Abstract
Purpose
Melanoma is the most invasive and deadly form of skin cancer. Few agents are available for treating advanced disease to enable long-term patient survival, which is driving the search for new compounds inhibiting deregulated pathways causing melanoma. Akt3 is an important target in melanomas since its activity is increased in ~70% of tumors, decreasing apoptosis in order to promote tumorigenesis.
Experimental Design
Since naturally occurring products can be effective anti-cancer agents, a library was screened to identify Akt3 pathway inhibitors. Isothiocyanates were identified as candidates but low potency requiring high concentrations for therapeutic efficacy made them unsuitable. Therefore, more potent analogs called isoselenocyanates were created using the isothiocyanate backbone but increasing the alkyl chain length and replacing sulfur with selenium. Efficacy was measured on cultured cells and tumors by quantifying proliferation, apoptosis, angiogenesis, toxicity and Akt3 pathway inhibition.
Results
Isoselenocyanates significantly decreased Akt3 signaling in cultured melanoma cells and tumors. Compounds having 4–6 carbon alkyl side chains with selenium substituted for sulfur, called ISC-4 and ISC-6 respectively, decreased tumor development by ~60% compared to corresponding isothiocyanates, which had no effect. No changes in animal body weight or in blood parameters indicative of liver, kidney or cardiac related toxicity were observed with isoselenocyanates. Mechanistically, isoselenocyanates ISC-4 and ISC-6 decreased melanoma tumorigenesis by causing an ~3-fold increase in apoptosis.
Conclusions
Synthetic isoselenocyanates are therapeutically effective for inhibiting melanoma tumor development by targeting Akt3 signaling to increase apoptosis in melanoma cells with negligible associated systemic toxicity.
Keywords: melanoma, isothiocyanates, isoselenocyanates, selenium, Akt3, PRAS40, tumorigenesis, apoptosis
INTRODUCTION
Dacarbazine (DTIC) is one of the approved chemotherapeutic agents for metastatic melanoma but is relatively ineffective with an overall response rates of 5–20% (1, 2). This is equally true of most currently available therapeutic strategies for metastatic melanoma patients, including surgery, immuno-, radio- and chemotherapy, which are ineffective long-term treatments for individuals suffering from advanced disease (3, 4). This is a serious problem since the incidence of melanoma remains unchecked, increasing at a rate of ~4% per year and is predicted to affect 1 in 50 US citizens by 2010. As a direct result of a lack of effective therapeutics, the current prognosis for patients with metastatic disease remains very poor with average survival ranging from 6–10 months (2, 5).
To develop more effective melanoma therapeutics, agents targeting proteins promoting disease development are being developed (6–11). Akt3 is one example, which is activated in ~70% of melanomas (6–8). While three Akt isoforms known as Akt1/PKBα, Akt2/PKBβ and Akt3/PKBγ (12, 13) are expressed in melanocytes and melanoma cells, Akt3 is the predominantly active family member (6). Increased AKT3 gene copy number and/or loss of a negative regulatory phosphatase called PTEN leads to Akt3 activation (6, 14), which reduces melanoma cell apoptosis mediated through caspase-3 to promote melanoma development (6). Recently, PRAS40 was identified as an important downstream protein in the Akt3 signaling cascade regulating cellular apoptosis (7). Inhibiting PRAS40 or Akt3 using siRNA-based approaches increased cellular apoptosis to similar levels causing a significant reduction in the tumorigenic potential of melanoma cells (7). A second function for Akt3 in early melanoma development has also been reported recently (15). Akt3 has been shown to phosphorylate a constitutively active mutant form of B-Raf, called V600EB-Raf, and in so doing, lowers the activity of the mutant protein to levels that promote rather than inhibit melanoma tumor progression (15). Therefore targeting Akt3 would also have the added consequence of increasing V600EB-Raf activity to levels that would be inhibitory to growth. While these studies demonstrate the therapeutic potential of targeting the Akt3 signaling cascade to inhibit melanoma development, no clinical agent is available for inhibition of Akt3 signaling in melanoma.
Isothiocyanates are naturally occurring compounds found in cruciferous vegetables having anti cancer properties (16–19), protecting against murine tumorigenesis induced by environmental carcinogens such as polycyclic aromatic hydrocarbons and nitrosamines (20, 21). Certain studies suggest the mechanism of action of isothiocyanates is by inhibiting the PI3 kinase pathway (22, 23). Selenium is also an effective chemopreventive agent by modulating Akt activity (24–26). Selenium deficiency occurs frequently in cancer patients including those diagnosed with metastatic melanoma (27). Recently, selenium has been shown to induce destabilization of Akt activity in prostate cancer cells (28, 29). Therefore, incorporating selenium into the structure of compounds could increase compound efficacy and these compounds would be safe since melanoma patients frequently have selenium deficiency.
In this study, isothiocyanate analogs having a longer carbon chain lengths and selenium substituted for sulfur have been developed and therapeutic efficacy for killing cultured melanoma cells or inhibiting tumor development in animals evaluated. While increasing chain length did not increase tumor inhibitory potency of sulfur containing isothiocyanates, incorporation of selenium with increasing chain length significantly enhanced anti-tumor potency by elevating rates of tumor cell apoptosis. Thus, novel isoselenocyanates have been developed that target Akt3 signaling in melanoma cells leading to robust anti-melanoma activity.
MATERIALS AND METHODS
Cell lines and culture conditions
The human fibroblast cells (FF2441) and metastatic melanoma cell lines UACC 903 and 1205 Lu were maintained in DMEM (Invitrogen, Carlsbad, CA), supplemented with 10% FBS (Hyclone, Logan, UT) at 37°C with 5% CO2 atmosphere in a humidified incubator. Vertical growth phase (VGP) melanoma cell line WM115 was maintained in Tu2% medium as described previously (6).
Western blot analysis
For Western blot analysis, floating and adherent cells treated with compounds or control vehicle (DMSO) were harvested by addition of lyses buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM sodium orthovanadate, 0.1mM sodium molybdate, 1mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, and 5 μg/ml leupeptin. Whole cell lysates were centrifuged (≥10,000 X g) for 10 min at 4°C to remove cell debris. Protein concentrations were quantitated using the BCA assay from Pierce (Rockford, IL), and 30 μg of lysate loaded per lane onto NuPAGE Gels from Life Technologies (Carlsbad, CA). Following electrophoresis, samples were transferred to a polyvinylidene difluoride membrane (Pall Corporation, Pensacola, FL). Blots were probed with antibodies according to each supplier’s recommendations: phosphorylated-PRAS40 (Thr246) from Invitrogen (Carlsbad, CA); Erk2, α-enolase and secondary antibodies conjugated with horseradish peroxidase from Santa Cruz Biotechnology (Santa Cruz, CA); and Immunoblots were developed using the enhanced chemiluminescence detection system (Pierce Biotechnology, Rockford, IL).
