Abstract
Resistant starch (RS) is a fermentable fiber that decreases dietary energy density and results in fermentation in the lower gut. The current studies examined the effect of RS on body fat loss in mice. In a 12 week study (study 1), the effect of two different types of RS on body fat was compared with two control diets (0% RS) in C57Bl/6J mice: regular control diet or the control diet that had equal energy density as the RS diet (EC). All testing diets had 7% (wt/wt) dietary fat. In a 16 week study (study 2), the effect of RS on body fat was compared with EC in C57BL/6J mice and two obese mouse models (NONcNZO10/LtJ or Non/ShiLtJ). All mice were fed control (0% RS) or 30% RS diet for 6 weeks with 7% dietary fat. On the 7th week, the dietary fat was increased to 11% for half of the mice, and remained the same for the rest. Body weight, body fat, energy intake, energy expenditure, and oral glucose tolerance were measured during the study. At the end of the studies, the pH of cecal contents was measured as an indicator of RS fermentation. Results: Compared with EC, dietary RS decreased body fat and improved glucose tolerance in C57BL/6J mice, but not in obese mice. For other metabolic characteristics measured, the alterations by RS diet were similar for all three types of mice. The difference in dietary fat did not interfere with these results. The pH of cecal contents in RS fed mice was decreased for C57BL/6J mice but not for obese mice, implying the impaired RS fermentation in obese mice. Conclusion: 1) decreased body fat by RS is not simply due to dietary energy dilution in C57Bl/6J mice, and 2) along with their inability to ferment RS; RS fed obese mice did not lose body fat. Thus, colonic fermentation of RS might play an important role in the effect of RS on fat loss.
Keywords: Resistant starch, Fiber, Dietary energy density, Short chain fatty acids, Obesity, Food intake
Introduction
Since obesity and overweight are serious health problems worldwide, methods that are easily accepted by the general public to decrease body fat are urgently needed. Dietary fiber has been widely used as an effective way to decrease calorie intake and maintain a healthy body weight (1, 2). Many dietary fibers are indigestible and non-fermentable; and therefore, are simply used to dilute dietary energy density. In contrast, resistant starch (RS) is a different kind of dietary fiber. Resistant starch is indigestible in the small intestine and arrives intact in the large intestine, where it is fermented by microflora to produce short chain fatty acids (3, 4). Incorporating RS into the diet can decrease body fat storage and increase insulin sensitivity (3, 5–8) in humans. However, opposite results have also been reported (9, 10). These equivocal results might be due to differences in dietary components, texture, and energy content between control diets and RS diets used. In contrast to human studies, the effect of resistant starch on lowering body fat is consistent in animal models (11–15). In these animal studies, regular cornstarch in the control diet was replaced by resistant starch in the RS diet. Thus, control diets and RS diets have the same gross energy density, but their metabolizable energy densities are different. Metabolizable energy of the diet is defined as gross energy of the diet minus the energy lost in the feces, urine and combustible gases (16). Because resistant starch is less digestible and metabolizable than regular starch, there is more energy loss to microflora in fermentation and in the feces of RS fed animals. Therefore, the metabolizable energy densities of RS diets are lower than the control diets used in those animal studies
Dietary energy density is important for controlling body weight (17, 18). There is a general assumption that resistant starch, if it does reduce body fat, does so simply by diluting the energy density of the diet. The uniqueness of dietary resistant starch on body fat loss beyond energy dilution was not addressed in those previous studies. We have shown that dietary resistant starch can decrease body fat in rats even when compared with the control diet that has the same metabolizable energy density (19–22). To study the mechanism for body fat loss by resistant starch beyond energy dilution, we plan to use several mice strains with certain gene deletions. However, the fat loss beyond energy dilution by RS has not been confirmed in the mouse model, especially in the obese mouse model. Thus, the following two studies were conducted to examine if dietary RS decreases body fat in lean and obese mice.
Experimental Methods
We first tested the effect of resistant starch on body fat loss in C57BL/6J mice (study 1). Two different control diets were used in this study: a regular AIN-93G rodent diet, and a modified AIN-93 control diet that has equal metabolizable energy density as the RS diet. This experimental design allows us to compare the RS diet with control diets that either have higher or the same metabolizable energy density as RS diet. In study 2, we tested the effects of RS on body fat loss and related metabolic characteristics in two obese mouse models: polygenic obese diabetes mice (NoNcNZO10/LtJ), and Non/ShiLtJ mice. C57BL/6J mice were also included as a positive control. Two levels of dietary fat (7% and 11%) were used in study 2 to examine if the level of dietary fat could alter the effect of resistant starch on body fat. More metabolic related characteristics were measured in study 2, including food intake, body weight, body fat, glucose tolerance, respiratory quotient, energy expenditure, and physical activity. Because colonic fermentation of resistant starch increases the weight of the cecum and decreases the pH of cecal contents, we also measured these characteristics as indicators of RS fermentation in the large intestine.
Animals, housing conditions and diets
Male C57BL/6J mice, polygenic obese mice (NoNcNZO10/LtJ, Jackson lab stock no. 4456), and Non/ShiLtJ mice (Jackson Lab stock no. 2423) were obtained from Jackson Laboratory (Bar Harbor, ME) at 5–7 weeks of age. Animals were housed in shoe box cages (Study 1) or suspended wire-bottom cages (Study 2) in a humidity and temperature controlled room (22±2°C, 65–67% humidity) on a 12:12h light:dark cycle with free access to food and water. Wire-bottom cages were used for measuring food spillage in study 2. All mice were fed semi-purified powder diets prepared in our lab. The control (0% RS) and RS diets were prepared based on the AIN-93 diet formula for laboratory rodents (23). After quarantine, mice were fed the control diet and acclimated to experimental conditions until their body weights were stable (at least for 7 days). Then, mice were divided into different dietary treatment groups based on their body weight. Animal protocols were approved by the Pennington Biomedical Research Center and the Louisiana State University Animal Care and Use Committees.
Study 1
The purpose of study 1 was to examine if dietary resistant starch is more effective in decreasing body fat than simple energy dilution in C57BL/6J mice. Two control diets were used: a regular AIN-93 rodent diet (CC, 3.7 kcal/g metabolizable energy) and a modified CC diet with metabolizable energy density equal to RS diet (EC, 3.3kcal/g metabolizable energy). Two different types of RS were also tested in Study 1: RS2 (High amylose resistant cornstarch, Hi- Maize 260®), and RS3 (Retrograded resistant cornstarch, Novelose® 330). Both types of RS diets had 3.3 kcal/g metabolizable energy density.
