Abstract
Purpose
To evaluate the safety of, immune response induced by, and efficacy of treatment with lapuleucel-T (APC8024) in patients with HER-2/neu-expressing tumors. Lapuleucel-T is an investigational active immunotherapy product consisting of autologous peripheral blood mononuclear cells, including antigen presenting cells, which are cultured ex-vivo with BA7072, a recombinant fusion antigen consisting of portions of the intra- and extra-cellular regions of HER-2/neu linked to granulocyte-macrophage colony-stimulating factor (GM-CSF).
Experimental Design
Patients with metastatic breast, ovarian, or colorectal cancer whose tumors expressed HER-2 were eligible. Patients underwent leukapheresis in week 0 and received lapuleucel-T infusions in weeks 0, 2, and 4. Patients who achieved a partial response or had stable disease through week 48 were eligible for re-treatment using the same protocol and dose as their initial treatment.
Results
Eighteen patients were enrolled and treated. Patients demonstrated an immune response to the immunizing antigen (BA7072) at week 8 compared to week 0 as measured by T lymphocyte proliferation and interferon gamma enzyme-linked immunospot (ELISPOT) assay. Therapy was well tolerated. The majority (94.7%) of adverse events associated with treatment were grade 1 or 2. Two patients experienced stable disease lasting more than 48 weeks.
Conclusions
Autologous active cellular immunotherapy with lapuleucel-T stimulated an immune response specific to the immunizing antigen and appeared to be well tolerated. Further clinical studies to assess the clinical benefit for patients with HER/2-neu-expressing breast, ovarian, and colorectal cancer are warranted.
INTRODUCTION
In the immune system, antigen presenting cells (APCs) initiate the antigen-specific immune response. They are responsible for uptake, processing, and presentation of antigens to T cells in the context of human leukocyte antigen molecules and co-stimulatory factors. Clinical trials have tested antigen-loaded APCs for treatment of B cell lymphoma (1), prostate cancer (2 – 4), melanoma (5), colorectal cancer (6, 7), and breast cancer (8). Results of these preliminary trials suggest that antigen-loaded APCs are well tolerated by most patients, and that treatment stimulates T and B cell immune responses to the target tumor antigen. Furthermore, recent results from a double-blind, placebo-controlled Phase 3 clinical trial in men with metastatic, androgen independent prostate cancer suggest that this type of approach may confer a survival advantage to men who were assigned to receive the active cellular immunotherapy, sipuleucel-T (9).
HER-2/neu (synonyms: erbB-2, c-erbB-2) is a member of the epidermal growth factor receptor (EGFR) group, which also includes HER1 (EGFR-1), HER3, and HER4. HER-2/neu encodes a 185,000 MW transmembrane glycoprotein that contains an extracellular binding domain and an intracellular domain that possesses tyrosine kinase activity (10). Gene amplification and/or protein overexpression of HER-2/neu has been demonstrated in a number of tumor types, including breast, ovarian, endometrial, bladder, lung, and colorectal cancer and has been correlated with higher-grade tumors and/or less favorable outcomes (11 – 13).
HER-2/neu is an attractive target for immunotherapy given its high level of expression in some patients and the role it may play in tumor progression (10). Although HER-2/neu is expressed at low levels by some normal tissues, treatment with immunological agents targeting HER-2/neu have generally not led to autoimmune complications (8, 14, 15). HER-2/neu has been validated as a cancer target, with clinical trials of trastuzumab (Herceptin®), the monoclonal antibody targeting HER-2/neu, demonstrating clinical benefit in breast cancer in both the adjuvant and metastatic settings (16). An active immunotherapy approach designed to elicit a durable cellular immune response against HER-2/neu-expressing tumors could complement a passive immunotherapeutic approach. In particular, an active immunotherapeutic approach could be effective in tumors with lower levels of antigen expression, where trastuzumab has not been shown to be effective (16); antigenic spread of the immune response could lead to targeting of a broader spectrum of tumor antigens; and the mechanism of action, being independent of the need to block HER-2/neu signaling, could potentially circumvent some mechanisms of trastuzumab resistance (17). A potential synergy between trastuzumab and immunization against HER-2/neu has been suggested by studies in which pretreatment of tumor cells with trastuzumab resulted in increases in specific cytotoxicity by peptide-stimulated cytotoxic T lymphocytes (18).
In this report we describe the results of a Phase 1 trial of lapuleucel-T (APC8024), a novel, cell-based immunotherapy designed to stimulate cellular immune responses against HER-2/neu. Lapuleucel-T contains autologous APCs loaded with a recombinant antigen that comprises extensive HER-2/neu sequences (HER500) linked to a granulocyte-macrophage colony-stimulating factor (GM-CSF) domain (to target antigen to APCs). This study evaluated the safety, immunologic activity, and efficacy of this therapy in patients with HER-2/neu-expressing metastatic breast, ovarian, or colorectal cancer. This study also evaluated the feasibility of manufacturing 3 lapuleucel-T products from a single leukapheresis procedure. Previous studies with lapuleucel-T (8) in breast cancer and with a similar product in prostate cancer, sipuleucel-T (9), have utilized 3 leukapheresis collections followed approximately 2 days later by infusion with fresh study product.