Synthesis of isothiocyanates (ITC), isoselenocyanates (ISC) and phenylhexyl selenocyanate (PHSC)
BITC and PEITC were purchased from Sigma-Aldrich (St. Louis, MO). PBITC and PHITC were synthesized by using previously reported methodology (30). Isoselenocyanates were synthesized using a described methoda. Briefly, a solution of triphosgene (5.0 mmol) in CH2Cl2 (15 mL) was added over a refluxing mixture of formamides (10.0 mmol), triethylamine (43.0 mmol) and 4 Å molecular sieves in CH2Cl2 (35 mL). Mixture was then refluxed for an additional 2.5 h. Selenium powder (20 mmol) was then added and resulting mixture refluxed for 6–8 h. Mixture was cooled, filtered, and solvent evaporated yielding a crude mixture, which was purified by silica gel column chromatography generating pure isoselenocyanates. 6-phenylhexylselenocyanate (PHSC) was prepared by reacting 0.3 g (1.25 mmol) of 6-phenylhexylbromide with 0.19 g (1.32 mmol) of KSeCN in 10 mL of acetonitrile, in a nitrogen atmosphere. After stirring overnight at room temperature, the residue was partitioned between EtOAc and water. The organic phase was separated, washed with brine and water, dried over MgSO4, filtered and the solvent evaporated to yield 0.26 g (74% yield). Compounds identities were confirmed by NMR as well as Mass Spectra analysis and purity (>99%) quantified by HPLC analysis.
SiRNA protein knockdown studies
Duplexed “Stealth” siRNA from Invitrogen (Carlsbad, CA) were: AKT3- 5′-GGA CUA UCU ACA UUC CGG AAA GAU U-3′ and scrambled- 5′-AAU UCU CCG AAC GUG UCA CGU GAG A-3′. Nucleofection using Amaxa Nucleofector (Koeln, Germany) was used to introduce siRNA into UACC 903 cells (Reagent R, program K17). SiRNA (100 pmoles) against Akt3 or scrambled siRNA or buffer were nucleofected into 1X106 UACC 903 cells, which were then replated in DMEM supplemented with 10% FBS and allowed to recover for 1.5 days. Transfection efficiency was >90% with ~80% viability. For animal experiments, thirty-six hours later, 1X106 viable UACC 903 cells in 0.2 ml of DMEM supplemented with 10% FBS were injected subcutaneously into the left and right flanks of 3-to-4 wk old female Athymic Nude-Foxn1nu mice. Dimensions of developing tumors were measured on alternate days using calipers up to day 17.5. To test duration of siRNA-mediated knockdown, protein lysates were collected at 2, 4, 6, and 8 d following nucleofection and measured for Akt3 protein expression by Western blot analysis and quantitated by densitometry as described previously (6, 14).
Cell viability, proliferation, apoptosis determination and cell cycle analysis
Viability and IC50 of melanoma cells following treatment with compounds was measured using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega, Madison, WI). Briefly, 5 X 103 melanoma (UACC 903, 1205 Lu or WM115) or human fibroblast (FF2441) cells per well in 100 μL DMEM containing 10% FBS were grown in a 96-well plate for 24 or 76 h and treated with either control DMSO vehicle or increasing concentrations (2–100 μM) of compounds for 24 h. Cellular viability compared to vehicle control treated cells was measured using the MTS assay. IC50 values for each compound in respective cell lines was determined from three independent experiments using GraphPad Prism version 4.01 (GraphPad software, San Diego, CA).
Cellular proliferation and apoptosis rates were measured by seeding 5 X 103 cells/well in 96-well plates, followed by treatment for 24 h with each respective agent. Proliferation and apoptosis were measured using a BrdUrd ELISA kit (Roche Applied Sciences, Indianapolis, IN) or Apo-ONE Homogenous caspase-3/7 Assay kit (Promega Corporation, Madison, WI), respectively.
Cell cycle analysis was undertaken by plating 1.5 X 106 melanoma cells in 100-mm culture dish and following treatment with respective compounds for 24 h, total cells (floating and adherent) were trypsinized, centrifuged (500 X g, for 5 min) and treated with 1 mL of propidium iodide staining solution containing 100 μg/mL PI; Sigma, St Louis, MO), 20 μg/mL Ribonuclease A (Roche Applied Sciences, Indianapolis, IN) 3 μg/mL Triton X-100 dissolved in 0.1% (w/v sodium citrate for 30 m at 4°C (31). Cells were analyzed using the FACScan analyzer (Becton Dickinson, San Jose, CA) and data processed using ModFit LT software (Verity software house, Topsham, ME).
Tumorigenicity assessment, knockdown of protein expression and measurement of proliferation/apoptosis rates in tumors
Animal experimentation was performed according to protocols approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University College of Medicine. Tumor kinetics were measured by subcutaneous injection of 2.5–5X106 1205 Lu or UACC 903 melanoma cells in 0.2 ml of DMEM supplemented with 10% FBS above both left and right rib cages of 3-to-4 wk old female Athymic Nude-Foxn1nu mice (Harlan Sprague Dawley, Indianapolis, IN). Six days later when a fully vascularized tumor (50–75 mm3) had formed, mice were randomly divided in to DMSO vehicle control and experimental (BITC, PEITC, PBITC, PHITC, ISC-1, ISC-2, ISC-4 or ISC-6) groups (5 mice/group; 2 tumors/mouse) and treated i. p. with isothiocyanate or isoselenocyanate compounds (2.5 or 0.76 μmoles (equivalent to 3 ppm selenium) / 20g mice in 50 μL DMSO vehicle) on Monday, Wednesday and Friday for ~ 3 weeks. Control mice received an equivalent volume of the vehicle. Body weight (grams) and dimensions of the developing tumors (mm3) were measured at the time of treatment.
