Abstract
Objectives
To determine risk factors associated with reduced adult height in survivors of childhood acute lymphoblastic leukemia (ALL).
Study design
Cross-sectional study. Attained adult height was determined among 2,434 ALL survivors participating in the Childhood Cancer Survivor Study, a cohort of five-year survivors of common pediatric cancers diagnosed from 1970–1986, and compared with 3,009 siblings.
Results
All survivor treatment exposure groups (chemotherapy alone, chemotherapy with cranial or craniospinal radiotherapy) had decreased adult heights and an increased risk of adult short stature (height standard deviation score < −2) compared with siblings (p<0.001). Compared with siblings, the risk of short stature for survivors treated with chemotherapy alone was elevated (OR 3.4, 95% CI 1.9, 6.0). Among survivors, significant risk factors for short stature included diagnosis of ALL prior to puberty, higher dose cranial radiotherapy (≥20 Gy versus <20 Gy), any radiotherapy to the spine, and female sex.
Conclusions
Survivors of childhood ALL are at increased risk of adult short stature, including those treated with chemotherapy alone. Risk is highest for those treated with cranial and craniospinal radiotherapy at a young age.
Keywords: chemotherapy, epidemiology, growth deficiency, late effects, radiation, survivorship
Cranial and craniospinal radiotherapy were commonly used in the 1970s and early 1980s to treat as well as to prevent the spread of acute lymphoblastic leukemia (ALL) to the central nervous system (CNS) in children. While radiotherapy was effective, it was associated with adverse endocrine and neurocognitive outcomes(1). As a result, over the past three decades, radiotherapy doses have been reduced or eliminated in an attempt to decrease these adverse long-term outcomes, and have been replaced by more intensive chemotherapy.
Several studies have examined growth in ALL survivors. Growth deficits have been reported consistently following doses of ≥24 Gy cranial radiotherapy, but the data are less consistent for doses <20 Gy(2–17). The effect on loss of stature was greater in children who also received radiotherapy to the spine, secondary to direct inhibition of vertebral growth(13). For most studies in which the impact of chemotherapy without radiotherapy was examined, growth suppression during treatment was followed by catch-up growth(2, 3, 10, 11, 15, 18). However, catch-up growth has not been observed consistently across studies(6, 14).
We hypothesized that adult survivors of childhood ALL would have shorter adult heights than their siblings and that cranial or craniospinal radiotherapy would be a significant risk factor, in a dose-dependent manner. We also hypothesized that the risk for decreased adult height among ALL survivors treated with chemotherapy alone would be smaller than that conferred by cranial or craniospinal radiotherapy. Therefore, we compared the attained adult height of a large population of pediatric ALL survivors enrolled in the Childhood Cancer Survivor Study (CCSS) with a sibling cohort to determine more precisely the risk factors associated with adult short stature.
Methods
Childhood Cancer Survivor Study description
The CCSS is a resource cohort study that was established to evaluate hypotheses associated with long-term health related outcomes in childhood cancer survivors. Specifics concerning the methodology and subject accrual for this cohort have been reported in detail(19). Briefly, the cohort was constructed from rosters of all children treated for most forms of childhood cancer at each of 26 institutions in the United States and Canada (see Appendix). Inclusion criteria included: 1) diagnosis of one of the following forms of childhood cancer before 21 years of age: leukemia, Hodgkin and non-Hodgkin lymphoma, neuroblastoma, soft-tissue sarcoma, bone cancer, malignant CNS tumor, or kidney tumor; 2) initial treatment at one of the collaborating institutions between January 1, 1970 and December 31, 1986; and 3) survival for at least 5 years following diagnosis.
The Human Subjects Committee at each participating institution reviewed and approved the CCSS protocol. Beginning August 1, 1994, all cohort members (or parents of patients under 18 years of age) completed a baseline questionnaire that included information on demographic and socioeconomic characteristics, health conditions and health related behaviors, family history of cancer, inherited conditions and congenital anomalies, and reproductive history. Two follow-up questionnaires have been sent since to all participants as well. For those patients who survived for 5 years following the initial cancer diagnosis and subsequently died, a family member completed the baseline questionnaire. Medical records were reviewed and abstracted for cancer diagnosis and treatment data including chemotherapy and radiotherapy exposures using a standardized protocol(19).