MATERIALS AND METHODS
Patients
Patients from the Mayo Clinic (Rochester, MN), the University of California at San Francisco Mount Zion Cancer Center (San Francisco, CA), and Swedish Medical Center (Seattle, WA) were enrolled in this study. Patients with advanced adenocarcinomas of the breast, ovary, endometrium, or gastrointestinal tract were eligible if their tumors expressed HER-2/neu ≥ 1+ as assessed by immunohistochemistry (IHC) using the HercepTest® IHC kit (Dako Cytomation, Carpentaria, CA), or were HER-2/neu-positive as assessed by fluorescent in situ hybridization (FISH). Patients had disease progression following standard chemotherapy and/or hormonal therapy. Such chemotherapy and/or hormonal therapy was completed at least 1 month prior to enrollment. For patients previously treated with trastuzumab, such therapy was discontinued ≥ 6 weeks prior to enrollment. Concurrent bisphosphonate therapy was permitted provided such therapy began > 30 days prior to leukapheresis. Other eligibility criteria included Eastern Cooperative Oncology Group performance status of 0 or 1; life expectancy > 16 weeks; adequate cardiac, hematologic, renal, and hepatic function; and negative serology for HIV-1/2, human T-lymphotropic virus-1/2, and hepatitis B and C. Females of child bearing potential had a negative serum pregnancy test within the 7 days prior to leukapheresis. The study was subject to ethical review according to local institutional guidelines. Each patient provided written informed consent prior to initiation of study procedures.
Treatment plan and evaluation
Patients underwent a single leukapheresis collection in week 0 followed by open-label treatment with lapuleucel-T in weeks 0, 2, and 4. Patients were then followed until disease progression (as assessed by the Investigator) or week 48, whichever occurred first.
The study evaluated 3 cohorts of patients treated with lapuleucel-T that was prepared with different BA7072 antigen concentrations (10 µg/mL or 40 µg/mL) and different product configurations (fresh lapuleucel-T followed by 2 infusions of cryopreserved lapuleucel-T or 3 infusions of cryopreserved lapuleucel-T; Table 1).
Table 1.
Study Cohorts
| Cohort | BA7072 Concentration In Vitro (µg/mL) |
Nucleated Cells Infused |
Product Configuration |
Disease Type (No. Patients) |
Total No. Patients |
||||
|---|---|---|---|---|---|---|---|---|---|
| Breast | Ovarian | Colorectal | |||||||
| 1 | 10 | 1 × 109 | Fresh and Cryopreserveda |
1 | 3 | 1 | 5 | ||
| 2 | 40 | 1 × 109 | Fresh and Cryopreserveda |
2 | 1 | 2 | 5 | ||
| 3 | 40 | 1 × 109 | Cryopreserved Onlyb |
8 | 0 | 0 | 8 | ||
One infusion of fresh lapuleucel-T followed by 2 infusions of cryopreserved lapuleucel-T.
Three infusions of cryopreserved lapuleucel-T.
Patients were monitored for adverse events, immune response, and disease progression throughout their participation in the study. To estimate the strength of the patient’s T cell and B cell immune responses to BA7072, blood was collected at weeks 0, 4, 8, 16, and at the off-study visit for patients who discontinued between weeks 8 and 16. Physical examinations and laboratory evaluations were performed at baseline, weeks 2, 4, 8, 12, 16, and every 8 weeks thereafter until study completion. A multiple-gated acquisition (MUGA) scan or echocardiogram was performed at baseline and at week 12, or at the off-study visit if the patient discontinued prior to week 12. Staging evaluations, consisting of computed tomography, magnetic resonance imaging, or x-rays of known masses, occurred at baseline, weeks 8 and 16, and every 16 weeks thereafter until study completion. When disease progression (assessed by the Investigator) occurred, patients underwent a physical examination, laboratory tests, and staging evaluation.
Patients who experienced tumor regression or stabilization (defined as stable disease, partial response, or complete response by week 16 and no progression by week 48) were offered the option of re-treatment with 3 doses of lapuleucel-T (using the same antigen concentration and product configuration as initially provided) at the time of disease progression or at week 48, whichever occurred first. Re-treated patients were followed for an additional 16 weeks.
Product manufacture
To prepare lapuleucel-T, patients underwent a standard 1.5- to 2.0-L blood volume mononuclear cell leukapheresis during week 0 to harvest peripheral blood mononuclear cells (PBMCs). Prior leukocyte mobilization with a colony-stimulating factor was not performed. The leukapheresis collection was transported to a cell processing center on the day of leukapheresis.
Antigen presenting cell precursors were isolated from the leukapheresis collection by buoyant density centrifugation. The mononuclear cell fractions containing APC precursors were then cultured for 32 to 48 hours in the presence of 250 ng/mL ionomycin and a predetermined concentration (10 µg/mL or 40 µg/mL) of the BA7072 antigen in order to allow the APCs to differentiate and process the antigen. BA7072 is a recombinant fusion protein containing sequences from both the intra- and extra-cellular domains of HER-2 (designated HER500) linked to GM-CSF and produced in a baculovirus system. Ionomycin, a calcium ionophore, induces activation of APCs (19). After culture, cells were washed to remove free antigen and ionomycin.
Following manufacture, lapuleucel-T was separated into 3 aliquots of approximately 1 × 109 cells each. For patients in cohorts 1 and 2, an aliquot was transported to the study center for infusion approximately 2 days following leukapheresis. The remaining 2 aliquots were cryopreserved for infusion during weeks 2 and 4. For patients in cohort 3, the APCs were separated into 3 aliquots and cryopreserved for infusion during weeks 0, 2, and 4 (Table 1).