Adult male C57BL/6J mice were randomly assigned to one of four diet treatment groups (n=10–20) for a 12 week study: 1) CC, 2) EC, 3) RS2, and 4) RS3. The detailed diet composition is listed in Table 1.
Table 1.
Composition of control and resistant starch diets used in Study 1
| Ingredient1,2 | kJ/g | kcal/g | Control diets | Resistant starch diets | ||
|---|---|---|---|---|---|---|
| Control | EC5 | RS2 | RS3 | |||
| Cornstarch | 15.4 | 3.50 | 531 | 420 | 0 | 77 |
| Resistant starch 2 | 11.7 | 2.80 | 0 | 0 | 531 | 0 |
| Resistant starch 3 | 11.1 | 2.65 | 0 | 0 | 0 | 454 |
| Cellulose | 0.0 | 0.00 | 50 | 161 | 0 | 50 |
| Sucrose | 16.8 | 4.00 | 100 | 100 | 100 | 100 |
| Casein | 15.0 | 3.58 | 200 | 200 | 200 | 200 |
| Soybean oil | 35.4 | 8.45 | 70 | 70 | 70 | 70 |
| Mineral mix3 | 3.5 | 0.84 | 35 | 35 | 35 | 35 |
| Vitamin mix3 | 16.2 | 3.87 | 10 | 10 | 10 | 10 |
| Choline chloride | 0.0 | 0.0 | 1.3 | 1.3 | 1.3 | 1.3 |
| L-cystine | 16.8 | 4.00 | 3.0 | 3.0 | 3.0 | 3.0 |
| Metabolizable energy4 | 1000g/kg (3.7kcal/g) | 1000g/kg (3.3kcal/g) | 1000g/kg (3.3kcal/g) | 1000g/kg (3.3kcal/g) | ||
| Fat content | 7%(wt/wt) or 18% (cal/cal) | 7%(wt/wt) or 18% (cal/cal) | 7%(wt/wt) 18% (cal/cal) | 7%(wt/wt) 18% (cal/cal) | ||
| Resistant starch content6, 7 | 0% | 0% | 30% | 25% | ||
Amioca ® cornstarch (100% amylopectin, which has 0% RS)), resistant starch2 (Hi-Maize 260®), and resistant starch3 (Novelose® 330) were provided by National Starch and Chemical Company (Bridgewater, NJ).
Other ingredients are purchased from Dyets Inc. (Bethlehem, PA).
Mineral mix and Vitamin mix are both AIN-93G.
Metabolizable energy values listed are obtained from National Starch and Chemical Company (Novelose® 330) or our unpublished data based on bomb calorimetry for Amioca® cornstarch and Hi-Maize 260®. The metabolizable energy values for the rest of the ingredients are obtained from Dyets Inc.
EC diet was similar to the regular control diet but extra cellulose was added to match its metabolizable energy density to the RS diets.
The resistant starch content in the diet was calculated based on the amount of Hi-Maize® (56% resistant starch) and Novelose® (55% resistant starch) used. The resistant starch contents in Hi-Maize and Novelose were determined by Englyst method and provided by National Starch and Chemical Company.
RS contents were slightly different between the RS2 and RS3 diets on a weight basis. This difference is due to the difference in metabolizable energy value between the RS2 and RS3. To make the RS2 and RS3 diets have equal metabolizable energy densities, the RS contents are 30% for the RS2 diet and 25% for the RS3 diet on a weight basis.
After 12 weeks on their respective treatment diets, mice were killed by CO2 inhalation. Fat pads (epididymal fat, perirenal, and retroperitoneal fat) from the abdominal cavity were dissected and combined as total abdominal fat. Body fat results were expressed as percent of total abdominal fat divided by final disemboweled body weight. Because RS fed mice have a significantly higher gastrointestinal (GI) tract weight than mice fed the control diet, the disemboweled body weight was calculated as body weight minus total full GI tract weight and was used for calculation of percentage body fat. Liver weight and spleen weight were also recorded for each mouse. Full and empty cecal weights were recorded and cecal contents weight was calculated as full cecal weight minus empty cecal weight. The pH and short chain fatty acids of cecal contents were measured as described previously (20).
Study 2
The purpose of study 2 was to determine if dietary resistant starch also decreases body fat in obese mouse models and the potential mechanism involved. Because fermentation of resistant starch is attenuated in animals fed a higher fat diet (unpublished observation), the high fat diet induced obese mouse model was not used here. Instead, two genetic obese mouse models were used: NONcNZO10/LtJ mice (Obese 1, Jackson Lab stock number 4456) and Non/ShiLtJ mice (Obese 2, Jackson Lab stock number 2423). NoNcNZO10/L is a polygenic mouse model of obesity-induced diabetes, and also a model that most closely mimics human obesity ( 24). Non/ShiLtJ mice are controls for NoNcNZO10/L mice, which are recommended by Jackson Lab as these two mouse models have similar genetic backgrounds (24). Non/ShiLtJ mice are also moderately obese, but the obesity is adult onset and they do not develop diabetes in the early stage of life (25). For Obese 1 mice to develop hyperglycemia, 10–11% dietary fat is required (24), but the control diet used in study 1 only has 7 % dietary fat. Thus, two dietary fat levels (7% and 11%) were used in study 2 for both control and RS diets. Besides these two obese mouse models, C57BL/6J mice were used as a positive control in study 2. RS2 was used as the resistant starch source in study 2 because less adverse effects (bloating and flatulence) were observed in RS2 fed mice compared to the mice fed RS3 (unpublished observation). The detailed composition of the diet is listed in Table 2.
Table 2.