To ascertain mechanism underlying tumor inhibition, 5X106 UACC 903 cells were injected into nude mice, 6-days later mice were treated i. p. with PBITC or PHITC (0.76 μmoles), ISC-4 or ISC-6 (0.76 μmoles, equivalent to 3 ppm selenium) on alternate days. Size and time matched tumors were harvested at days 11 and 13 to assess changes in cell proliferation and apoptosis. A small portion of the tumor was also flash frozen in liquid nitrogen, pulverized and lysed in protein lysis buffer (600–800 μl, 50 mM Tris-HCl, pH 7.5 containing 0.1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 50 mM sodium fluoride, 10 mM sodium β-glycerol phosphate, 5 mM sodium pyrophosphate, 1 mM activated sodium orthovanadate, protease inhibitor cocktail from Sigma and 0.1% (v/v) 2-mercaptoethanol). Protein concentration was determined using Bio-Rad protein assay reagent (Bio-Rad laboratories, Hercules, CA), analyzed by Western blotting to measure levels of pAkt and downstream pPRAS40 in tumors and quantitated by densitometry as described previously (6, 14).
Cell proliferation and apoptosis were measured in formalin-fixed, paraffin-embedded tumor sections using the TUNEL TMR Red Apoptosis kit from Roche (Manheim, Germany) or purified mouse anti-human Ki-67 from PharMingen (San Diego, CA), respectively. A minimum of 6 different tumors with 4–6 fields per tumor was analyzed and results represented as the average ± S.E.M.
Toxicity assessments
4-to-6 wks old female nude mice (Harlan Sprague Dawley, Indianapolis, IN) were injected i. p. with either control DMSO vehicle, PBITC or PHITC (0.76 μmoles) or ISC-4 or ISC-6 (0.76 μmoles equivalent to 3 ppm Se) on Monday, Wednesday and Friday for 3 weeks. Animals were sacrificed by CO2 asphyxiation and blood collected from each animal in plasma separator tubes with lithium heparin (BD, Franklin Lakes, NJ) following cardiac puncture and analyzed for SGOT (serum glutamic oxaloacetic transaminase), SGPT (serum glutamate pyruvate transaminase), alkaline phosphatase, glucose and creatinine to ascertain liver, heart, kidney and pancreas related toxicity. For morphological examination of blood cells, whole blood was collected in microtainer tubes containing K2EDTA (BD, Franklin Lakes, NJ) and RBC, WBC, lymphocytes, monocytes, eosinophils, platelets, total hemoglobin and hematocrit percentage analyzed. Blood was also microscopically examined for segregates, polychromatin bodies, and smudge cells. A portion of liver, heart, kidney, spleen, intestine, pancreas and adrenal from each animal was formalin fixed and paraffin-embedded to examine toxicity-related changes in cell or organ morphology by H&E staining.
Statistical analysis
Statistical analysis was carried out using Prism 4.0 (GraphPad Software). One-way or Two-way Analysis Of Variance (ANOVA) was used for groupwise comparisons, followed by the Tukey’s or Bonferroni’s post hoc tests. All the data represented as ± S.E.M. Results were considered significant at a P value less than 0.05 (95% CI).
RESULTS
siRNA-mediated inhibition of Akt3 signaling reduced the tumorigenic potential of melanoma cells
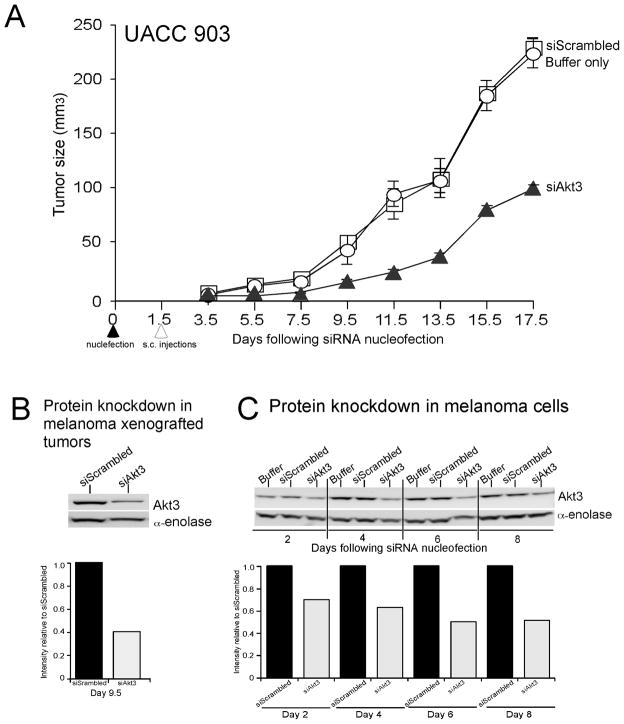
To confirm prior studies documenting the therapeutic potential of inhibiting Akt3 signaling in melanoma tumorigenesis, a siRNA-based approach was initially used to inhibit protein expression and thereby activity (6, 7, 15). UACC 903 cells were nucleofected with siRNA targeting Akt3, or a scrambled siRNA or a buffer control using the Amaxa nucleofection system. 36 h later, viable cells were subcutaneously injected in to nude mice and tumor development measured at every other day. Decreased expression (activity) of Akt3 reduced the tumorigenic potential of melanoma cells by ~60% (One Way ANOVA; P< 0.001) compared to control cells nucleofected with scrambled siRNA or nucleofection buffer (Fig. 1A). A tumor removed from animals at day 9.5 showed significantly less Akt3 protein than control (scrambled siRNA) tumors demonstrating effective knockdown of Akt3 protein expression (Fig. 1B). Duration of Akt3 protein knockdown following exposure to siRNA targeting Akt3 persisted up to 8-d in culture (Fig. 1C) as reported previously (6). Thus, targeting Akt3 signaling led to significant melanoma tumor inhibition, which has laid the foundation to search for pharmacological agents that could inhibit melanoma development by reducing the activity of this important signaling cascade involved in ~70% of sporadic melanomas (6).
Figure 1. siRNA-mediated inhibition of Akt3 expression/activity inhibits melanoma tumor development.
A. siRNA (100 pmoles) against Akt3 (▲), scrambled siRNA (△) or nucleofection buffer (○) were introduced into UACC 903 (1X106) melanoma cells and 36 h later viable cells were injected subcutaneously into nude mice. Result shows that reduced expression/activity of Akt3 decreases the tumorigenic potential of melanoma cells by ~60% compared to control cells nucleofected with scrambled siRNA or nucleofection buffer; error bars, S.E.M. B. Western blot and quantitation analysis of tumor protein lysates harvested 9.5 d after cells were nucleofected and subcutaneously injected into animals. Decreased Akt3 protein expression was observed in tumors confirming siRNA-mediated inhibition. α-enolase served as a control for protein loading. C. siRNA-mediated knockdown of Akt3 protein persists up to 8 d in cultured cells. One million UACC 903 cells were nucleofected with 100 pmoles of siAkt3 and replated in culture dishes, and protein lysates harvested 2, 4, 6, and 8 d later for Western blot analysis to determine Akt3 protein levels in cells. Decreased Akt3 protein expression compared to controls was observed up to 8 d after nucleofection into cells. α-enolase served as a control for protein loading on Western blots, whereas scrambled siRNA served as a control for RNA interference specificity.