ALL survivors and sibling controls
Of 5,814 ALL survivors eligible for the CCSS, 811 were lost to follow-up despite tracking, 801 declined participation, and 47 were pending contact at the time of analysis. Prior analysis found no significant differences between participants and non-participants with respect to sex, cancer diagnosis, age at diagnosis, age at contact, and type of cancer treatment(19). The rate of non-participation was significantly higher among next-of-kin of deceased as opposed to living patients(19). Survivors were excluded from this analysis if diagnosed after 17 years of age, or if they had a recurrence of their primary leukemia, developed a second malignant neoplasm, or underwent hematopoietic stem cell transplant before 18 years of age, leaving 2,990 survivors available for analysis. Those who lacked self or proxy-reported adult height data (defined as the tallest height recorded at age ≥18 years) or had incomplete radiotherapy exposure data also were excluded, resulting in a final study cohort of 2,434 (2,384 alive at time of study enrollment).
A cohort of 5,857 siblings was randomly selected from all eligible CCSS cases. At the time of this analysis, 3,846 siblings had agreed to participate and were recruited to serve as a comparison group. If a cancer survivor had more than one sibling, the sibling of closest age was selected for participation. Among siblings, 3,009 were ≥18 years of age at the time of study enrollment and had self-reported adult height data. Of these siblings, 818 were siblings of ALL survivors, with the remainder being related to other CCSS cases.
Exposure and outcome assessment
Cumulative chemotherapy doses were available for selected agents, which included anthracyclines (daunorubicin and doxorubicin summed), cyclophosphamide, cytarabine, epipodophyllotoxins (etoposide and teniposide summed), and methotrexate (intravenous, intramuscular, and intrathecal doses). Systemic and intrathecal doses were classified separately. To examine dose-response, each agent was categorized into none, low, medium, and high doses based on tertiles or previously published cut-points when available(20). While systemic doses were adjusted for the patient’s body surface area at the time of administration, intrathecal doses were not. For asparaginase, corticosteroids, oral methotrexate, mercaptopurine, thioguanine, and vincristine, dosage information was not available, only exposure recorded “yes” or “no.” Scores for overall chemotherapy intensity were created based on the cumulative number of drugs received as well as their dose (if known), with higher scores assigned to patients exposed to higher levels of individual agents. All agents were analyzed individually and in combination, along with overall treatment duration (<2.5, 2.5–3.5, and >3.5 years) and measures of chemotherapy intensity.
CNS radiotherapy doses were abstracted in 5 Gy increments. Ninety-five percent of survivors (1,511 of 1,584) who received cranial radiotherapy were treated with doses between 15 to 29 Gy to the brain and 88% of survivors (180 of 204) who received spinal radiotherapy received doses between 10 to 24 Gy to the spine. Remaining patients received radiotherapy to the brain and spine outside these ranges, but their numbers were too small to allow meaningful stratification. As a result, CNS radiotherapy doses were categorized into <20 Gy and ≥20 Gy doses. Exclusion of survivors exposed to <15 Gy or ≥30 Gy did not affect reported estimates. Radiotherapy exposures occurring after age 17 were excluded from analysis.
Height was assessed both in absolute terms as well as by standard deviation scores (SDS). Raw heights were converted to SDS by Epi Info (version 3.3.2, Centers for Disease Control) based on the Centers for Disease Control Year 2000 growth charts. In general, one SDS change in height was approximately 7 cm for both males and females.
Statistical analysis
T-tests were used for mean height comparisons between survivor subgroups. Multiple linear regression was applied to simultaneously examine multiple factors that contributed to changes in height SDS among survivors. Multiple logistic regression was used to examine risk of clinical short stature (height SDS, dichotomized at −2) among survivors. To assess potential contributions of puberty, a surrogate variable was created a priori in the absence of information on pubertal timing: pubertal status at diagnosis was dichotomized at age 8 for females and age 10 for males. The final models adjusted for sex, age at diagnosis, ethnicity, and were stratified by the surrogate variable for pubertal status at diagnosis, given clear evidence from an exploratory analysis that radiation effects on growth differed according to pubertal status. Year of diagnosis and year of birth did not affect the outcomes and were not included in the final models. When regression models were analyzed including survivors who had missing radiation exposures as a separate group, results did not change significantly. To account for potential within-family correlation between survivors and siblings, generalized estimating equations were used whenever survivors were compared with siblings, and adjusted for sex, ethnicity, and pubertal status at diagnosis. All reported estimates represent adjusted values, unless otherwise indicated. Analyses were done using STATA (version 9, StataCorp).