Composition of control and resistant starch diets used in Study 2
| Ingredient (g/kg)1,2 |
7% dietary fat |
11% dietary fat |
||
|---|---|---|---|---|
| Control | RS | Control | RS | |
| Corn starch1,3 | 424.5 | 0 | 432.5 | 0 |
| Resistant starch1,3 | 0 | 530.7 | 0 | 540.7 |
| Sucrose | 100 | 100 | 100 | 100 |
| Casein | 200 | 200 | 200 | 200 |
| Soybean oil | 70 | 70 | 70 | 70 |
| lard | 0 | 0 | 40 | 40 |
| Cellulose | 156.2 | 50 | 108.2 | 0 |
| Mineral mix (AIN-93G) | 35 | 35 | 35 | 35 |
| Vitamin mix (AIN-93G) | 10 | 10 | 10 | 10 |
| Choline chloride | 1.3 | 1.3 | 1.3 | 1.3 |
| L-cystine | 3.0 | 3.0 | 3.0 | 3.0 |
| Metabolizable energy | 1000g/kg (3.3kcal/g) | 1000g/kg (3.3kcal/g) | 1000g/kg (3.7kcal/g) | 1000g/kg (3.7kcal/g) |
| Fat content | 7%(wt/wt) or 18% (cal/cal) | 7%(wt/wt) or 18% (cal/cal) | 11%(wt/wt) 26% (cal/cal) | 11%(wt/wt) 26% (cal/cal) |
| Resistant starch content4 | 0% | 30% | 0% | 30% |
Amioca ® cornstarch (100% amylopectin, which has 0% RS) and resistant starch (Hi-Maize 260®) were used for control and resistant starch diets respectively. Both starches were provided by National Starch and Chemical Company (Bridgewater, NJ).
Other ingredients are purchased from Dyets Inc. (Bethlehem, PA).
Metabolizable energy values were 3.5 kcal/g for Amioca® cornstarch (National Starch and Chemical Company) and 2.8 kcal/g for resistant starch Hi-Maize 260® (unpublished data based on bomb calorimetry).
The resistant starch content in the diet was calculated based on the amount of Hi-Maize® (56% resistant starch) used. The resistant starch content in Hi-Maize® was determined by Englyst method and provided by National Starch and Chemical Company.
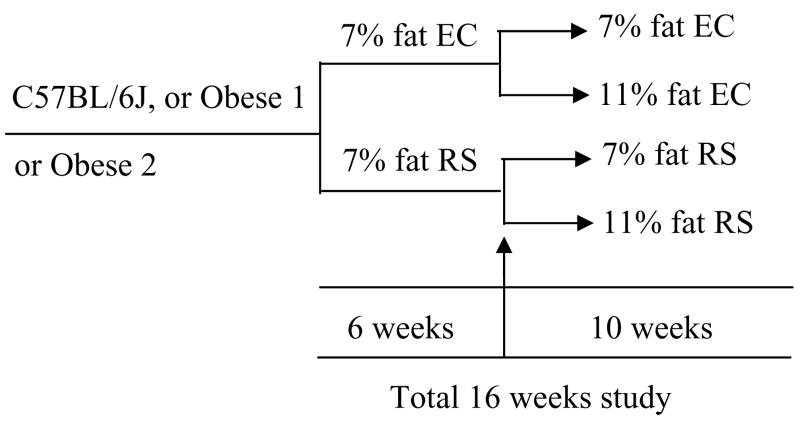
The experimental design is schematically illustrated in Fig. 1.
Figure 1.
Schematic illustration of design for study 2.
C57BL/6J, Obese 1, and Obese 2 mice (32 for each mouse model) were fed control (0% RS) or the 30% RS diet containing 7% fat for 6 weeks (n=16 for each diet group). At the 7th week, the dietary fat was increased to 11% for half of the mice in each group, and the rest remained on the 7% fat diet (n=8). The study ended after 16 weeks. Food intake and body weight were measured twice per week for the entire study. Body fat was measured by NMR (26) before the study, at the 5th and 14th weeks; and by fat dissection at the end of study. Fasting blood glucose and fasting insulin were measured at the 6th week of the study. Respiratory quotient, energy expenditure, and physical activity were measured using indirect calorimetric chambers for the mice on 7% dietary fat during the 7th–9th week and for the mice on 11% dietary fat during the10th – 12th week. Oral glucose tolerance was measured for all mice at the 14 th week. The pH of cecal contents was measured at the end of study.
Food intake, body weight and body fat measurements
Food intake was recorded by measuring food jar weight and spillage for each mouse while the mice were in regular wire-bottom cages. When mice were placed in metabolic chambers, the mice should eat the food from a special tunnel and there should be no spillage in the metabolic cage. However, we observed that some mice moved food from the tunnel and ate in the metabolic cages. Such eating behavior caused unexpected food spillage. Thus, the food intake data from metabolic chambers were not used. Cumulative food intake was calculated for a three week period, prior to mice being placed in metabolic chambers for all groups. During this time frame, mice had well adapted to the cages, food jars, and powder diets. There were no new environmental factors that could influence food intake. Based on this paradigm, the food intake data were collected for mice on 7% dietary fat for a period from the 3rd to 6th week of the study, and for mice on 11% dietary fat for a period from the 7th to 10th week of the study.
Body fat was measured during the study by NMR (26) (Bruker minispec Live Mice Analyzer model mq7.5, LF50, Bruker Optics, Inc., Woodlands, TX), and by dissection of epididymal, perirenal, and retroperitoneal fat pads at the end of study. Body fat is calculated as fat mass divided by body weight for NMR results, and as the sum of epididymal, perirenal, and retroperitoneal fat divided by disemboweled body weight at the end of study.
Body weight and full GI weight were also recorded at the end of the study. The disemboweled body weight was calculated as body weight minus full GI weight for each mouse.
Similar to Study 1, the fermentation of RS should increase full and empty cecum weight, and decrease pH of cecal contents. Thus, full and empty cecal weights, and the pH of cecal contents were measured for Obese 1 and Obese 2 mice. Because we already confirmed RS is fermented in C57Bl/6J mice in Study 1, the same fermentation related measurements were not repeated for C57Bl/6J mice in Study 2. Instead, after weighing the full GI tract, the whole cecum with cecal content for C57Bl/6J mice were collected for microbiota profile characterization. These data will be published separately.
Fasting glucose, fasting insulin and oral glucose tolerance test
For fasting glucose and fasting insulin measurements, all mice were fasted overnight. Blood was collected from the tip of tail (1–2 ul) directly to the test strip for the blood glucose measurement (FreeStyle Glucometer, Abbott. Alameda, CA), and (~50ul) in heparinized tubes for insulin measurements (Mouse insulin Ultrasensitive EIA, ALPCO Diagnostics, Salem, NH). For the oral glucose tolerance test (OGTT), mice were fasted for 5 hours during the light period and gavaged with glucose solution (2.1–2.2g glucose/kg of body weight). The blood glucose levels were measured at 0, 15, 30, 45, 70, and 150 minutes after the glucose gavage, and the area under the curve (AUC) during the OGTT was examined. Insulin levels were not measured during the OGTT test due to the limited blood volume collected at each time point and frequent blood collection.