Immune response evaluation
Cellular immune responses were measured by quantifying T cell proliferation in response to BA7072 and HER500 antigens. These assays were performed in 96-well plates, with PBMCs from each patient acting as both APCs and responding (proliferating) T cells. Antigens (BA7072 and HER500) were added at 10 µg/mL. Assays were incubated for 6 days, with 3H-thymidine added for the final 18 hours. Proliferation was assessed by the amount of 3H-thymidine taken up by the cells, and was expressed as a stimulation index (SI), defined as the median 3H-thymidine uptake (in counts per minute) of the experimental wells (PBMC + antigen) divided by the median uptake of the control wells (PBMC alone).
Interferon gamma (IFNγ) production in response to the BA7072 and HER500 antigens was measured using the enzyme-linked immunospot (ELISPOT) assay. For this assay, membrane-coated plates were pre-coated with monoclonal antibody specific for IFNγ. PBMCs from each patient, along with 10 µg/mL whole protein (BA7072 or HER500), were added. If the cells produced IFNγ, it was captured by the membrane-bound antibody. After a 2-day incubation period, the cells were removed from the plate and plates were incubated with a second IFNγ-specific antibody and detection agents. The spots, indicating cells producing IFNγ, were counted using an automated counter. Results were reported as the median spots per 3 × 105 PBMC (the number of cells initially plated per well) minus the median for the control at that visit. The ELISPOT assay included separate positive assay controls comprising CMV peptide pool and influenza M1 peptide; the negative control was media alone. Patients were not HLA typed prior to enrollment.
Patients’ production of antibodies against BA7072, HER500, and GM-CSF were measured using enzyme-linked immunosorbent assays (ELISAs). ELISA plates were coated with antigen, blocked, and then incubated with patient serum. After washing, patient antibodies that remained bound to the antigen were detected using a secondary antibody. Results were reported as 1 divided by the titer, which is the highest dilution of serum with an absorbance greater than 0.2, if the background of the assay (in the absence of serum) was no greater than 0.1.
The level of HER-2/neu protein found in each patient’s blood was measured using a commercial ELISA-based kit (Dako Cytomation, Carpenteria, CA). Results were reported as ng of HER-2/neu protein/mL serum.
Statistical methods
Patients who underwent at least 1 leukapheresis procedure were included in the safety and efficacy evaluation. In general, descriptive statistics were provided for each response variable within each group of interest. Descriptive statistics for immune response parameters were provided for all patients who received at least 1 infusion of lapuleucel-T and for whom data were available for at least one week 0 and one week 8 or earlier immune monitoring time point.
The pre-treatment immune data were compared to post-treatment data at each time point. Proliferation responses were quantified by calculating the SI Ratio (i.e., dividing the SI for each week by the SI at Week 0) at Weeks 4, 8, and 16. ELISPOT responses were quantified by subtracting the median number of spots reported at Week 0 from the median number of spots reported at Weeks 4, 8, and 16. The Wilcoxon signed rank test was used to calculate two sided p-values.
The immune response difference between antigen concentrations and between product configurations were tested using the Wilcoxon rank-sum test. Two sided nominal p-values associated with each test were provided to assess potential trends. Kaplan-Meier estimates were used to describe the distribution of time to disease progression.
RESULTS
Baseline Demographics, Disease Characteristics, and Patient Disposition
Eighteen patients were enrolled in this study. Patients were 2 males (11.1%) and 16 females (88.9%) between 34 and 76 years of age. The median age was 56 years. Seventeen patients (94.4%) were Caucasian. Eleven patients (61.1%) had metastatic breast cancer, 3 (16.7%) had metastatic colorectal cancer, and 4 (22.2%) had metastatic ovarian cancer. The majority of patients had an Eastern Cooperative Oncology Group score of 0 (11 patients, 61.1%). All patients received chemotherapy prior to study enrollment; 17 patients (94.4%) received chemotherapy for metastatic disease, and 1 patient (5.6%) received chemotherapy as adjuvant therapy. Nine patients (50.0%) were treated with trastuzumab prior to study enrollment; all 9 of these patients had metastatic breast cancer. Patient demographics are presented in Table 2.
Table 2.