RESULTS
Basic demographic and clinical characteristics of the survivor and sibling cohorts are summarized in Table I. Survivors who met inclusion criteria for this study, but had missing adult height or radiation exposure data, differed significantly from survivors with full exposure data in the percentage of women (46.4% versus 51.1%), representation of ethnic minorities (non-Caucasian, 21.6% versus 11.7%), and older age at diagnosis (>4 years, 56.6% versus 48.7%).
Table I.
Selected characteristics of cases (5-year ALL survivors) and controls (siblings).
| Characteristic | Survivors n=2,434 |
Siblings n=3,009 |
% exposed | |
|---|---|---|---|---|
| Age at last follow-up, years | ||||
| Median | 27 | 31 | ||
| Range | 18, 47 | 18, 56 | ||
| Female, % | 51.1 | 52.7 | ||
| Ethnicity, %* | Chemotherapy, % exposed | |||
| Caucasian, non-Hispanic | 88.3 | 88.9 | Asparaginase | 91.3 |
| Black, non-Hispanic | 2.9 | 2.3 | Cyclophosphamide | 44.9 |
| Hispanic/Latino | 5.1 | 3.1 | Cytarabine, systemic | 33.5 |
| Asian/Native/Pacific | 2.0 | 1.4 | Cytarabine, intrathecal | 28.6 |
| Islander | ||||
| Other | 1.4 | 1.0 | Daunorubicin | 23.3 |
| Diagnosis age, % | Dexamethasone | 11.1 | ||
| 0–4 years | 51.3 | Doxorubicin | 20.9 | |
| 5–9 years | 28.5 | Etoposide | 5.5 | |
| 10–14 years | 14.6 | Mercaptopurine | 93.0 | |
| 15–18 years | 5.6 | Methotrexate, systemic | 97.7 | |
| Year of diagnosis, % | Methotrexate, intrathecal | 92.1 | ||
| 1970–75 | 21.9 | Prednisone | 97.6 | |
| 1976–80 | 29.3 | Teniposide | 10.0 | |
| 1981–86 | 48.8 | Thioguanine | 12.8 | |
| Vincristine | 98.9 | |||
| Radiotherapy, % exposed | ||||
| No exposure | 34.9 | |||
| Cranial <20Gy | 29.0 | |||
| Cranial ≥20Gy | 27.7 | |||
| Craniospinal <20Gy | 1.6 | |||
| Cranial ≥20Gy, Spinal | 2.6 | |||
| <20Gy | ||||
| Craniospinal ≥20Gy | 4.2 |
ALL, acute lymphoblastic leukemia
Percentages do not add up to 100 because of missing values
When mean adult heights and height SDS stratified by sex and treatment exposures were examined (Table II), all survivor treatment groups, including those treated with chemotherapy alone, had decreased adult height and height SDS compared with siblings (p<0.001). There was no significant height difference between siblings related to ALL survivors and siblings related to other CCSS survivors (i.e. diagnoses other than ALL).
Table II.
Adult height (centimeters) and height SDS stratified by sex and treatment exposures.