Development of isothiocyanate analogs with longer alkyl chain lengths and selenium substituted for sulfur
In order to identify a pharmacological agent inhibiting Akt3 activity and melanoma cell survival, a natural product library was screened and isothiocyanates identified as possible candidates. However, the parent isothiocyanate compound had low potency in vivo requiring high concentrations for efficacy. Therefore, more potent analogs were created that could serve as therapeutic agents using the isothiocyanate backbone. The goal was to identify optimal carbon chain length for maximal tumor inhibition by comparing arylalkyl isothiocyanate compounds with increasing alkyl chain length and by replacing sulfur with selenium. Figure 2A shows the structures of isothiocyanates containing 1(benzyl), 2 (phenethyl), 4 (phenylbutyl) and 6 (phenylhexyl) carbon spacers. Corresponding isosteric selenium analogs are also shown in which sulfur was replaced with selenium. Finally, a 6 carbon selenocyanate, called phenylhexyl selenocyanate (PHSC), was created to serve as a control to show that selenium alone was not accounting for inhibition but rather that the structure of the compound containing selenium was critical for tumor reduction (Fig. 2A).
Figure 2. Characterization of isothiocyanates and isoselenocyanates as inhibitors of melanoma.
A. Structures of isothiocyanates and selenium containing isoselenocyanates. Chemical structures of isothiocyanates containing 1(benzyl), 2 (phenethyl), 4 (phenylbutyl) and 6 (phenylhexyl) carbon spacers and corresponding isosteric selenium analogs in which sulfur was replaced with selenium. A 6-carbon selenocyanate (phenylhexyl selenocyanate, PHSC), served as a control to show compound structure and not selenium caused the inhibitory effect. B and C. Comparison of melanoma cell survival following exposure to isothiocyanates versus isoselenocyanates. Cell viability was measured using the MTS assay and IC50 (μM) values plotted against carbon chain length. 5X103 melanoma cells were plated in 96-well plates and allowed to attach for 24 h. Increasing concentrations of isothiocyanates and selenium analogs were added in culture medium. Values represent averages of percentage of control DMSO treated cells. API-2 and PHSC served as an Akt inhibitor and selenium control respectively. Results show that ISC-4 and ISC-6 were the most effective inhibitors. D. ISC-4 kills melanoma cells at 2–4 fold lower concentrations than normal cells. 5X103 normal human fibroblasts (FF2441) or metastatic melanoma cells (UACC 903) were plated in 96-well plates in 100 μl DMEM media containing 10% FBS and grown for 72 and 36 h respectively. Exponentially growing cells were treated with increasing concentrations (2–100 μM) of ISC-4 or PBITC for 12 and 24 h and IC50 (μM) values determined. ISC-4 was found to consistently inhibit melanoma cells growth at concentrations 2–4 fold lower than fibroblast cells (One way ANOVA ***P<0.001); error bars, S.E.M.
Isoselenocyanates are more effective at inhibiting cultured melanoma cells than isothiocyanates
Initially, the MTS assay was used to quantify viable cells of three human melanoma cell lines (UACC 903, 1205 Lu and WM115) following treatment with increasing concentrations of each agent to measure the IC50 of respective compounds. Figure 2B shows a representative example of this analysis where inhibitory effectiveness of the 4 carbon PBITC and ISC-4 as well as 6 carbon PHITC and ISC-6 were compared to DMSO vehicle, Akt inhibitor API-2 (1,5-Dihydro-5-methyl-1-b-D-ribofuranosyl-1,4,5,6,8-penta azaacenaphthylen-3-amine) (7, 32) or control PHSC. Selenium containing ISC-4 and ISC-6 were more effective at inhibiting growth of melanoma cells than sulfur containing PBITC, PHITC, control PHSC or API-2. Figure 2C shows a detailed comparison where the IC50 of 1, 2, 4 or 6 carbon isothiocyanates are compared to selenium containing analogs in three independently derived melanoma cell lines, UACC 903, 1205 Lu and WM115. A general trend was observed in which increasing carbon chain length and substitution of selenium for sulfur decreased the IC50 for all cell lines but the differences were subtle. Increased potency ranged from 30–70% with increasing chain length and/or sulfur substituted for selenium. Thus, isothiocyanate analogs with longer alkyl chain lengths and sulfur substituted for selenium had increased killing efficiency for cultured melanoma cells.
Isoselenocyanates inhibits melanoma cell growth more effectively than normal cells
Sensitivity of melanoma and normal cells to PBITC or ISC-4 was compared to determine whether cancer cells were more sensitive to the compounds. Normal human fibroblast, FF2441, and melanoma (UACC 903) cells were treated with 2–100 μM of PBITC or ISC-4 and IC50 measured at 12 and 24 h (Fig. 2D). Consistently, 2–4-fold higher drug concentrations were required to kill fibroblasts compared to melanoma cells (Fig. 2D). Thus, cultured cancer cells are more sensitive to PBITC or ISC-4 than normal cells.
Isoselenocyanates have increased in vivo potency compared to corresponding isothiocyanates and effectively reduce melanoma development
Effectiveness of isoselenocyanates for inhibiting the growth of pre-existing tumors was evaluated in nude mice. UACC 903 melanoma cells having high Akt3 signaling activity were injected subcutaneously and 6 days later when a vascularized tumor had developed, mice were injected intraperitoneally with 2.5 μmoles of each isothiocyanate or 0.76 μmoles of isoselenocyanate (Fig. 3A). While 3-fold less isoselenocyanate was administered, ~50% tumor inhibition was observed at day 24, which indicates enhanced tumor inhibitory effectiveness of selenium containing analogs. Increasing carbon chain length of isothiocyanates seemed to be less effective at tumor inhibition (Fig. 3A; left panel). In contrast, increasing carbon chain length of isoselenocyanates was associated with more effective tumor inhibition (P<0.001, One-way ANOVA, ISC-1 vs. ISC-2, ISC-4, ISC-6) (Fig. 3A; right panel). Thus, PBITC and PHITC reduced tumor development but at concentrations 3-fold higher than corresponding isoselenocyanates. Therefore, 4–6 carbon chain isoselenocyanates appeared to be the most robust inhibitors of melanoma tumorigenesis. Based on these findings subsequent studies focused on comparing ISC-4 and ISC-6 to PBITC and PHITC, respectively.