Indirect calorimetry
Mice were placed in metabolic chambers (Oxymax, Columbus Instruments, Columbus, OH) at room temperature (23°C) for 7 total days. The first three days served as adaptation. Oxygen consumption (VO2), carbon dioxide expiration (VCO2), respiratory exchange rate (RQ: VCO2/VO2), energy expenditure [EE (kcal/hr) = 3.815 + 1.2329 (VCO2/VO2)], and total physical activity (XTOT: represent all movement recorded by the counts of light beam breaks) were recorded for the last four days continuously. The data collected from the middle 48 hours of the four days data collection period were used for analysis. Due to large food spillage, the food intake data collected during this period were not used.
Exclusion criteria
The data were not used for calculations if a mouse lost more than 5 g of its body weight within a week during the study, or the body weight gain for the entire study period was less than 4 g for obese mice, nor or a negative body weight gain for C57Bl/6J mice.
Statistical analysis
Results are presented as mean ± SEM. For Study 1, one-way ANOVA followed by F-protected LSD was used for analyzing all data. Differences were considered significant if p<0.05. For Study 2, because the aim of the study was to examine diet effects within each type of mice, rather than compare the differences among three types of mice, a two-way ANOVA with dietary fat and starch as two independent factors was used to analyze data within each type of mice. The only exception is analysis of physical activity. Because the two way ANOVA model is not significant for all three types of mice, 2 × 2 × 2 way ANOVA was used for physical activity data analysis. Fasting glucose, fasting insulin, and HOMA index were analyzed by T-tests between control and RS groups within each type of mice. For the OGTT, the area under the curve (AUC) was determined by calculating the entire AUC (‘total area’ AUC method). SAS 9.1 for Windows was used for analysis.
Results
Study 1
The results from all measurements are summarized in Table 3 for study 1.
Table 3.
Body weight, body fat, indicators of fermentation and SCFA in cecal contents for four groups of mice at the end of Study 11,2.
| Measurements | CC | EC | RS2 | RS3 | |
|---|---|---|---|---|---|
| Body weight | Body weight (g) | 30.1±0.5 | 29.3±0.5 | 28.8±0.7 | 28.4±0.4 |
| Disemboweled BW3(g) | 28.4±0.5 a | 27.0±0.5 b | 26.0±0.7 bc | 25.3±0.4 c | |
| Body fat | Total abdominal fat (mg) | 1512±101 a | 1261±77 b | 1050±66 bc | 861±65 c |
| Body fat4 (%) | 5.28±0.27 a | 4.64±0.21 ab | 4.03±0.17 b | 3.37±0.21 c | |
| Indicators of fermentation | Full GI tract weight (mg) | 1739±69 a | 2304±70 b | 2785±102 c | 3079±107 c |
| Full cecum weight (mg) | 244±17 a | 281±17 a | 832±85 b | 888±56 b | |
| Empty cecum weight (mg) | 70.9±3.1 a | 84.0±3.4 a | 155±15 b | 158±7 b | |
| Cecal content weight (mg) | 173±15 a | 198±15 a | 677±84 b | 730±50 b | |
| Cecal content pH | 8.08±0.06 a | 8.28±0.05 a | 7.69±0.08 b | 7.64±0.10 b | |
| SC FA 6 | Total SCFA5 | 57.5±12.7a | 32.8±3.3a | 171±46ab | 243±73 b |
| Acetate | 36.5±8.5 a | 21.0±2.1 a | 119±34 ab | 160±47 b | |
| Propionate | 9.4±1.9 a | 4.9±0.5 a | 35±10 ab | 59±21 b | |
| Butyrate | 5.9±1.7 a | 3.2±0.4 a | 10.8±2.6 ab | 17.7±5.2 b | |
Each group has 12–16 mice except for RS2. Due to an unexpected disaster, there were five mice left in the RS2 group at the end of the study.
Values with different superscripts across rows indicate they are statistically different (P<0.05).
Disemboweled body weight is calculated as body weight minus full gastrointestinal weight for each mouse.
Body fat (%) is calculated as total abdominal fat (g) divided by disemboweled body weight (g).
Total SCFA includes SCFA with carbon numbers: C2, C3, C4, IC4, C5, IC5, C6, C7, C8
The unit for SCFA measurement is SCFA (μmoles) in cecal contents (total g)
Compared with mice fed CC diet, mice in both RS groups had significantly lower disemboweled body weight and body fat (P<0.01 for disemboweled body weight, retroperitoneal fat, epididymal fat and total abdominal fat). Compared with mice fed EC diet, mice in RS2 and RS3 groups also had lower disemboweled body weight and body fat, but reductions were statistically significant only for the RS3 group. Notably, this might be due to the small number of mice in the RS2 group (n=5), which resulted from an unexpected disaster that affected the rest of the mice in this group during the study. Compared with mice fed the CC diet, mice in the EC group also had lower body weight and body fats, but their percentages of body fat were similar to the mice in CC group. Both types of RS are fermented in the cecum as indicated by higher cecal content weights, empty cecal weights, lower cecal content pH and higher amounts of short chain fatty acids (SCFA) in RS groups compared to both CC and EC groups (P<0.01).
Study 2
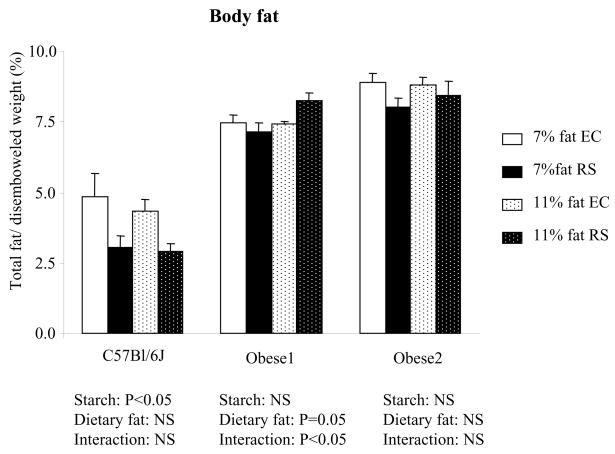
Body fat is summarized in Table 4 for data obtained by NMR during the study and in Figure 2 for data obtained by fat dissection at the end of study.