| Exposure group | Male | Height (cm) | SDS | Female | Height (cm) | SDS | |
|---|---|---|---|---|---|---|---|
| n | mean ± SD | mean ± SD | n | mean ± SD | mean ± SD | ||
| Siblings | 1422 | 180.1 ± 6.9 | 0.47 ± 0.97 | 1587 | 165.5 ± 7.2 | 0.33 ± 1.11 | |
| Survivors | |||||||
| Chemotherapy alone | 379 | 178.5 ± 7.7*† | 0.24 ± 1.09*† | 471 | 164.1 ± 7.3*† | 0.12 ± 1.13*† | |
| Chemotherapy, cranial RT <20Gy | 366 | 175.2 ± 8.1 | −0.21 ± 1.12 | 339 | 160.4 ± 8.1 | −0.45 ± 1.24 | |
| Chemotherapy, cranial RT ≥20Gy | 339 | 174.6 ± 7.9 | −0.30 ± 1.10 | 336 | 157.3 ± 8.1 | −0.93 ± 1.24 | |
| Chemotherapy, craniospinal RT <20Gy | 21 | 171.5 ± 9.1 | −0.75 ± 1.27 | 17 | 156.2 ± 8.8 | −1.10 ± 1.36 | |
| Chemotherapy, cranial RT ≥20Gy, spinal RT <20Gy | 31 | 168.9 ± 9.8 | −1.09 ± 1.36 | 33 | 156.1 ± 9.2 | −1.11 ± 1.42 | |
| Chemotherapy, craniospinal RT ≥20Gy | 55 | 168.5 ± 10.0 | −1.16 ± 1.39 | 47 | 155.7 ± 7.7 | −1.17 ± 1.18 |
RT, radiotherapy; SD, standard deviation; SDS, standard deviation score
P<0.001 between siblings and survivors treated with chemotherapy alone
P<0.001 for all comparisons between survivors treated with chemotherapy alone and subgroups treated with radiotherapy
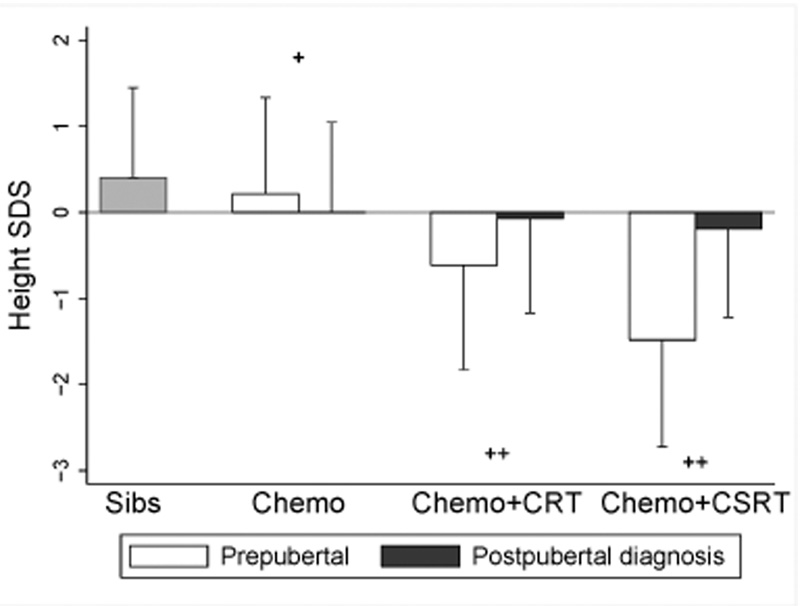
The effects of radiotherapy on adult height SDS differed between survivors who were pre- versus postpubertal at diagnosis (Figure). Amongst survivors diagnosed prior to puberty, height SDS was decreased at all doses of cranial and craniospinal radiotherapy compared with survivors treated with chemotherapy alone in a dose-dependent fashion (trend, p<0.001). For example, the differences in adult height SDS between survivors exposed to chemotherapy only versus those also exposed to <20 Gy cranial radiotherapy or ≥20 Gy craniospinal radiotherapy were −0.71 (95% CI −0.84, −0.58) and −1.80 (95% CI −2.08, −1.53), respectively. The difference in height SDS between survivors treated with <20 Gy versus ≥20 Gy cranial radiotherapy also was significant (−0.31, 95% CI −0.45, −0.17). While survivors exposed to any dose of craniospinal radiotherapy experienced similar levels of height loss, the height SDS of these survivors were on average −0.88 (95% CI −1.08, −0.68) lower than those treated with cranial radiotherapy alone. Among survivors diagnosed after pubertal onset, a significant negative impact on height SDS was not seen at any cranial or craniospinal radiotherapy dose compared with chemotherapy alone. However, on average the adult height SDS of survivors treated after pubertal onset remained shorter than siblings (−0.42, 95% CI −0.51, −0.32).
Figure.
Height standard deviation scores (mean + SD) across exposure groups stratified by pubertal status at ALL diagnosis with siblings as comparison. T-test for differences in height SDS between pre- and postpubertal survivors were significant (+, p<0.05; ++, p<0.001). Test of trend across treatment exposure groups (chemotherapy alone, chemotherapy with cranial radiotherapy (CRT), and chemotherapy with craniospinal radiotherapy (CSRT)) was significant for prepubertal survivors (p<0.001), but not postpubertal ones (p=0.37).
