal et al. 2001) and studies with 125I-MCDF showed that this compound bound the AhR and induced formation of a nuclear AhR complex in cancer cells (Piskorska-Pliszczynska et al. 1991). However, results of RNA interference studies with iAhR (Figs. 6A and 6B) demonstrate that loss of the AhR only partially reversed the antiproliferative effects of MCDF on BT474 and MDA-MB-468 cells. Thus, the anticancer activity of MCDF in ER-negative breast cancer cells is both AhR-dependent and AhR-independent, and current studies are focused on the molecular mechanisms associated with both pathways and application of MCDF and other SAhRMs for treatment of ER-negative breast cancer.
Supplementary Material
Ah-responsiveness and ERα expression in BT474 and MCF-7 cells. Cells were treated with DMSO (D), 10 nM TCDD, 5 µM MCDF, 10 µM diindolylmethane (DIM), and 10 nM 17β-estradiol (E2, MCF-7 cells only) and after 24 hr, whole cell lysates were analyzed by western blots for ERα, AhR and CYP1A1 (loading control was β-actin).
Acknowledgments
Funding: The financial assistance of the Dow Chemical Company, the National Institutes of Health (ES04917), and Texas A&M AgriLife is gratefully acknowledged.
Footnotes
Declaration of Interest: The authors declare that there are no conflicts of interest.
REFERENCES
- Abdelrahim M, Smith R, III, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol. Pharmacol. 2003;63:1373–1381. doi: 10.1124/mol.63.6.1373. [DOI] [PubMed] [Google Scholar]
- Astroff B, Zacharewski T, Safe S, Arlotto MP, Parkinson A, Thomas P, Levin W. 6-Methyl-1,3,8-trichlorodibenzofuran as a 2,3,7,8- tetrachlorodibenzo-p-dioxin antagonist: inhibition of the induction of rat cytochrome P-450 isozymes and related monooxygenase activities. Mol. Pharmacol. 1988;33:231–236. [PubMed] [Google Scholar]
- Bannister R, Biegel L, Davis D, Astroff B, Safe S. 6-Methyl-1,3,8-trichlorodibenzofuran (MCDF) as a 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonist in C57BL/6 mice. Toxicology. 1989;54:139–150. doi: 10.1016/0300-483x(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Buzdar AU. Advances in endocrine treatments for postmenopausal women with metastatic and early breast cancer. Oncologist. 2003;8:335–341. doi: 10.1634/theoncologist.8-4-335. [DOI] [PubMed] [Google Scholar]
- Castro-Rivera E, Wormke M, Safe S. Estrogen and aryl hydrocarbon responsiveness of ECC-1 endometrial cancer cells. Mol. Cell. Endocrinol. 1999;150:11–21. doi: 10.1016/s0303-7207(99)00041-6. [DOI] [PubMed] [Google Scholar]
- Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol. Rev. 2001;53:25–71. [PubMed] [Google Scholar]
- Demonty G, Bernard-Marty C, Puglisi F, Mancini I, Piccart M. Progress and new standards of care in the management of HER-2 positive breast cancer. Eur. J. Cancer. 2007;43:497–509. doi: 10.1016/j.ejca.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Fernandez-Salguero P. The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metab Dispos. 1998;26:1194–1198. [PubMed] [Google Scholar]
- Harris M, Zacharewski T, Astroff B, Safe S. Partial antagonism of 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated induction of aryl hydrocarbon hydroxylase by 6-methyl-1,3,8-trichlorodibenzofuran: mechanistic studies. Mol. Pharmacol. 1989;35:729–735. [PubMed] [Google Scholar]
- Hobday TJ, Perez EA. Molecularly targeted therapies for breast cancer. Cancer Control. 2005;12:73–81. doi: 10.1177/107327480501200202. [DOI] [PubMed] [Google Scholar]
- Howell A, Pippen J, Elledge RM, Mauriac L, Vergote I, Jones SE, Come SE, Osborne CK, Robertson JF. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma: a prospectively planned combined survival analysis of two multicenter trials. Cancer. 2005;104:236–239. doi: 10.1002/cncr.21163. [DOI] [PubMed] [Google Scholar]
- Jana NR, Sarkar S, Ishizuka M, Yonemoto J, Tohyama C, Sone H. Comparative effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on MCF-7, RL95-2, and LNCaP cells: role of target steroid hormones in cellular responsiveness to CYP1A1 induction. Mol. Cell. Biol. Res. Commun. 2000;4:174–180. doi: 10.1006/mcbr.2001.0275. [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kociba RJ, Keyes DG, Beger JE, Carreon RM, Wade CE, Dittenber DA, Kalnins RP, Frauson LE, Park CL, Barnard SD, Hummel RA, Humiston CG. Results of a 2-year chronic toxicity and oncogenicity study of 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) in rats. Toxicol. Appl. Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- Koliopanus A, Kleeff J, Xiao Y, Safe S, Zimmerman A, Buchler MW, Friess H. Increased aryl hydrocarbon receptor expression offers a potential therapeutic target in pancreatic cancer. Oncogene. 2002;21:6059–6070. doi: 10.1038/sj.onc.1205633. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Denison MS, Novak H, Vorderstrasse BA, Harrer N, Neruda W, Reichel C, Woisetschlager M. Activation of the aryl hydrocarbon receptor is essential for mediating the anti-inflammatory effects of a novel low-molecular-weight compound. Blood. 2008;112:1158–1165. doi: 10.1182/blood-2007-08-109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaskill EJ, Dixon JM. Neoadjuvant use of endocrine therapy in breast cancer. Breast J. 2007;13:243–250. doi: 10.1111/j.1524-4741.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- McDougal A, Wilson C, Safe S. Inhibition of 7,12-dimethylbenz[a]anthracene-induced rat mammary tumor growth by aryl hydrocarbon receptor agonists. Cancer Lett. 1997;120:53–63. doi: 10.1016/s0304-3835(97)00299-1. [DOI] [PubMed] [Google Scholar]