Patient Characteristics
| Characteristic | No. Patients | |
|---|---|---|
| Received treatment | 18 | |
| Median age, years (range) | 56 (34 – 76) | |
| Race | ||
| Caucasian | 17 | 94.4% |
| Asian | 1 | 5.6% |
| Eastern Cooperative Oncology Group (ECOG) performance status | ||
| 0 | 11 | 61.1% |
| 1 | 6 | 33.3% |
| Unknown | 1 | 5.6% |
| Estrogen receptor | ||
| Positive | 7 | 38.9% |
| Negative | 5 | 27.8% |
| Unknown | 6 | 33.3% |
| Progesterone receptor | ||
| Positive | 6 | 33.3% |
| Negative | 6 | 33.3% |
| Unknown | 6 | 33.3% |
| HER-2/neu | ||
| 1+ | 6 | 33.3% |
| 2+ | 3 | 16.7% |
| 3+ | 9 | 50.0% |
| Prior chemotherapy for metastatic disease | ||
| Yes | 17 | 94.4% |
| No | 1 | 5.6% |
| Median time between last chemotherapy administration and first infusion of lapuleucel-T, days (range) |
17 | 148 (39 – 755) |
| Prior trastuzumab | ||
| Yes | 9 | 50.0% |
| No | 9 | 50.0% |
| Prior hormonal therapy | ||
| Yes | 7 | 38.9% |
| No | 11 | 61.1% |
| Prior radiation therapy | ||
| Yes | 11 | 61.1% |
| No | 7 | 38.9% |
All patients received at least 1 infusion of lapuleucel-T. Patients were assigned sequentially to 1 of 3 cohorts. Table 1 presents the BA7072 antigen concentration level, product configuration, and number of patients from each of the 3 disease types enrolled. One patient in cohort 3 developed disease progression and was removed from the study prior to receiving the third infusion of lapuleucel-T. Two patients had stable disease at the end of study participation (week 48), and 1 of these patients elected to undergo re-treatment with lapuleucel-T as allowed per protocol using the same BA7072 antigen concentration and product configuration as the initial treatment. The re-treated patient had stable disease at week 72 when she completed the extended follow-up period.
Safety
Adverse events (AEs) are summarized for all patients (Table 3). Fatigue and rigors were the most commonly reported AEs occurring in 66.7% and 44.4% of patients, respectively; the majority of events of fatigue (8 of 12, 66.7%) and rigors (7 of 8, 87.5%) occurred within 1 day of infusion with lapuleucel-T. Other events that occurred in more than 25% of patients included arthralgia, headache, nausea, and vomiting. The AE distribution appeared well balanced between the BA7072 antigen concentration groups (10 µg/mL or 40 µg/mL) and between the disease type groups (breast, colorectal, or ovarian). The incidence of treatment-associated AEs following infusion with fresh lapuleucel-T appeared similar to that following infusion with cryopreserved lapuleucel-T. Treatment-associated AEs were defined as any AE that occurred within 1 day of infusion with lapuleucel-T (including cryopreserved lapuleucel-T). It was noted that patients who were initially treated with fresh lapuleucel-T were more likely to experience a treatment-associated AE following their second or third infusions (with cryopreserved lapuleucel-T, treatment-associated AEs in 80.0% and 50.0%, respectively) than were patients treated only with cryopreserved lapuleucel-T (treatment-associated AEs in 50.0% and 42.9% following the second and third infusions, respectively).
Table 3.
Adverse Events Experienced by Two or More Patients by Decreasing Frequency
| Preferred Term | Breast Cancer N = 11 n (%) |
Colorectal Cancer N = 3 n (%) |
Ovarian Cancer N = 4 n (%) |
Total N = 18 n (%) |
|---|---|---|---|---|
| Any adverse event | 11 (100.0) | 3 (100.0) | 4 (100.0) | 18 (100.0) |
| Fatigue | 6 (54.5) | 3 (100.0) | 3 (75.0) | 12 (66.7) |
| Rigors | 2 (18.2) | 3 (100.0) | 3 (75.0) | 8 (44.4) |
| Arthralgia | 5 (45.5) | 1 (33.3) | 0 (0.0) | 6 (33.3) |
| Headache | 5 (45.5) | 1 (33.3) | 0 (0.0) | 6 (33.3) |
| Nausea | 3 (27.3) | 1 (33.3) | 2 (50.0) | 6 (33.3) |
| Vomiting | 2 (18.2) | 1 (33.3) | 2 (50.0) | 5 (27.8) |
| Myalgia | 2 (18.2) | 1 (33.3) | 1 (25.0) | 4 (22.2) |
| Abdominal distension | 2 (18.2) | 0 (0.0) | 1 (25.0) | 3 (16.7) |
| Abdominal pain | 3 (27.3) | 0 (0.0) | 0 (0.0) | 3 (16.7) |
| Anaemia | 3 (27.3) | 0 (0.0) | 0 (0.0) | 3 (16.7) |
| Back Pain | 1 (9.1) | 1 (33.3) | 1 (25.0) | 3 (16.7) |
| Diarrhoea | 2 (18.2) | 1 (33.3) | 0 (0.0) | 3 (16.7) |
| Anorexia | 0 (0.0) | 1 (33.3) | 1 (25.0) | 2 (11.1) |
| Anxiety | 1 (9.1) | 1 (33.3) | 0 (0.0) | 2 (11.1) |
| Breast Mass | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (11.1) |
| Cough | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (11.1) |
| Depression | 0 (0.0) | 1 (33.3) | 1 (25.0) | 2 (11.1) |
| Hypokalaemia | 1 (9.1) | 0 (0.0) | 1 (25.0) | 2 (11.1) |
| Lymphadenopathy | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (11.1) |
| Oedema | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (11.1) |
| Pain in limb | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (11.1) |
| Pain | 1 (9.1) | 0 (0.0) | 1 (25.0) | 2 (11.1) |
| Pyrexia | 0 (0.0) | 1 (33.3) | 1 (25.0) | 2 (11.1) |
| Tachycardia | 1 (9.1) | 0 (0.0) | 1 (25.0) | 2 (11.1) |
| Upper respiratory tract infection | 2 (18.2) | 0 (0.0) | 0 (0.0) | 2 (11.1) |
Four patients developed serious adverse events (SAEs) during this study. All 4 SAEs were judged by the Investigator as unrelated to treatment with lapuleucel-T. These SAEs consisted of acquired pyloric stenosis, fecal impaction and hydronephrosis, urosepsis, and disease progression. At the time of the SAE, the patient with acquired pyloric stenosis was noted as having infiltrating adenocarcinoma in the stomach and hypodense lesions consistent with metastases in the liver. The patient was withdrawn from the study due to disease progression and died approximately 3 months later. No other patient died during the study.