Table 4.
Body fat measured by NMR during Study 2 for three types of mice1
| Mice | % fat in diet | Diet | Beginning | 6th week of study | 14th week of study |
|---|---|---|---|---|---|
| C57BJ/6J | EC | 6.61 ± 0.51 | 8.19 ± 1.08 | 12.26 ± 2.72 | |
| 7% | RS | 6.08 ± 0.51 | 6.59 ± 0.80 | 8.59 ± 1.49* | |
| EC | N/A | N/A | 11.23 ± 1.49 | ||
| 11% | RS | N/A | N/A | 8.27 ± 0.86* | |
| Obese 1 | EC | 14.60 ± 0.91 | 23.30 ± 1.65 | 26.44 ± 1.11 | |
| 7% | RS | 14.34 ± 0.62 | 21.26 ± 1.16 | 23.76 ± 1.89 | |
| EC | N/A | N/A | 28.74 ± 1.19 | ||
| 11% | RS | N/A | N/A | 28.87 ± 1.20 | |
| Obese 2 | EC | 13.17 ± 0.64 | 25.86 ± 1.06 | 26.18 ± 1.63 | |
| 7% | RS | 12.89 ± 0.62 | 24.95 ± 0.85 | 24.18 ± 1.23 | |
| EC | N/A | N/A | 29.66 ± 1.90 | ||
| 11% | RS | N/A | N/A | 27.24 ± 1.38 | |
Body fat is calculated as percentage of fat mass divided by body weight
P< 0.05 compared to the EC diet.
Figure 2.
Body fat measured at the end of study for mice fed control diet or RS diet in Study 2. Body fat was calculated as percentage of the sum of epididymal fat, perirenal fat, and retroperitoneal fat divided by disemboweled body weight. Values are mean ± SEM with n=6 to 8 per group.
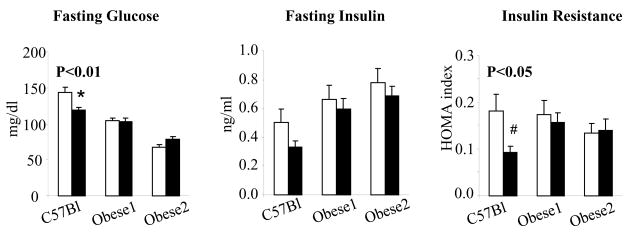
The two obese mice were fatter than the C57BL/6J mice. Body fat was significantly lower in the RS fed mice than controls at the 14th week of the study as measured by NMR (P<0.05) and at the end of the study as measured by fat dissection (P<0.05) for C57BL/6J mice only, but not for obese mice. Increasing dietary fat from 7% to 11% led to increased body fat for Obese 1 mice (P=0.05). At the 6th week of the study, overnight fasting glucose levels were lower in RS fed mice than the EC fed mice for C57BL/6J mice (P<0.01), but not for obese mice (Figure 3).
Figure 3.
Assessment of insulin sensitivity by fasting glucose, fasting insulin and HOMA index for mice fed 7% fat control diet (□) or 7% fat RS diet (■) at 6th week of Study 2. Values are mean ± SEM with n=14 to 16 per group. * P<0.01 and # P<0.05 compared to the mice fed control diet within the same genotyping group.
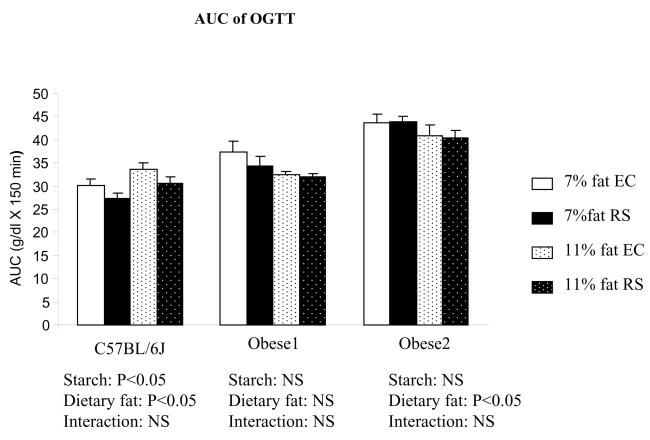
Fasting insulin in RS fed C57Bl/6J mice was also lower than EC-fed C57BL/6J mice, but the difference did not reach statistical significance. Insulin resistance, indicated by HOMA index, was decreased in RS fed C57Bl/6J mice (p<0.05); and again, obese mice did not show improvements for the same measurement. Similar to the fasting glucose and fasting insulin results, the area under the curve from OGTT conducted in the14th week of the study was also decreased for RS fed C57Bl/6J mice (P<0.05), but not for obese mice (Figure 4).
Figure 4.
Area under the curve of the glucose tolerance test was improved in C57Bl/6J mice fed RS diet at 14th week of study 2. Values are mean ± SEM with n=7 to 8 per group.
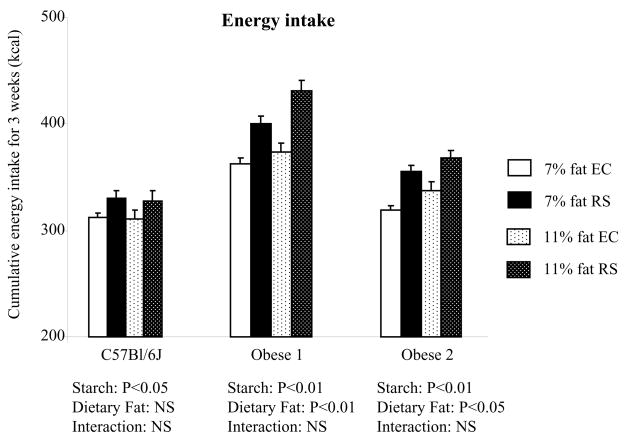
Three weeks of cumulative food intake was significantly higher in RS fed mice compared to EC fed mice for all three types of mice tested. Increasing dietary fat from 7% to 11% also further increased food intake in the two obese mouse models, but not in C57Bl/6J mice (Figure 5).
Figure 5.
Cumulative food intake for mice fed control diet or RS diet in Study 2. Food intake was measured from 3rd to 6th week period for mice on 7% fat diet (n=14–16) and from 7th to 10th week for mice on 11% fat diet (n=7–8). Values are mean ± SEM.