All survivor exposure groups were at significantly greater risk of adult clinical short stature (height SDS < −2) compared with siblings (OR 12.5, 95% CI 8.1, 19.2). This included survivors exposed only to chemotherapy (OR 3.4, 95% CI 1.9, 6.0), an association that was not affected by adjustment for sex, ethnicity, pubertal status at diagnosis, age at diagnosis, or treatment era.
Risk of short stature also appeared to be greater for survivors diagnosed prior to puberty versus older patients (Table III). Among prepubertal survivors, risk was greater among those who received cranial radiotherapy doses ≥20 Gy versus <20 Gy (OR 1.9, 95% CI 1.3, 2.8), and any craniospinal dose versus cranial doses ≥20 Gy (OR 3.1, 95% CI 2.0, 4.8). Different doses of craniospinal radiotherapy were associated with similar effects, regardless of the dose to the brain alone. Survivors diagnosed after pubertal onset and treated with radiotherapy did not have an increased risk of adult short stature compared to the chemotherapy only group, except for a borderline association among those treated with ≥20 Gy cranial radiotherapy (OR 2.8, 95% CI 0.9, 8.5).
Table III.
Risk of short stature (adult height SDS < −2).
| Exposure group | SDS < − 2 n |
SDS ≥ − 2 n |
OR* | 95% CI |
|---|---|---|---|---|
| Survivors, prepubertal diagnosis | ||||
| Chemotherapy alone | 19 | 670 | 1.0 | referent |
| Chemotherapy, cranial RT <20Gy | 48 | 468 | 4.1† | 2.3, 7.1 |
| Chemotherapy, cranial RT ≥20Gy | 77 | 417 | 7.7† | 4.5, 13.0 |
| Chemotherapy, craniospinal RT <20Gy | 9 | 17 | 24.9† | 9.4, 65.9 |
| Chemotherapy, cranial RT ≥20Gy, spinal RT | 11 | 32 | 14.4 | 6.0, 34.5 |
| <20Gy | ||||
| Chemotherapy, craniospinal RT ≥20Gy | 27 | 47 | 30.0 | 15.0, 59.9 |
| Survivors, postpubertal diagnosis | ||||
| Chemotherapy alone | 5 | 156 | 1.0 | referent |
| Chemotherapy, cranial RT <20Gy | 8 | 181 | 1.4 | 0.4, 4.5 |
| Chemotherapy, cranial RT ≥20Gy | 13 | 168 | 2.8 | 0.9, 8.5 |
| Chemotherapy, craniospinal RT <20Gy | 0 | 12 | 0 | 0, - |
| Chemotherapy, cranial RT ≥20Gy, spinal RT | 2 | 19 | 3.4 | 0.6, 21.1 |
| <20Gy | ||||
| Chemotherapy, craniospinal RT ≥20Gy | 1 | 27 | 1.3 | 0.1, 12.1 |
| Siblings | 25 | 2894 | 0.3‡ | 0.2, 0.5 |
RT, radiotherapy; SDS, standard deviation score
Adjusted for sex, age at diagnosis, and ethnicity
Significantly different from stratum above (p<0.05)
Adjusted for sex and ethnicity, compared with chemotherapy alone (all ages)
No chemotherapy agent analyzed—anthracyclines, cyclophosphamide, cytarabine, epipodophyllotoxins, and intrathecal methotrexate—showed a consistent dose-effect or a trend suggestive of a dose relationship with adult height SDS or risk of short stature when analyzed individually, in combination, or according to overall treatment intensity and duration (Table IV; online). The dose-effect on height outcome of asparaginase, corticosteroids, thiopurines, and vincristine could not be assessed because exposure was common among all survivors and cumulative doses were not available. However, when exposure (yes/no) to these agents was included in the overall analysis, no modification of effect was seen.
Lastly, there was an increased proportion of female survivors with adult short stature (12.5%; 155 of 1,243) compared with male survivors (5.5%; 65 of 1,191) and female siblings (1.1%; 18 of 1,587). In the regression analyses, after adjusting for age and pubertal status at diagnosis, ethnicity, and radiotherapy exposures, female survivors had an increased risk of short stature (OR 3.0, 95% CI 2.2, 4.2) as well as decreased height SDS (−0.27, 95% CI −0.37, −0.18) compared to male survivors.