No patient experienced an AE that resulted in an intervention, withdrawal of test drug, dose reduction, or significant additional concomitant medications other than those previously described as SAEs. No marked hematological, biochemical, or other laboratory abnormalities were noted. The only cardiac disorder reported during the study was tachycardia not otherwise specified, which was seen in 2 patients: 1 patient with breast cancer who had been previously treated with trastuzumab and1 patient with ovarian cancer who had not been previously treated with trastuzumab. Ten patients underwent serial left ventricular ejection fraction (LVEF) imaging. Overall, no trend in LVEF changes were noted among patients who underwent serial imaging. Four patients had absolute declines of LVEF ranging from 9.4% to 13.0%; two of the patients had received prior trastuzumab treatment, 2 had not received prior trastuzumab treatment. Two patients had absolute improvement of LVEF ranging from 11.2% to 14.0%; one of the patients had received prior trastuzumab treatment, 1 had not received prior trastuzumab treatment.
Based on a review of AE and laboratory data, there was no evidence of autoimmune complications that developed during this study. There were no reports of autoimmune reactions, and no evidence of autoimmune or inflammatory changes in vital organs were noted based on hematologic and biochemistry monitoring. There was no evidence of neutropenia or leukopenia following treatment with lapuleucel-T. Furthermore, there was no observed development of antibodies against GM-CSF (n = 10 patients for whom GM-CSF titers were measured at both baseline and week 8; a negative titer was defined as < 16 fold increase versus baseline).
Immunologic activity
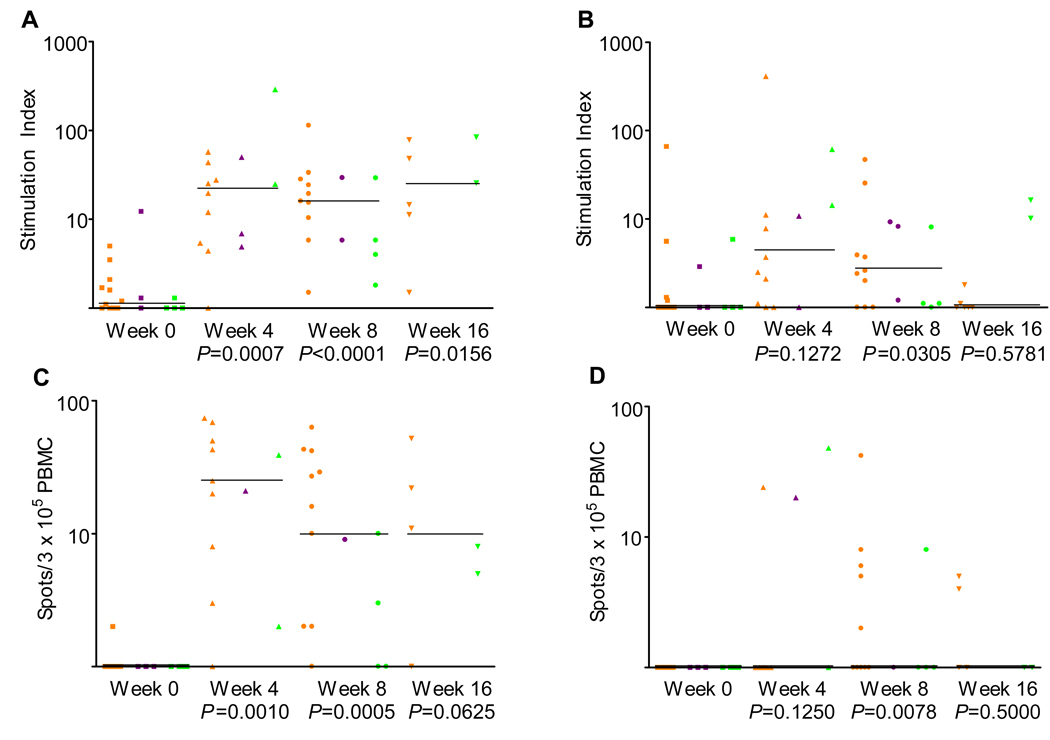
Immune response data for all patients were evaluated using in vitro proliferation responses to the BA7072 and HER500 antigens. An immune response was observed to the immunizing antigen (BA7072) at week 8 compared to week 0 (P < 0.0001). A difference in response (week 0 to week 8) to the tumor-specific region of the immunizing antigen (HER500) was observed (P = 0.0305), although the response was not as robust as that to BA7072. A visual display of individual data points observed at week 0, 4, 8, and 16 to the 10 µg/mL concentrations of BA7072 and HER500 are presented in Figure 1. Immune responses to BA7072 were generally greater in magnitude than those to HER500. For example, the median in vitro proliferation stimulation index ratio at week 8 to BA7072 was 11.2 compared with a stimulation index ratio of 1.7 to HER500.
Figure 1.