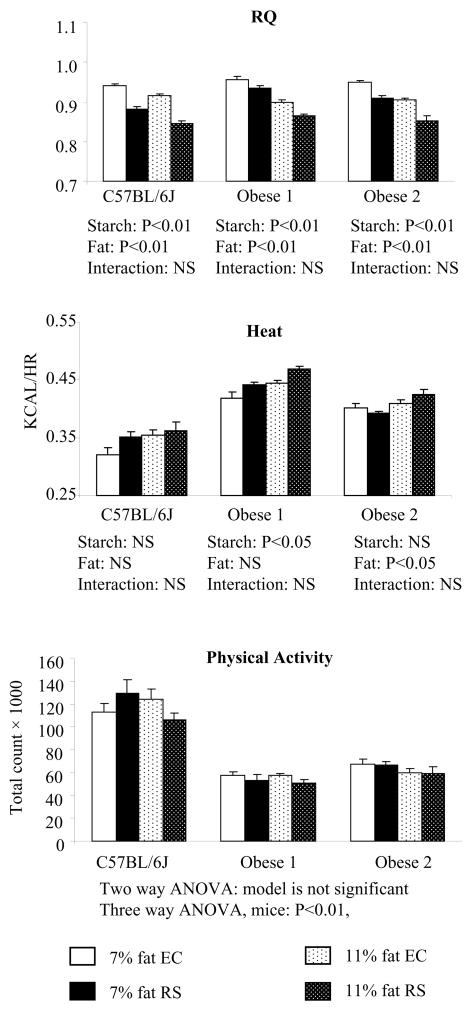
Figure 6 summarizes metabolic characteristics obtained from indirect calorimetry. Respiratory exchange rate (RQ: VCO2/VO2) was lower in RS fed mice than the controls. Increasing dietary fat further lowered RQ for all three types of mice tested. All mice had significantly lower RQ during the light cycle than in the dark cycle (data not shown). Energy expenditure, or heat, was not different between EC group and RS groups for C57Bl/6J mice and Obese 2 mice, but was higher in the RS group than the EC group for Obese 1 mice. Increasing dietary fat from 7% to 11% did not change heat production in C57Bl/6J mice or Obese 1 mice, but heat production was increased in Obese 2 mice when the dietary fat was increased. Physical activity was not different between the RS and control diets for all mice. However, the two obese mice strains did have less physical activity than the C57Bl/6J mice. Additionally, the two types of obese mice also had a higher heat production than the lean mice. Because the aim of the current study is to investigate the response to the RS diet in lean and obese mice, and not to compare the physiology differences among the three types of mice used, the difference in heat production and physical activity between lean and obese mice was not considered as all comparisons were made within the same type of mice.
Figure 6.
RQ, heat, and physical activity measurements from indirect calorimetric chambers for lean and obese mice in Study 2. Data were collected for 48 hours for the mice on 7% dietary fat during the 7th–9th weeks and for the mice on 11% dietary fat during the10th – 12th weeks. Values are mean ± SEM with 6–8 mice per dietary group.
Body weight and full GI tract weight recorded at the end of study is summarized in Table 5. RS fed C57Bl/6J mice had significantly higher GI weights than the controls (P<0.01). Due to smaller numbers of mice in each group compared to the number of mice used in study 1, the decreased disemboweled body weight did not reach the statistical significance for RS fed C57Bl/6J mice.
Table 5.
Body weight and disemboweled body weight at the end of Study 2.
| Mice | % fat in diet | Diet | Body weight (g) | Full GI tract weight (g) | Disemboweled body weight (g) |
|---|---|---|---|---|---|
| C57BJ/6J | EC | 30.8 ± 1.7 | 1.60 ± 0.09 | 29.2 ± 1.7 | |
| 7% | RS | 31.0 ± 1.2 | 2.18 ± 0.17** | 28.9 ± 1.2 | |
| EC | 31.2 ± 1.0 | 1.51 ± 0.09 | 29.7 ± 0.9 | ||
| 11% | RS | 30.9 ± 1.2 | 2.41 ± 0.14** | 28.5 ± 1.2 | |
| Obese 1 | EC | 41.5 ± 1.2 | 2.13 ± 0.07 | 39.3 ± 1.2 | |
| 7% | RS | 39.4 ± 2.0 | 2.25 ± 0.08* | 37.1 ± 2.0 | |
| EC | 41.7 ± 1.5 | 1.98 ± 0.10 | 39.7 ± 1.4 | ||
| 11% | RS | 44.2 ± 1.2 | 2.35 ± 0.10* | 41.8 ± 1.2 | |
| Obese 2 | EC | 38.7 ± 1.3 | 2.06 ± 0.10 | 36.6 ± 1.3 | |
| 7% | RS | 39.8 ± 1.1 | 2.17 ± 0.10 | 37.6 ± 1.1 | |
| EC | 40.4 ± 1.4 | 1.91 ± 0.06 | 38.5 ± 1.4 | ||
| 11% | RS | 40.0 ± 0.6 | 2.27 ± 0.13 | 37.8 ± 0.6 | |
and ** P< 0.05 or P<0.01 compared to relative controls in the same dietary fat and type of mouse.
As indicators of RS fermentation, empty cecal weight, the weight of the cecal contents and the pH were measured for two types of obese mice and the results are listed in Table 6. Unlike the results we observed in Study 1, the RS diet and EC diet groups did not show significant differences in both cecal pH and empty cecal weights for the two obese mouse models. Although there is a significantly higher full cecum and cecal contents weight for RS fed Obese 1 mice on the 11% fat diet, the pH of their cecal contents was not decreased compared to their relative controls. Thus, the increased cecal contents in this group were simply due to large amounts of food remaining in the cecum. The lack of data for C57BL/6J mice is due to their cecal samples being used for analysis of whole microbiota population profile in RS fed mice. Such results will be published separately. However, we did observe two types of bacteria, lactic acid bacteria and Clostridium Spp, were significantly higher in RS fed C57Bl/6J mice than the control diet fed C57Bl/6J mice. The increases of these two types of bacteria represent the occurrence of fermentation and indicate the increased short chain fatty acids in the cecal contents (27–29)
Table 6.