- McDougal A, Wormke M, Calvin J, Safe S. Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective Ah receptor modulator. Cancer Res. 2001;61:3901–3907. [PubMed] [Google Scholar]
- Morrow D, Qin C, Smith R, III, Safe S. Aryl hydrocarbon receptor-mediated inhibition of LNCaP prostate cancer cell growth and hormone-induced transactivation. J. Steroid Biochem. Mol. Biol. 2004;88:27–36. doi: 10.1016/j.jsbmb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Moulder S, Hortobagyi GN. Advances in the treatment of breast cancer. Clin. Pharmacol. Ther. 2008;83:26–36. doi: 10.1038/sj.clpt.6100449. [DOI] [PubMed] [Google Scholar]
- Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, Fujii-Kuriyama Y, Kato S. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–566. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J, Astroff B, Zacharewski T, Harris M, Rosengren R, Morrison V, Safe L, Safe S. Mechanism of action of 2,3,7,8-tetrachlorodibenzo-p-dioxin antagonists: characterization of [125I]-6-methyl-8-iodo-1,3-dichlorodibenzofuran-Ah receptor complexes. Arch. Biochem. Biophys. 1991;284:193–200. doi: 10.1016/0003-9861(91)90283-o. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol: evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons. Examinations of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Safe S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and related environmental antiestrogens: characterization and mechanism of action. In: Naz RK, editor. Endocrine Disruptors. edn 2nd. Boca Raton, FL: CRC Press; 2005. pp. 249–287. [Google Scholar]
- Safe S, McDougal A. Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers. Int. J. Oncol. 2002;20:1123–1228. [PubMed] [Google Scholar]
- Safe S, Qin C, McDougal A. Development of selective aryl hydrocarbon receptor modulators (SARMs) for treatment of breast cancer. Expert Opinion on Investigational Drugs. 1999;8:1385–1396. doi: 10.1517/13543784.8.9.1385. [DOI] [PubMed] [Google Scholar]
- Safe S, Wormke M. Inhibitory aryl hydrocarbon-estrogen receptor α cross-talk and mechanisms of action. Chem. Res. Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semiglazov VF, Semiglazov VV, Dashyan GA, Ziltsova EK, Ivanov VG, Bozhok AA, Melnikova OA, Paltuev RM, Kletzel A, Berstein LM. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244–254. doi: 10.1002/cncr.22789. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, DeVito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr., Wade JL, III, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, Caskill-Stevens W, Ford LG, Jordan VC, Wolmark N. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- Wang W, Porter W, Burghardt R, Safe S. Mechanism of inhibition of MDA-MB-468 breast cancer cell growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Carcinogenesis. 1997;18:925–933. doi: 10.1093/carcin/18.5.925. [DOI] [PubMed] [Google Scholar]
- Wang X, Thomsen JS, Santostefano M, Rosengren R, Safe S, Perdew GH. Comparative properties of the nuclear Ah receptor complex from several human cell lines. Eur. J. Pharmacol. 1995;293:191–205. doi: 10.1016/s0922-4106(05)80044-6. [DOI] [PubMed] [Google Scholar]
- Whitlock JP., Jr. Mechanistic aspects of dioxin action. Chem. Res. Toxicol. 1993;6:754–763. doi: 10.1021/tx00036a003. [DOI] [PubMed] [Google Scholar]
- Whitlock JP, Okino ST, Dong L, Ko HP, Clarke-Katzenberg R, Ma Q, Li H. Induction of cytochrome P4501A1: a model for analyzing mammalian gene transcription. FASEB J. 1996;10:809–818. doi: 10.1096/fasebj.10.8.8666157. [DOI] [PubMed] [Google Scholar]
- Wormke M, Castro-Rivera E, Chen I, Safe S. Estrogen and aryl hydrocarbon receptor expression and crosstalk in human Ishikawa endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 2000;72:197–207. doi: 10.1016/s0960-0760(00)00030-3. [DOI] [PubMed] [Google Scholar]
- Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S. The aryl hydrocarbon receptor mediates degradation of the estrogen receptor α through activation of proteasomes. Mol. Cell. Biol. 2003;23:1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Safe S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced porphyria in genetically inbred mice: partial antagonism and mechanistic studies. Toxicol. Appl. Pharmacol. 1989;100:208–216. doi: 10.1016/0041-008x(89)90307-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ah-responsiveness and ERα expression in BT474 and MCF-7 cells. Cells were treated with DMSO (D), 10 nM TCDD, 5 µM MCDF, 10 µM diindolylmethane (DIM), and 10 nM 17β-estradiol (E2, MCF-7 cells only) and after 24 hr, whole cell lysates were analyzed by western blots for ERα, AhR and CYP1A1 (loading control was β-actin).