T cell proliferation data for (A) BA7072 and (B) HER500 at 10 µg/mL. Interferon gamma (IFN-γ;) enzyme-linked immunospot (ELISPOT) data for (C) BA7072 and (D) HER500 at 10 µg/mL. Data are shown for each sample. Median response for each time point is indicated by the line. Orange, purple, and green marks represent breast, colon, and ovarian cancer patients, respectively. P-values associated with the change at each week from baseline were calculated using the Wilcoxon signed rank test.
No appreciable difference in immune response was noted between patients treated with lapuleucel-T manufactured with the 2 antigen concentrations (10 µg/mL or 40 µg/mL). There was also no appreciable difference in immune response noted between patients treated with the 2 product configurations (fresh lapuleucel-T followed by 2 infusions of cryopreserved lapuleucel-T or 3 infusions of cryopreserved lapuleucel-T). Immune responses were observed in patients with all 3 tumor types (breast, colorectal, or ovarian). Although there were fewer immune samples obtained at week 16 than at week 8, there was evidence of continued immune response noted at the week 16 time point.
Immune response data were also evaluated using in vitro IFNγ production responses (measured by ELISPOT) to the BA7072 and HER500 antigens. An immune response was observed to the immunizing antigen (BA7072) at week 8 compared to week 0 (P = 0.0005). A difference in response (week 0 to week 8) to the tumor-specific region of the immunizing antigen (HER500) was also observed (P = 0.0078. The absolute median value for the Week 8 ELISPOT anti-HER500 response was 1 spot per 3 × 105 PBMCs (range 0 – 42) versus 0 spots per 3 × 105 PBMCs at baseline (range 0 – 1), although the response was not as robust as that to BA7072. A visual display of individual data points observed at week 0, 4, 8, and 16 to the 10 µg/mL concentrations of BA7072 and HER500 are presented in Figure 1. Consistent with the proliferation assay results, no appreciable differences in immune responses measured by ELISPOT were noted between patients treated with lapuleucel-T manufactured with different BA7072 antigen concentrations, between those treated with the different product configurations, or between patients with different tumor types.
The magnitude of immune response appeared to be similar between groups based on level of HER2 expression by IHC (1+, 2+, or 3+). Of note, the highest proliferative response to BA7072 was observed in a patient whose tumor was HER2 1+ by IHC (Data not shown).
Circulating antibodies to HER500, BA7072 antigen, and GM-CSF were assessed in each patient using ELISAs. A total of 40% of patients had a > 16-fold increase in antibody response to BA7072; no changes in the antibody response to HER500 or GM-CSF were noted.
Serum levels of circulating HER-2/neu were assessed in each patient using a commercial ELISA-based kit. No reductions in median HER-2/neu levels over time were noted.
Efficacy
The estimated median time to disease progression was 12.8 weeks (observed range 3.9 to 71.9 weeks, including censored patients). Two patients, both with breast cancer, treated at the 40 µg/mL BA7072 antigen concentration, and treated with 3 infusions of cryopreserved lapuleucel-T, had stable disease at the end of the follow-up period (week 48). One of these patients was re-treated approximately 1 year following initial treatment at the same antigen concentration and product configuration as previously administered. The re-treated patient had stable disease at the time follow-up was completed 16 weeks following re-treatment (week 72). Of the 16 patients who developed disease progression prior to week 48, 5 experienced short periods of stable disease following treatment with lapuleucel-T. A clinical efficacy summary by individual patient is presented in Table 4.
Table 4.
Clinical Efficacy Summary by Individual Patient
| Cohort No.a |
Hormone Receptor Status |
HER/2-neu by | Disease Type | Lesion Site(s) | Time to Progression (weeks) |
Best Response |
|
|---|---|---|---|---|---|---|---|
| IHC | FISH | ||||||
| 1 | ER+/PR− | 2+ | Positive | Breast | Chest wall, lymph nodes, skin | 19.3 | PD |
| 1 | NA | 1+ | NA | Colorectal | Lung | 12.9 | PD |
| 1 | ER+/PR+ | 1+ | NA | Ovarian | Liver, pleura, peritoneum | 12.3 | PD |
| 1 | NA | 1+ | NA | Ovarian | Chest wall, lymph nodes | 18.3 | SD |
| 1 | NA | 2+ | Negative | Ovarian | Lymph nodes, lung | 12.1 | PD |
| 2 | ER−/PR+ | 3+ | NA | Breast | Lung | 11.1 | PD |
| 2 | NA | 1+ | Positive | Colorectal | Liver, spleen, lymph nodes, peritoneum |
8.1 | PD |
| 2 | NA | 1+ | NA | Colorectal | Liver, lung | 8.0 | PD |
| 2 | NA | 1+ | Negative | Ovarian | Lung, mesentery | 15.7 | SD |
| 2 | ER−/PR− | 3+ | Positive | Breast | Bone, liver | 16.7 | SD |
| 3 | ER+/PR+ | 3+ | Positive | Breast | Lung | 3.9 | PD |
| 3 | ER+/PR+ | 3+ | NA | Breast | Bone, lymph nodes, lung, chest wall | 12.7 | PD |
| 3 | ER−/PR− | 3+ | NA | Breast | Breast, liver, bone | 16.9 | SD |
| 3 | ER−/PR− | 3+ | NA | Breast | Breast | 26.0 | SD |
| 3 | ER−/PR− | 3+ | NA | Breast | Breast, bone, lymph nodes | 8.0 | PD |
| 3 | ER−/PR+ | 2+ | NA | Breast | Bone, uterus, chest wall | 71.9b,c | SD |
| 3 | ER−/PR− | 3+ | Positive | Breast | NAd | 49.4b | SD |
| 3 | ER+/PR+ | 3+ | NA | Breast | Breast, liver | 8.1 | PD |
Abbreviations: IHC, immunohistochemistry; FISH, fluorescent in situ hybridization; ER, estrogen receptor; PR, progesterone receptor; PD, progressive disease; SD, stable disease; NA, not available.