DISCUSSION
This report represents the largest cohort of childhood ALL survivors evaluated for adult height to date. While the negative impact of ALL therapy on final height has been previously documented (2–17), we utilized this large population of survivors to determine more precisely risk factors associated with adult short stature. We found that, as a group, these survivors drawn from the CCSS cohort were at increased risk of adult short stature compared with siblings. In particular, we found clear differences in height outcomes between survivors treated with higher doses of cranial radiotherapy (≥20 Gy versus <20 Gy), as well as between those treated with <20 Gy cranial radiotherapy versus chemotherapy alone. Those who received any form of spinal radiotherapy had the shortest adult heights among all survivor groups. Prior studies had not been consistent in finding differences in final height between survivors treated with 18 Gy versus 24 Gy cranial radiotherapy(3–5, 7, 12, 15–17), or between 18 Gy and treatment with chemotherapy alone(3, 6, 14). These studies had relatively few subjects who reached adult height (N <200), limiting their statistical power to detect differences.
The mechanism by which cranial radiotherapy results in short stature is not entirely clear. While cranial radiotherapy can affect growth hormone secretion, especially at doses ≥24 Gy(16, 21, 22), the evidence for growth hormone deficiency following 18 Gy doses has been inconsistent(9, 23). The duration of the pubertal growth spurt and peak growth velocity may also be decreased following cranial radiotherapy as a result of growth hormone deficiency(8). However, the degree of hormone deficiency does not always correlate with the degree of growth retardation(4), suggesting the contribution of other etiologic mechanisms.
A second mechanism through which cranial radiotherapy may be causing short stature is its effects on pubertal timing. ALL patients exposed to cranial radiotherapy appear to be at increased risk of earlier puberty, particularly females treated at a young age(17, 24, 25). The combination of growth hormone insufficiency and earlier pubertal onset is associated with shorter adult stature(26). Consistent with these hypotheses, our study found that risk of short adult stature was greater among those diagnosed with ALL at younger ages, and that girls appeared to be affected more than boys.
Contrary to some(2, 3, 10, 11, 15, 18) but not all previous studies(6, 14), we found that compared with siblings, patients treated with chemotherapy alone had mildly shorter mean adult heights and a three-fold increased risk of short adult stature across age groups, pubertal status, and secular time periods, even after adjustment for possible demographic confounding variables. Differences in results between this study and others may stem from differences in the definition of “adult/final” heights and in comparison groups. For example, earlier termination of follow-up may obscure significant growth changes occurring in later adolescence and use of older population norms or midparental heights may not reflect secular trends towards increased height. Also, many earlier studies predominantly enrolled patients who were exposed to cranial radiotherapy, leaving relatively few unirradiated patients for analysis, thus diminishing power to detect differences.
Given that the treatment of ALL involves using multiple chemotherapeutic agents simultaneously, it has been difficult to separate the contribution of individual drugs to adult short stature. Studies that have analyzed chemotherapy exposures by dose “intensity”(14) or protocol(6, 11, 13) have not identified specific drugs associated with growth suppression. With the chemotherapy information available in this study, we could not isolate specific agents or factors, such as treatment duration or intensity, associated with short stature. As most patients with ALL are currently treated exclusively with chemotherapy, future analyses should focus on understanding better the relationships between adult height and chemotherapy doses, duration, and type.
Limitations of this study included the use of self or proxy-reported height data, lack of longitudinal growth information, and the use of surrogate pubertal status. Self-reported heights have been well-validated and correlate closely with measured heights(27). Any bias introduced by self-reported values tends to overestimate true height by at most 1 to 2 cm(27), and therefore is unlikely to explain our study’s findings. While we did not have longitudinal growth data that allowed us to compare height SDS at the beginning of treatment with subsequent values, we used conservative final height criteria and sampled a much larger number of adult survivors compared with earlier studies resulting in greater power to detect differences between exposure groups. The use of sibling controls also helped validate the significant differences found. It also is unlikely that misclassification of pubertal status accounted for the differences found between younger and older age groups in this study, as the results did not change significantly when different age cutoffs were used.
Supplementary Material
ACKNOWLEDGMENT
This research utilized the CCSS, a resource supported by the National Cancer Institute to promote and facilitate research on long-term survivors of cancer diagnosed in childhood and adolescence. Investigators may apply to use the CCSS by proposing an analysis of existing data or proposing new initiatives that would utilize the cohort (including specimens within the biorepository). Interested investigators are encouraged to visit the CCSS website at www.cancer.umn.edu/ccss to learn more about this unique resource.