Figure 3. Isoselenocyanates are effective inhibitors of melanoma tumor development.
A. Isoselenocyanates inhibit melanoma development at concentrations 3-fold less than corresponding isothiocyanates. Effect of isothiocyanates and isoselenocyanates on melanoma tumor development was measured by subcutaneous injection of 5 million UACC 903 cells and after 6-d when small vascularized palpable tumors were observed, mice were treated i. p. with isothiocyanates (2.5 μmoles) or isoselenocyanates (0.76 μmoles, equivalent to 3 ppm selenium) 3 times per week. Bar graph represents means of melanoma tumor volume (n=10) as the percentage of vehicle treated tumors at d 24; error bars, S.E.M. Results show that similar tumor inhibition required 3-fold less isoselenocynate than isothiocyante compound. B and C. Isoselenocyanates decrease melanoma tumor development by 50–60% compared to corresponding isothiocyanates at similar concentrations. Effect of isoselenocyanates on tumor development was measured by subcutaneous injection of 2.5 or 5 million1205 Lu or UACC 903 melanoma cells, respectively, and after 6-d, mice were treated i. p. 3 times per week with ISC-4 or ISC-6 (0.76 μmoles, equivalent to 3 ppm selenium) and compared to animal treated with PBITC or PHITC (0.76 μmoles), respectively; error bars, S.E.M. ISC-4 and ISC-6 reduced tumor development by 50–60% compared to PBITC, PHITC or DMSO control treated mice.
UACC 903 and 1205 Lu melanoma cells having high Akt3 signaling activity were injected subcutaneously and following 6 days when tumor angiogenesis had occurred, mice were exposed to 0.76 μmoles representative isothiocyanate compound PBITC versus ISC-4 or PHITC versus ISC-6, 3 times per week and tumor development measured (Figs. 3B & 3C). Animals were also weighed to ascertain possible toxicity. While PBITC and PHITC are ineffective at reducing tumor burden of UACC 903 (Fig. 3B) or 1205 Lu (Fig. 3C) at this concentration, ISC-4 and ISC-6 led to significant (P<0.001; Two-way ANOVA) reductions in tumor size beginning from day 13 for UACC 903 cells or from day 10 for 1205 Lu cells. Thus, isoselenocyanates are effective at reducing melanoma tumor development by 50–60% at significantly lower concentrations than corresponding isothiocyanates, which is similar to siRNA mediated inhibition of Akt3 (Fig. 1A).
Synthetic isoselenocyanate compounds causes negligible organ related toxicity following systemic administration
Systemic toxicity of PBITC, PHITC, ISC-4 or ISC-6 administration was evaluated in nude mice. Body weights of mice treated with isothiocyanate or isoselenocyanate compounds compared to control DMSO vehicle showed no significant differences between groups (Fig. 4A). Furthermore, blood parameters (SGOT, SGPT, alkaline phosphatase, blood urea, glucose and creatinine) indicative of systemic toxicity did not detect significant liver, kidney or cardiac related toxicity (Fig. 4B). Levels of cellular metabolites basal urea nitrogen (BUN), creatinine and glucose in animals were also not significantly different between ISC-4 or PBITC treated and control animals. Histological examination of hematoxylin and eosin stained vital organ sections, including the liver (Fig. 4C), revealed that ISC-4 treatment did not significantly alter cell morphology or structure of kidney, adrenal, lung, spleen, heart, pancreatic or intestinal tissue (data not shown). Similar results were observed following treatment with ISC-6 in animals (data not shown). Thus, treatment using synthetic selenium containing analogs of isothiocyanate ISC-4 or ISC-6 led to negligible associated systemic toxicity with significant therapeutic potential.
Figure 4. No major organ related toxicity was associated with isoselenocyanate treatment.
A. Body weight of animals was measured at the time of treatment to ascertain weight related toxicity in animals. No significant changes in body weight were observed, suggesting negligible systemic toxicity. B. Levels of SGOT, SGPT, alkaline phosphatase, glucose and creatinine were analyzed in blood collected from animals treated with PBITC, ISC-4 or DMSO vehicle. No significant differences were observed, indicating negligible vital organ related toxicity, error bars, S.E.M. C. Histological analysis of liver tissue shows no liver associated toxicity following isoselenocyanate treatment. H&E stained sections (400X) of liver tissue showing no significant differences in liver histology that would be indicative of damage caused following isoselenocyanate treatment.
Isoselenocyanates decreased Akt3 signaling in cultured melanoma cells and tumors
Cells were next treated with isoselenocyanates ISC-4 and ISC-6 and effect on Akt3 signaling examined by Western blotting. Both compounds inhibited Akt3 signaling as demonstrated through decreased pAkt and downstream pPRAS40 levels (Fig. 5A). However, ISC-4 and ISC-6 were effective at lower concentrations, completely inhibiting the pathway at ~10 μM compared to corresponding isothiocyanates requiring ≥15 μM for similar inhibition (Fig. 5A). As reported previously, Akt3 pathway inhibition led to apoptosis, which was indicated by high levels of cleaved PARP (Fig. 5A) (7). Higher cleaved PARP levels were observed at lower concentrations of ISC-4 than corresponding isothiocyanate PBITC suggesting isoselenocyanate compounds were more effective than corresponding sulfur containing isothiocyanates. Similar Akt pathway inhibition by ISC-4 or ISC-6 (not shown) also occurred for human melanoma cell lines 1205 Lu and WM115 (Fig. 5B).
Figure 5. Inhibition of Akt3 signaling mediated by isoselenocyanates induces apoptosis in melanoma cells.