Cohort 1: 10 µg/mL BA7072 antigen concentration; fresh lapuleucel-T × 1, cryopreserved lapuleucel-T × 2
Cohort 2: 40 µg/mL BA7072 antigen concentration; fresh lapuleucel-T × 1, cryopreserved lapuleucel-T × 2
Cohort 3: 40 µg/mL BA7072 antigen concentration; cryopreserved lapuleucel-T × 3
Patient censored; patient reached the end of the follow-up period without developing disease progression.
Patient was re-treated at approximately 1 year.
Patient had prior surgical resection of sternal and lung metastases.
DISCUSSION
This Phase 1 study investigated the safety and immunologic activity of lapuleucel-T, a novel, cell-based immunotherapy consisting of APCs activated with a recombinant fusion protein (BA7072) containing HER-2 sequences and designed to stimulate cellular immune responses against HER-2/neu.
In addition to passive immunotherapy such as trastuzumab, a number of previous studies of active immunologic therapies targeting HER-2/neu have been reported, including peptide approaches (20 – 23) and dendritic cell based vaccines (24). These approaches appear to be well-tolerated and induce robust immune responses. In addition, evidence of clinical activity has been reported in breast cancer in the adjuvant setting (21). Possible advantages of the approach pursued here compared with peptide-based approaches include the potential to induce immune responses to multiple HER-2/neu epitopes, and the ability to include patients without regard to HLA haplotype. A previous study of an approach similar to lapuleucel-T, but targeting the tumor antigen prostatic acid phosphatase, has shown evidence of survival prolongation in men with advanced prostate cancer (9).
In contrast to the toxicities associated with many other cancer treatments, lapuleucel-T therapy was generally well tolerated. The most common AEs reported in the study were fatigue and rigors. Most AEs were mild or moderate, were of short duration, and occurred soon after infusion of lapuleucel-T. The overall toxicity profile was generally similar to that reported for a previous study with lapuleucel-T in breast cancer (8) and that of sipuleucel-T in men with advanced prostate cancer (9). When AE incidence rates from this study were compared with those of the sipuleucel-T study, rigors and pyrexia appeared to occur more frequently in patients treated with sipuleucel-T than with lapuleucel-T (rigors 62.2% vs. 44.4%, and pyrexia 34.1% vs. 11.1%). Conversely, headache, nausea, and vomiting appeared to occur more frequently in patients treated with lapuleucel-T in this study compared with sipuleucel-T (headache 33.3% vs. 17.1%, nausea 33.3% vs. 14.6%, and vomiting 27.8% vs. 12.2%).
Immune responses to the immunizing antigen BA7072 were observed in this study. Responses to HER500 were also observed, but the magnitude of responses was lower.
The findings that proliferative immune responses were observed across all groups based on the level of HER2 expression, suggest that this form of active cellular immunotherapy may be applicable to patient populations where passive immunotherapeutic approaches targeting HER2 are not effective.
Immune responses were observed in patients receiving both configurations of lapuleucel-T (fresh lapuleucel-T followed by 2 infusions of cryopreserved lapuleucel-T, or 3 infusions of cryopreserved lapuleucel-T), which demonstrated the feasibility of this manufacturing approach. However, the administration of cryopreserved lapuleucel-T did not appear to result in as robust an immune response as that observed in the Park et al study (8) which used only infusions of fresh lapuleucel-T. For example, the median proliferative response to BA7072 measured at Week 8 was 60.1 in the previous study, as compared with 15.4 in this study. The reason for the differences in immune responses is not known at this time. A possible reason for the difference could be the formulation of lapuleucel-T; the patients in this study received either 2 or 3 infusions of cryopreserved lapuleucel-T, whereas all infusions administered in the previous study were fresh lapuleucel-T.
An important aspect of this study was the inclusion of patients with HER-2 expressing malignancies other than breast cancer. Immune responses to lapuleucel-T were observed in patients with colorectal and ovarian cancers as well as in those with breast cancer.
A limitation of this study was the small number of patients enrolled. With the number of variables in the study design, including the concentrations of BA7072, the different product configurations (fresh and cryopreserved lapuleucel-T), and tumor types included, the study was not adequately powered to draw definitive conclusions regarding the comparative immunologic activity or efficacy of lapuleucel-T between groups.
Evidence of anticancer activity in this trial included short-term disease stabilization in 5 patients, and long-term disease stabilization lasting 48 weeks or more in 2 patients. The 2 patients with long-term disease stabilization both had breast cancer and were treated with 3 infusions of cryopreserved lapuleucel-T. One of these patients was also retreated with 3 infusions of lapuleucel-T approximately 1 year after initial treatment. This patient had stable disease at the time of the last follow-up visit, which occurred 72 weeks after initial treatment.