Cecal weight and pH of cecal contents at the end of Study 2
| Mice | % fat in diet | Diet | Full Cecum weight (mg) | Empty Cecum weight (mg) | Cecum content(mg) | pH of cecal content |
|---|---|---|---|---|---|---|
| C57BJ/6J | EC | N/A | N/A | N/A | N/A | |
| 7% | RS | |||||
| EC | N/A | N/A | N/A | N/A | ||
| 11% | RS | |||||
| Obese 1 | EC | 238 ± 21 | 66 ± 7 | 172 ± 20 | 7.73 ± 0.15 | |
| 7% | RS | 296 ± 18* | 62 ± 4 | 234 ± 17* | 8.06 ± 0.16 | |
| EC | 212 ± 15 | 70 ± 9 | 140 ± 13 | 7.64 ± 0.19 | ||
| 11% | RS | 314 ± 25* | 72 ± 7 | 246 ± 23* | 7.99 ± 0.16 | |
| Obese 2 | EC | 328 ± 53 | 87 ± 10 | 241 ± 43 | 8.23 ± 0.16 | |
| 7% | RS | 395 ± 54 | 83 ± 7 | 313 ± 48 | 7.85 ± 0.13 | |
| EC | 267 ± 16 | 67 ± 9 | 197 ± 15 | 8.01 ± 0.16 | ||
| 11% | RS | 442 ± 85 | 94 ± 14 | 348 ± 81 | 7.95 ± 0.25 | |
P< 0.01 compared to controls
In summary, the data obtained from study 2 can be categorized into two types: data observed only in RS fed lean mice and data observed in all types of RS fed mice. Body fat and glucose homeostasis were altered by dietary RS for C57BL/6J mice, but not for the other two types of obese mice. Food intake, RQ, energy expenditure, and physical activity were altered in the same direction by dietary RS for all three types of mice tested. Also, the two types of obese mice show an impaired ability to ferment dietary resistant starch in the large intestine.
Discussion
The current studies support the conclusion that fermentation and dilution of energy density might both contribute to resistant starch’s effects on body fat loss in mice. Also, dietary resistant starch is more effective in lowering body fat than simply diluting dietary energy density. But the effect was only observed in lean mice, not in two types of obese mice tested. Interestingly, body fat loss, as well as improved glucose tolerance, was associated with colonic fermentation of resistant starch. Lean mice can ferment resistant starch and obese mice cannot. Although the detailed mechanisms remain to be learned, our data indicate certain actions of resistant starch are associated with its colonic fermentation. This speculation is also supported by the Udagawa group. They reported dietary resistant starch lost its effect on lowering serum cholesterol when there was a high level of cholesterol in the diet. Coincidently, resistant starch was not fermented in the gut when the dietary cholesterol was high in their study (8, 10). Thus, any factor that may affect colonic fermentation of resistant starch should be considered for the clinical application of resistant starch.
Colonic fermentation of resistant starch may also cause some discomfort, which can be a concern for the clinical use of dietary fermentable fiber. In our studies, we have used two types of resistant starch at the concentration of 30% (wt/wt) in diets. RS2 fed mice initially have soft feces but the symptoms are generally ameliorated after a few days of adaptation. No other side effects are observed in RS2 fed animals. Thus, the RS2 was used for study 2. In study 2, all RS fed mice had greater food intake than controls, suggesting no abdominal discomfort for RS fed mice, as the discomfort would likely reduce food consumption. Although the mice tolerated the 30% RS diet well, a further dose-response study is still needed to test the minimal amount of RS in the diet that still leads to body fat loss.
Besides fermentation and dilution of metabolizable energy density, resistant starch also decreases glycemic response of the diet, which should lower the postprandial insulin concentration. The lower insulin should cause less glucose uptake into insulin sensitive tissues and facilitate lipolysis. However, these changes only had a minor contribution to the fat loss caused by dietary resistant starch in current studies. The available carbohydrates in the EC diet are only 1.14 fold higher compared to the RS diet in our study. Thus, the immediate postprandial glycemia difference between the EC diet and RS diet are expected to be minimal.
Study 2 also showed some unexpected results, which are contradictory to our general assumptions. For example, although RQ was decreased in RS fed obese mice, they did not lose body fat. Three weeks’ cumulative food intakes were also increased in all RS fed mice, regardless if the mice lost body fat or not. Thus, food intake and RQ for RS fed mice might be influenced by particular factors, which are independent of the RS effect on body fat. Full cecal weights and cecal content weights indicate that the RS is reaching the large intestine as expected for the obese mice. However, the RS is unexpectedly not being fermented, demonstrated by no decrease in the pH of cecal contents.
An unanswered question is why the RS is not fermented in the obese mice? One speculation is the difference in the gut microbiota profile between lean and obese mice (30). As prebiotic (a non-digestible food ingredient that stimulates the growth or activity of beneficial bacteria in the gut), dietary resistant starch might stimulate growth of specific types of microflora in the cecum of lean mice. If those types of microflora are absent in the obese mice, RS cannot be fermented.
Regardless, the overall bacteria number in general could still be increased in RS fed mice. The higher number of microflora requires their host organism, or RS fed mice, to consume more energy from the diet. This might be a reason that all RS fed mice increased food intake, regardless if they lost body fat or not.
The lower RQ in all RS fed mice might also partially result from the metabolic consequence of gut microflora in RS fed mice. The current calorimetry method measured VO2 and VCO2 that generated from both mice and microbiota inside of the mice. Thus, an isotopic tracer method would be required to more accurately evaluate the metabolic changes in RS fed mice.
The level of dietary fat used in study 2 did not affect the ability of RS fed lean mice to lose body fat. However, caution should be given in interpreting this result. The dietary fat levels used in study 2 were at the recommended levels for the AIN-93 diet, and the recommendation from Jackson Lab for obese mice. The 7% (18% of energy) and 11% (26% of energy) dietary fat levels are considered very low or a moderate dietary fat diet for humans. Although dietary fat used at these levels did not change the response to RS diet in lean mice, the effect of resistant starch with higher levels of dietary fat is still unclear and needs further investigation.
Obese mouse models used in the current study have similar genetic backgrounds. Based on the Jackson Lab description, Non/ShiLtJ mice (Obese 2 mice) have the same genetic background as NoNcNZO10/LtJ mice (Obese 1 mice), but diabetes is not developed in their early life stage. We did see that Obese 2 mice had a lower fasting glucose than Obese 1 mice at the 6th week of the study. However, at the end of the study, the two types of obese mice were very similar in terms of metabolic characteristics measured.