A. Western blot analysis of cells treated with ISC-4 or ISC-6 show decreased Akt3 signaling. Melanoma cells (UACC 903,1205 Lu or WM115) were exposed for 24 h to increasing concentrations (2.5 – 15 μM) of ISC-4 or ISC-6 and compared to PBITC, PHITC, or DMSO. Western blot analysis measuring activity of the Akt3 signaling pathway shows dose dependent decrease in pAkt (S473), downstream pPRAS40 (T246) and increase in cleaved PARP, indicating increased cellular apoptosis. Erk2 served as control for equal protein loading. B. ISC-4 decreases Akt3 singling in 1205Lu and WM115 melanoma cell lines. Western blot showing effect of ISC-4 treatment on Akt3 activity in 1205 Lu and WM115 cells. ISC-4 decreases pAkt levels and increases cellular apoptosis levels as indicated by elevated cleaved PARP protein. Erk2 served as a control for equal protein loading. C. ISC-4 decreases pAkt and downstream pPRAS40 levels in tumors. Densitometric quantitation of Western blot analysis of tumor protein lysates from animals treated with PBITC or ISC-4 and compared to DMSO vehicle treatment shows decreased relative expression of pAkt and downstream pPRAS40 normalized against α-enolase indicating decreased Akt3 signaling in tumors following treatment (***P<0.001; * P<0.05); error bars, S.E.M.
Western blot analysis of size and time matched tumors harvested at day 13 from animals treated with DMSO, PBIT or ISC-4 also showed significantly decreased phosphorylated (active) Akt (P<0.05; One-way ANOVA) and downstream PRAS40 (P<0.001; One-way ANOVA) in ISC-4 tumor lysate’s compared to DMSO control or PBITC treated tumors (Fig. 5C). Thus, isoselenocyanates ISC-4 and ISC-6 were more robust inhibitors of the Akt3 signaling cascade in cultured melanoma cells as well as in xenografted melanoma tumors than corresponding sulfur containing isothiocyanates.
Isoselenocyanates induced apoptosis in cultured melanoma cells as well as in xenografted melanoma tumors
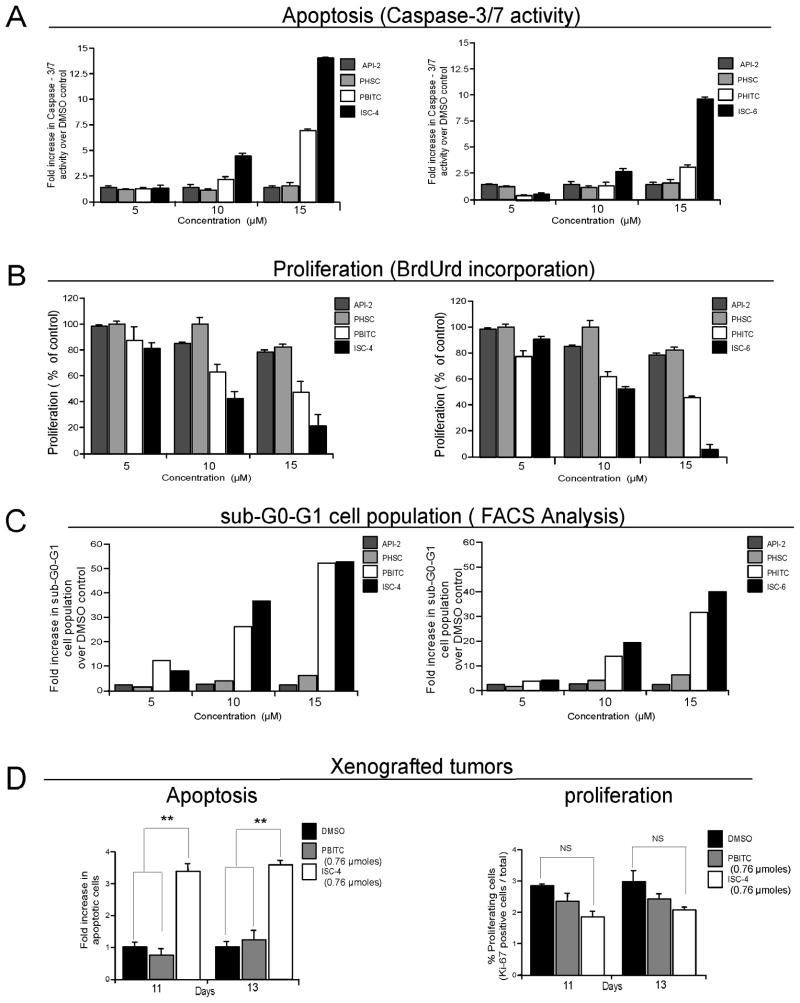
To identify the underlying mechanism by which isothiocyanates or isoselenocyanates inhibited melanoma cell survival, rates of apoptosis and proliferation were examined following treatment. In contrast to API-2 or PHSC, increasing concentrations of PBITC, ISC-4, PHITC or ISC-6 led to increased cellular apoptosis (Fig. 6A) and decreased proliferative potential (Fig. 6B) of UACC 903 melanoma cells. ISC-4 or ISC-6 was ~2-fold more effective than PBITC or PHITC at inducing apoptosis and inhibiting cellular proliferation (Figs. 6A & 6B). Cell cycle analysis of asynchronously growing UACC 903 cells showed a significant increase in the sub-G0-G1 population in PBITC or ISC-4 and PHITC or ISC-6 treated cells compared to controls (Fig. 6C), which is indicative of cellular apoptosis. Analysis of cells in each stage of the cell cycle, in Supplementary Table, showed a 30–40% decrease in the G0-G1 phase cells with a 50–60% increase in the G2-M phase cell population. Marginal changes were observed in S-phase cells. The most significant change was an ~15-fold increase in the sub-G0-G1 cell population indicating a dramatic increase in cellular apoptosis.
Figure 6. Isoselenocyanates increase cellular apoptosis in cultured cells and melanoma tumors.
A. Isoselenocyanates induce apoptosis in cultured melanoma cells. Levels of caspase-3/7 activity in cultured melanoma cells exposed to ISC-4 or ISC-6 were compared to PBITC, PHITC, API-2 and PHSC using the Apo-ONE homogeneous caspase-3/7 assay kit. Results show significant dose dependent increases in caspase-3/7 activity relative to DMSO vehicle treated cells. Results represent average of 3 independent experiments. B. Isoselenocyanates reduces proliferation of cultured melanoma cells. Proliferating UACC 903 cells, using a BrdUrd ELISA kit, were measured following 24 h treatment with 5–15 μM ISC-4 or ISC-6 and compared to PBITC, PHITC, API-2 and PHSC. Results show a dose dependent decrease in proliferating cells with maximal inhibition occurring following isoselenocyanate treatment. Values represent the average of the percentage of control DMSO treated cells from 3 independent experiments. C. Isoselenocyanates increase sub-G0-G1 cell population indicating increased cellular apoptosis. Asynchronously growing UACC 903 cells were treated with ISC-4, ISC-6, PBITC, PHITC, API-2 or PHSC, and 24 h later, cells stained with propidium iodide analyzed for cell cycle distribution using a FACScan analyzer. ISC-4 or ISC-6 treatment significantly increased the sub G0-G1 cell population indicating apoptotic cells. Results represent average of 2 independent experiments. D. Isoselenocyanate treatment increases levels of cellular apoptosis in melanoma tumors. Rates of apoptosis and proliferation in size and time matched tumors from mice treated i. p. with ISC-4 (3 ppm equivalent to 0.76 μmoles), starting 6 d after subcutaneous injection of cells and on alternate days thereafter up to d 13, were compared to mice treated with PBITC (0.76 μmoles) or DMSO (50 μl). Results show a 3-fold increase in number of apoptotic cells following treatment of UACC 903 tumors with ISC-4 at d 11 and 13 compared to PBITC or DMSO control. No statistically significant difference was observed in proliferation rate. Values represent means from 2 separate experiments with 4–6 fields analyzed from each of 6 tumors per experiment. (** P<0.01; NS, non significant); error bars, S.E.M.