This study was supported by grant U24-CA55727 (L.L. Robison, Principal Investigator) from the National Institutes of Health and funding provided to the University of Minnesota by the Children’s Center Research Fund.
ABBREVIATIONS
- ALL
acute lymphoblastic leukemia
- CCSS
Childhood Cancer Survivor Study
- CI
confidence interval
- CNS
central nervous system
- Gy
gray
- OR
odds ratio
- SDS
standard deviation score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors disclose no potential conflicts of interest.
The list of CCSS Institutions and Investigators is available at www.jpeds.com.
REFERENCES
- 1.Margolin JF, Steuber CP, Poplack DG. Acute lymphoblastic leukemia. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 489–544. [Google Scholar]
- 2.Birkebaek NH, Clausen N. Height and weight pattern up to 20 years after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1998;79:161–164. doi: 10.1136/adc.79.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bongers ME, Francken AB, Rouwe C, Kamps WA, Postma A. Reduction of adult height in childhood acute lymphoblastic leukemia survivors after prophylactic cranial irradiation. Pediatr Blood Cancer. 2005;45:139–143. doi: 10.1002/pbc.20334. [DOI] [PubMed] [Google Scholar]
- 4.Cicognani A, Cacciari E, Vecchi V, Cau M, Balsamo A, Pirazzoli P, et al. Differential effects of 18- and 24-Gy cranial irradiation on growth rate and growth hormone release in children with prolonged survival after acute lymphocytic leukemia. Am J Dis Child. 1988;142:1199–1202. doi: 10.1001/archpedi.1988.02150110077023. [DOI] [PubMed] [Google Scholar]
- 5.Clayton PE, Shalet SM, Morris-Jones PH, Price DA. Growth in children treated for acute lymphoblastic leukaemia. Lancet. 1988;1:460–462. doi: 10.1016/s0140-6736(88)91246-9. [DOI] [PubMed] [Google Scholar]
- 6.Dalton VK, Rue M, Silverman LB, Gelber RD, Asselin BL, Barr RD, et al. Height and weight in children treated for acute lymphoblastic leukemia: relationship to CNS treatment. J Clin Oncol. 2003;21:2953–2960. doi: 10.1200/JCO.2003.03.068. [DOI] [PubMed] [Google Scholar]
- 7.Davies HA, Didcock E, Didi M, Ogilvy-Stuart A, Wales JK, Shalet SM. Disproportionate short stature after cranial irradiation and combination chemotherapy for leukaemia. Arch Dis Child. 1994;70:472–475. doi: 10.1136/adc.70.6.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groot-Loonen JJ, van Setten P, Otten BJ, van't Hof MA, Lippens RJ, Stoelinga GB. Shortened and diminished pubertal growth in boys and girls treated for acute lymphoblastic leukaemia. Acta Paediatr. 1996;85:1091–1095. doi: 10.1111/j.1651-2227.1996.tb14223.x. [DOI] [PubMed] [Google Scholar]
- 9.Hata M, Ogino I, Aida N, Saito K, Omura M, Kigasawa H, et al. Prophylactic cranial irradiation of acute lymphoblastic leukemia in childhood: outcomes of late effects on pituitary function and growth in long-term survivors. Int J Cancer. 2001;(96 Suppl):117–124. doi: 10.1002/ijc.10348. [DOI] [PubMed] [Google Scholar]
- 10.Hokken-Koelega AC, van Doorn JW, Hahlen K, Stijnen T, de Muinck Keizer-Schrama SM, Drop SL. Long-term effects of treatment for acute lymphoblastic leukemia with and without cranial irradiation on growth and puberty: a comparative study. Pediatr Res. 1993;33:577–582. doi: 10.1203/00006450-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Katz JA, Pollock BH, Jacaruso D, Morad A. Final attained height in patients successfully treated for childhood acute lymphoblastic leukemia. J Pediatr. 1993;123:546–552. doi: 10.1016/s0022-3476(05)80948-5. [DOI] [PubMed] [Google Scholar]
- 12.Robison LL, Nesbit ME, Jr, Sather HN, Meadows AT, Ortega JA, Hammond GD. Height of children successfully treated for acute lymphoblastic leukemia: a report from the Late Effects Study Committee of Childrens Cancer Study Group. Med Pediatr Oncol. 1985;13:14–21. doi: 10.1002/mpo.2950130105. [DOI] [PubMed] [Google Scholar]