Breast, colorectal, and ovarian cancer comprise 3 of the 5 leading causes of death from cancer among women, and are expected to account for over 275,000 new cases in 2008 (25). Given the number of patients affected by these diseases, and the toxicities associated with standard treatments, new treatment options are needed. We conclude that treatment with lapuleucel-T, a novel, cell-based immunotherapy, stimulated an immune response specific to the immunizing antigen and appeared to be well tolerated. Further clinical studies to assess the clinical benefit for patients with HER/2-neu-expressing breast, ovarian, and colorectal cancer are warranted.
STATEMENT OF TRANSLATIONAL RELEVANCE.
Lapuleucel-T (APC8024) is an investigational autologous active cellular immunotherapy designed to stimulate an immune response against tumor cells expressing the cancer antigen HER-2/neu. Such an active cellular immunotherapy approach may complement and/or synergize with passive immunotherapy approaches targeting HER-2/neu, such as trastuzumab. This study provides evidence that lapuleucel-T leads to an antigen-specific immune response in patients with breast, ovarian, and colorectal cancer. The therapy was well tolerated and was associated with prolonged disease stabilization in some patients. These findings, if confirmed in future clinical trials, may lead to new immunotherapy options for patients with HER-2/neu-positive malignancies.
Acknowledgements
We are indebted to the patients who participated in this study. We thank study coordinators Kim Jensen, Mayo Clinic, Susan Steinmetz, Mayo Clinic, Jennafer Carlin, University of California San Francisco-Mount Zion Cancer Center, and Christine Reed, Swedish Medical Center for their contributions to the study. We thank Frank Valone for his work on the conceptualization and design of the clinical trial and Kim Miller for medical writing assistance with this manuscript.
Grant support: Supported in part by Grant No. U54-CA90788 from the National Institutes of Health, by Grants No. NCI P50-CA 58207, U54-CA90788, U01 CA111234-01, and the Lauder Fund.
Footnotes
Disclosure of Potential Conflicts of Interest
The conduct of this trial was supported by Dendreon Corporation.
REFERENCES
- 1.Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 2.Murphy G, Tjoa B, Ragde H, Kenny G, Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371–380. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Burch PA, Breen JK, Buckner JC, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 4.Small EJ, Fratesi P, Reese DM, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 5.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 6.Arnaout AH, Dawson PM, Soomro S, et al. HER2 (c-erbB-2) oncoprotein expression in colorectal adenocarcinoma: an immunohistological study using three different antibodies. J Clin Pathol. 1992;45:726–727. doi: 10.1136/jcp.45.8.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morse MA, Deng Y, Coleman D, et al. A phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res. 1999;5:1331–1338. [PubMed] [Google Scholar]
- 8.Park JW, Melisko ME, Esserman LJ, Jones LA, Wollan JB, Sims R. Treatment with autologous antigen-presenting cells activated with the HER-2 based antigen lapuleucel-T: results of a phase I study in immunologic and clinical activity in HER-2 overexpressing breast cancer. J Clin Oncol. 2007;25:3680–3687. doi: 10.1200/JCO.2006.10.5718. [DOI] [PubMed] [Google Scholar]
- 9.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 10.Park JW, Neve RM, Szollosi J, Benz CC. Unraveling the biologic and clinical complexities of HER2. Clin Breast Cancer. 2008;8:392–401. doi: 10.3816/CBC.2008.n.047. [DOI] [PubMed] [Google Scholar]
- 11.Seidman JD, Frisman DM, Norris HJ. Expression of the HER-2/neu proto-oncogene in serous ovarian neoplasms. Cancer. 1992;70:2857–2860. doi: 10.1002/1097-0142(19921215)70:12<2857::aid-cncr2820701223>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–898. [PubMed] [Google Scholar]
- 13.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 14.Pegram MD, Konecny G, Slamon DJ. Use of HER2 for predicting response to breast cancer therapy. Dis of Breast. 1999;3:1–9. [Google Scholar]
- 15.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 16.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 17.Ritter CA, Perez-Torres M, Rinehart C, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 18.Mittendorf EA, Storrer CE, Shriver CD, Ponniah S, Peoples GE. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol. 2006;13:1085–1098. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 19.Czerniecki BJ, Carter C, Rivoltini L, et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J Immunol. 1997;159:3823–3837. [PubMed] [Google Scholar]
- 20.Disis ML, Goodell V, Schiffman K, Knutson KL. Humoral epitope-spreading following immunization with a HER-2/neu peptide based vaccine in cancer patients. J Clin Immunol. 2004;24:571–578. doi: 10.1023/B:JOCI.0000040928.67495.52. [DOI] [PubMed] [Google Scholar]
- 21.Holmes JP, Benavides LC, Gates JD, et al. Results of the first phase I clinical trial of the novel II-key hybrid preventive HER-2/neu peptide (AE37) vaccine. J Clin Oncol. 2008;26:3426–3433. doi: 10.1200/JCO.2007.15.7842. [DOI] [PubMed] [Google Scholar]
- 22.Peoples GE, Holmes JP, Hueman MT, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients: U.S. Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Clin Cancer Res. 2008;14:797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 23.Coveler AL, Goodell V, Webster DJ, et al. Common adjuvant breast cancer therapies do not inhibit cancer vaccine induced T cell immunity. Breast Cancer Res Treat. 2008;113:95–100. doi: 10.1007/s10549-008-9910-y. [DOI] [PubMed] [Google Scholar]
- 24.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 25.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]