To confirm that a similar mechanism led to tumor inhibition in animals following ISC-4 treatment, rates of apoptosis (TUNEL staining) and proliferation (Ki-67 immunohistochemistry) were compared in size and time matched melanoma tumors from ISC-4 or PBITC treated animals and compared to DMSO vehicle. Tumors harvested at day 11 and 13 from mice treated with ISC-4, showed ~3-fold (Fig. 6D, right panel, P< 0.01; One-way ANOVA) more TUNEL positive cells compared to control animals treated with DMSO or PBITC. Slightly fewer proliferating tumor cells were observed in ISC-4 treated tumors compared to PBITC, but this difference was not statistically significant (Fig. 6D, left panel, P> 0.05; One-way ANOVA). Thus, the superior anti-melanoma activity of ISC-4 relative to PBITC appears primarily to be due to an effect on tumor cell apoptosis rather than on cellular proliferation, which is consistent with effects observed following treatment of cultured cells (Fig. 5A & Supplementary Table).
DISCUSSION
For several decades, no substantial progress has been made in developing drugs effective for the long-term survival of patient’s with advanced-stage melanoma (33). Current systemic therapies for metastatic disease still achieve only a modest ~20% overall response rates, and duration of efficacy is typically months and not years (1, 33). The median progression-free survival following initiation of systemic therapy for stage IV melanoma is typically about 1.7 months, and the median survival is 6.2 months (34). Clearly, a pipeline of novel, more effective therapeutics is needed to increase the long-term survival of metastatic melanoma patients, which is the goal of this report.
Targeted agents that inhibit the activity of aberrant melanoma causing genes have potential to significantly increase patient survival (1, 9, 11). Agents of this type have been shown to be effective, for example: imatinib targeting receptor-type KIT tyrosine kinase and BCR-ABL tyrosine kinase in chronic myelogenous leukemia and gastrointestinal stromal tumors; bevacizumab targeting vascular endothelial growth factor (VEGF) in colorectal cancer; sunitinib targeting VEGF receptors, FMS-like tyrosine kinase 3, c-KIT & platelet-derived growth factor in renal cell carcinoma; and sorafenib targeting Raf kinases in renal cell as well as hepatocellular carcinomas (35–39). Thus, it is reasonable to assume that agents could be developed to inhibit proteins deregulated during melanoma development that would be more effective than currently available drugs for treating this disease.
The Akt3 pathway is an important pathway deregulated in ~70% of melanomas, important to therapeutically target alone or in combination with other targeted agents (6–8). A second key pathway is that of the MAP kinase signaling cascade, which is constitutively activated through Ras mutations in 10–15% and B-Raf mutations in ~60% of melanomas (40, 41). A T to A mutation at nucleotide 1799 of B-Raf, leads to substitution of a valine for a glutamic acid at codon 600 (V600E) in exon 15 in the vast majority of melanomas in which B-Raf is mutated (41). This alteration is acquired during development of sporadic melanomas and not inherited (41). Since B-Raf is the most mutated gene in melanomas, it is an attractive therapeutic target (40). Sorafenib, which was identified as a Raf kinase inhibitor, was initially hoped to be effective for treating melanoma (10, 40). However, off-target effects have limited its efficacy for treating melanoma. Sorafenib inhibits Raf, but it also decreases activity of VEGFR1, VEGFR2, VEGFR3, PDGFR Beta, Flt-3, p38, c-Kit and FGFR1 (42). Therefore, while it targets constitutively active mutant V600EB-Raf present in ~60% of metastatic melanomas, it is ineffective because its primary mechanism of action is as an angiogenesis inhibitor and not as a regulator of cellular proliferation, which is required for effective melanoma inhibition when targeting this aberrant signaling cascade (9, 10, 43). More specific B-Raf inhibitors are being developed and evaluated to circumvent these limitations but clinical efficacy is currently unknown (11).
Inhibitors of the Akt3 pathway were developed in this report by initially screening a natural product library for candidate anti-neoplastic compounds that inhibited this signaling cascade. The screen was based on reports showing that targeting Akt3 signaling significantly reduced the tumorigenic potential of melanoma cells (6, 7, 15). Naturally occurring isothiocyanates were identified as potential inhibitors of Akt3 signaling. Numerous naturally occurring compounds exhibiting anti-neoplastic properties are being exploited as potential chemotherapeutic agents (44, 45). Some are well established components of standard systemic chemotherapeutic regimens, such as the taxanes, vinca alkaloids, and camptothecins (44, 46, 47). However, no successful agent or combination of agents has been identified that dramatically extends melanoma patient survival (33). Thus, naturally occurring agents targeting key signaling pathways, such as Akt3, could be important breakthroughs for more effective melanoma therapies.
While naturally occurring isothiocyanates were found to inhibit Akt3 signaling, impractical quantities were required for melanoma anti-tumor activity. Therefore, replacing the sulfur group with selenium and lengthening the carbon chains was evaluated to enhance potency as therapeutic agents. The resulting family of compounds, called isoselenocyanates, had greater efficacy killing cultured cells as well as inhibiting tumor development in animals compared to sulfur containing isothiocyanates. Two isoselenocyanates, ISC-4 and ISC-6, had particularly robust anti-melanoma activity with enhanced potency due primarily to enhanced tumor cell apoptosis following treatment. Thus, isoselenocyantes represents a significant development in the natural product drug pipeline by targeting a key signaling cascade deregulated in ~70% of melanomas.