In summary, the findings from the current studies indicate that resistant starch is more effective than a simple energy dilution for body fat reduction in lean mice. And, colonic fermentation of resistant starch might play an important role in this regulation. Obese mice used in current study were unable to ferment resistant starch and did not lose body fat with the feeding of resistant starch. Because origin of obesity is only one of many factors that might influence the colonic fermentation of resistant starch, caution should be given to food industry, dietitian, and consumers: any factors that influence the colonic fermentation of resistant starch should be considered when using resistant starch in treatment of obesity and diabetes. Further studies are required to determine if the origin of obesity (genetic, dietary, or endocrine) has unique effects on gut mucosa and the fermentation environment that alters microflora and thereby the response to a dietary resistant starch.
Acknowledgments
This work is supported by NIH R21 DK073403 to Roy J Martin, June Zhou and Mike Keenan and by the LSU AgCenter Experiment Station Project to Mike Keenan
This paper was approved for publication by the Director of Louisiana Agricultural Experiment Station as manuscript no. XX-XX-XXXX.
References
- 1.Delzenne NM, Cani PD. A place for dietary fibre in the management of the metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2005;8(6):636–40. doi: 10.1097/01.mco.0000171124.06408.71. [DOI] [PubMed] [Google Scholar]
- 2.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21(3):411–8. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Brown IL. Applications and uses of resistant starch. J AOAC Int. 2004;87(3):727–32. [PubMed] [Google Scholar]
- 4.Kendall CW, Emam A, Augustin LS, Jenkins DJ. Resistant starches and health. J AOAC Int. 2004;87(3):769–74. [PubMed] [Google Scholar]
- 5.Higgins JA. Resistant starch: metabolic effects and potential health benefits. J AOAC Int. 2004;87(3):761–8. [PubMed] [Google Scholar]
- 6.de Deckere EA, Kloots WJ, van Amelsvoort JM. Resistant starch decreases serum total cholesterol and triacylglycerol concentrations in rats. J Nutr. 1993;123(12):2142–51. doi: 10.1093/jn/123.12.2142. [DOI] [PubMed] [Google Scholar]
- 7.Cassidy A, Bingham SA, Cummings JH. Starch intake and colorectal cancer risk: an international comparison. Br J Cancer. 1994;69(5):937–42. doi: 10.1038/bjc.1994.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Udagawa H, Kitaoka C, Sakamoto T, Kobayashi-Hattori K, Oishi Y, Arai S, Takita T. Serum cholesterol-decreasing effect of heat-moisture-treated high-amylose cornstarch in cholesterol-loaded rats. Biosci Biotechnol Biochem. 2008;72(3):880–4. doi: 10.1271/bbb.70656. [DOI] [PubMed] [Google Scholar]
- 9.de Roos N, Heijnen ML, de Graaf C, Woestenenk G, Hobbel E. Resistant starch has little effect on appetite, food intake and insulin secretion of healthy young men. Eur J Clin Nutr. 1995;49(7):532–41. [PubMed] [Google Scholar]
- 10.Udagawa H, Kitaoka C, Sakamoto T, Kobayashi-Hattori K, Oishi Y, Arai S, Takita T. Increase of Serum Cholesterol Levels by Heat-Moisture-Treated High-Amylose Cornstarch in Rats Fed a High-Cholesterol Diet. Lipids. 2008 doi: 10.1007/s11745-008-3191-4. [DOI] [PubMed] [Google Scholar]
- 11.Scribner KB, Pawlak D, Aubin CM, Majzoub J, Ludwig D. Long-term effects of dietary glycemic index on adiposity, energy metabolism and physical activity in mice. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90487.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.So PW, Yu WS, Kuo YT, Wasserfall C, Goldstone AP, Bell JD, Frost G. Impact of resistant starch on body fat patterning and central appetite regulation. PLoS ONE. 2007;2(12):e1309. doi: 10.1371/journal.pone.0001309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrnes SE, Miller JC, Denyer GS. Amylopectin starch promotes the development of insulin resistance in rats. J Nutr. 1995;125(6):1430–7. doi: 10.1093/jn/125.6.1430. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JA, Brown MA, Storlien LH. Consumption of resistant starch decreases postprandial lipogenesis in white adipose tissue of the rat. Nutr J. 2006;5:25. doi: 10.1186/1475-2891-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364(9436):778–85. doi: 10.1016/S0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]
- 16.Goranzon H, Forsum E, Thilen M. Calculation and determination of metabolizable energy in mixed diets to humans. Am J Clin Nutr. 1983;38(6):954–63. doi: 10.1093/ajcn/38.6.954. [DOI] [PubMed] [Google Scholar]
- 17.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105(5 Suppl 1):S98–103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 18.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59(5):129–39. doi: 10.1111/j.1753-4887.2001.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 19.Hegsted M, Francis AR, McCutcheon KL, Keenan MJ, O’Neil CE, Gillespie MS, Mekary RA, RJM Amylose resistant starch (RS) decreases body fat in rats. FASEB Journal. 2003;17:A335. [Google Scholar]
- 20.Keenan MJ, Zhou J, McCutcheon KL, Raggio AM, Bateman HG, Todd E, Jones CK, Tulley RT, Melton S, Martin RJ, Hegsted M. Effects of Resistant Starch, A Non-digestible Fermentable Fiber, on Reducing Body Fat. Obesity (Silver Spring) 2006;14(9):1523–34. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- 21.Shen L, Keenan MJ, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Zhou J. Dietary Resistant Starch Increases Hypothalamic POMC Expression in Rats. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295(5):E1160–6. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 24.Jackson LT. http://jaxmice.jax.org/strain/004456.html.
- 25.Jackson LT. http://jaxmice.jax.org/strain/002423.html.
- 26.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377(6):990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 27.Louis P, Flint HJ. Development of a semiquantitative degenerate real-time pcr-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol. 2007;73(6):2009–12. doi: 10.1128/AEM.02561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis P, McCrae SI, Charrier C, Flint HJ. Organization of butyrate synthetic genes in human colonic bacteria: phylogenetic conservation and horizontal gene transfer. FEMS Microbiol Lett. 2007;269(2):240–7. doi: 10.1111/j.1574-6968.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 29.Louis P, Scott KP, Duncan SH, Flint HJ. Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol. 2007;102(5):1197–208. doi: 10.1111/j.1365-2672.2007.03322.x. [DOI] [PubMed] [Google Scholar]
- 30.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]