- 13.Schriock EA, Schell MJ, Carter M, Hustu O, Ochs JJ. Abnormal growth patterns and adult short stature in 115 long-term survivors of childhood leukemia. J Clin Oncol. 1991;9:400–405. doi: 10.1200/JCO.1991.9.3.400. [DOI] [PubMed] [Google Scholar]
- 14.Sklar C, Mertens A, Walter A, Mitchell D, Nesbit M, O'Leary M, et al. Final height after treatment for childhood acute lymphoblastic leukemia: comparison of no cranial irradiation with 1800 and 2400 centigrays of cranial irradiation. J Pediatr. 1993;123:59–64. doi: 10.1016/s0022-3476(05)81537-9. [DOI] [PubMed] [Google Scholar]
- 15.Starceski PJ, Lee PA, Blatt J, Finegold D, Brown D. Comparable effects of 1800- and 2400-rad (18- and 24-Gy) cranial irradiation on height and weight in children treated for acute lymphocytic leukemia. Am J Dis Child. 1987;141:550–552. doi: 10.1001/archpedi.1987.04460050092038. [DOI] [PubMed] [Google Scholar]
- 16.Stubberfield TG, Byrne GC, Jones TW. Growth and growth hormone secretion after treatment for acute lymphoblastic leukemia in childhood. 18-Gy versus 24-Gy cranial irradiation. J Pediatr Hematol Oncol. 1995;17:167–171. doi: 10.1097/00043426-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Uruena M, Stanhope R, Chessells JM, Leiper AD. Impaired pubertal growth in acute lymphoblastic leukaemia. Arch Dis Child. 1991;66:1403–1407. doi: 10.1136/adc.66.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holm K, Nysom K, Hertz H, Muller J. Normal final height after treatment for acute lymphoblastic leukemia without irradiation. Acta Paediatr. 1994;83:1287–1290. doi: 10.1111/j.1651-2227.1994.tb13018.x. [DOI] [PubMed] [Google Scholar]
- 19.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 20.Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 21.Adan L, Souberbielle JC, Blanche S, Leverger G, Schaison G, Brauner R. Adult height after cranial irradiation with 24 Gy: factors and markers of height loss. Acta Paediatr. 1996;85:1096–1101. doi: 10.1111/j.1651-2227.1996.tb14224.x. [DOI] [PubMed] [Google Scholar]
- 22.Birkebaek NH, Fisker S, Clausen N, Tuovinen V, Sindet-Pedersen S, Christiansen JS. Growth and endocrinological disorders up to 21 years after treatment for acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 1998;30:351–356. doi: 10.1002/(sici)1096-911x(199806)30:6<351::aid-mpo9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 23.Melin AE, Adan L, Leverger G, Souberbielle JC, Schaison G, Brauner R. Growth hormone secretion, puberty and adult height after cranial irradiation with 18 Gy for leukaemia. Eur J Pediatr. 1998;157:703–707. doi: 10.1007/s004310050918. [DOI] [PubMed] [Google Scholar]
- 24.Leiper AD, Stanhope R, Kitching P, Chessells JM. Precocious and premature puberty associated with treatment of acute lymphoblastic leukaemia. Arch Dis Child. 1987;62:1107–1112. doi: 10.1136/adc.62.11.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills JL, Fears TR, Robison LL, Nicholson HS, Sklar CA, Byrne J. Menarche in a cohort of 188 long-term survivors of acute lymphoblastic leukemia. J Pediatr. 1997;131:598–602. doi: 10.1016/s0022-3476(97)70069-6. [DOI] [PubMed] [Google Scholar]
- 26.Oberfield SE, Sklar CA. Endocrine sequelae in survivors of childhood cancer. Adolesc Med. 2002;13:161–169. viii. [PubMed] [Google Scholar]
- 27.Gillum RF, Sempos CT. Ethnic variation in validity of classification of overweight and obesity using self-reported weight and height in American women and men: the Third National Health and Nutrition Examination Survey. Nutr J. 2005;4:27. doi: 10.1186/1475-2891-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.