Incorporating selenium into the structure of isoselenocyanates is a further significant development. Selenium plays a role in cancer chemoprevention but the exact mechanistic basis for inhibition remains to be identified (28, 29, 48, 49). Several clinical trials are examining the role of selenium in the prevention of colorectal cancer (NCT00078897), breast cancer (NCT00555386), lung cancer (NCT00008385), and bladder cancer (NCT00553345). Furthermore, incorporating selenium into the structure of drugs, as in the case of isoselenocyanates, can increased compound potency making ineffective agents better therapeutics. Recently, a selenium containing analog of the iNOS inhibitor PBIT, called PBISe, which is ineffective at killing melanoma cells was made >10-fold more potent by incorporating selenium into its structure to more effectively decrease melanoma tumor development in animals (50). Thus, incorporating selenium into the structure of cancer therapeutics is one feasible approach to increase the tumor inhibitory efficacy of therapeutic agents.
Since control compounds containing selenium had little effect on melanoma cell survival, isoselenocyanate-mediated inhibition of melanoma is independent of selenium, suggesting that the structure of the compound in combination with selenium is necessary for enhanced inhibitory activity. Compared to the chemopreventive effects of natural selenium enriched products, isoselenocyanates are unique in that the selenium containing compounds target Akt3 signaling in melanoma to promote apoptosis, block the growth of tumors, and are associated with negligible toxicity at biologically effective doses. Therefore, isoselenocyanates represent a promising adjunct to rational, targeted, single- or perhaps multi-agent therapy for advanced melanoma.
Supplementary Material
Acknowledgments
Grant support: This work was supported by The American Cancer Society [RSG-04- 053-01-GMC to G.P.R.]; National Institutes of Health [CA-127892-01A to G.P.R.]; National Institute of Health and National Cancer Institute contract [NO2-CB-56603 to S.A], The Foreman Foundation for Melanoma Research to G.P.R.; Elsa U. Pardee Foundation to A.S.; and Melanoma Research Foundation to A.S.
We thank Dr. Raghvendra Gowda for providing technical assistance.
Footnotes
Sharma AK, Sharma A, Desai D, Madhunapantula SV, Huh SJ, Robertson GP, Amin S. Synthesis and anticancer activity comparison of isoselenocyanates with isothiocyanates present in cruciferous vegetables (submitted)
STATEMENT OF TRANSLATIONAL RELEVANCE
This manuscript is clinically relevant since melanoma is the most invasive and deadly form of skin cancer with few agents available to treat advanced metastatic disease. Thus, research scientists are vigorously searching for new therapeutic agents targeting pathways important in melanoma to treat the disease. One gene involved in ~70% of sporadic melanomas is AKT3, promoting tumorigenesis by decreasing apoptosis. Here we detail identification of naturally occurring isothiocyanates, present in cruciferous vegetables, as inhibitors of the Akt3 pathway in melanoma. However, low potency requiring high concentrations for therapeutic efficacy made them unsuitable therapeutics. Therefore, more potent analogs have been developed using the isothiocyanate backbone but increasing the alkyl chain length and replacing sulfur with selenium to create compounds called isoselenocyanates. Isoselenocyanates decreased Akt3 signaling in cultured melanoma cells and tumors to significantly reduce melanoma tumor development without changes in animal body weight or in blood parameters indicative of liver, kidney or cardiac related toxicity.
References
- 1.Katipamula R, Markovic SN. Emerging therapies for melanoma. Expert Rev Anticancer Ther. 2008;8:553–60. doi: 10.1586/14737140.8.4.553. [DOI] [PubMed] [Google Scholar]
- 2.McDermott DF, Sosman JA, Gonzalez R, et al. Double-Blind Randomized Phase II Study of the Combination of Sorafenib and Dacarbazine in Patients With Advanced Melanoma: A Report From the 11715 Study Group. J Clin Oncol. 2008;26:2178–85. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 3.Helmbach H, Rossmann E, Kern MA, Schadendorf D. Drug-resistance in human melanoma. International Journal of Cancer. 2001;93:617–22. doi: 10.1002/ijc.1378. [DOI] [PubMed] [Google Scholar]
- 4.Markovic SN, Erickson LA, Rao RD, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364–80. doi: 10.4065/82.3.364. [DOI] [PubMed] [Google Scholar]
- 5.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–7. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 6.Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. doi: 10.1158/0008-5472.CAN-04-1399. [DOI] [PubMed] [Google Scholar]
- 7.Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res. 2007;67:3626–36. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- 8.Robertson GP. Functional and therapeutic significance of Akt deregulation in malignant melanoma. Cancer Metastasis Rev. 2005;24:273–85. doi: 10.1007/s10555-005-1577-9. [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, Tran MA, Liang S, et al. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–9. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 11.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–64. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 14.Stahl JM, Cheung M, Sharma A, Trivedi NR, Shanmugam S, Robertson GP. Loss of PTEN promotes tumor development in malignant melanoma. Cancer Res. 2003;63:2881–90. [PubMed] [Google Scholar]
- 15.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–39. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–50. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht SS. Chemoprevention by isothiocyanates. J Cell Biochem Suppl. 1995;22:195–209. doi: 10.1002/jcb.240590825. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Yao S, Li J. Vegetable-derived isothiocyanates: anti-proliferative activity and mechanism of action. Proc Nutr Soc. 2006;65:68–75. doi: 10.1079/pns2005475. [DOI] [PubMed] [Google Scholar]
- 19.El-Bayoumy K, Sinha R, Pinto JT, Rivlin RS. Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. J Nutr. 2006;136:864S–9S. doi: 10.1093/jn/136.3.864S. [DOI] [PubMed] [Google Scholar]
- 20.Miyoshi N, Uchida K, Osawa T, Nakamura Y. A link between benzyl isothiocyanate-induced cell cycle arrest and apoptosis: involvement of mitogen-activated protein kinases in the Bcl-2 phosphorylation. Cancer Res. 2004;64:2134–42. doi: 10.1158/0008-5472.can-03-2296. [DOI] [PubMed] [Google Scholar]
- 21.Chiao JW, Wu H, Ramaswamy G, et al. Ingestion of an isothiocyanate metabolite from cruciferous vegetables inhibits growth of human prostate cancer cell xenografts by apoptosis and cell cycle arrest. Carcinogenesis. 2004;25:1403–8. doi: 10.1093/carcin/bgh136. [DOI] [PubMed] [Google Scholar]
- 22.Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y. Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–90. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Bandura L, Drukala J, Wolnicka-Glubisz A, Bjornstedt M, Korohoda W. Differential effects of selenite and selenate on human melanocytes, keratinocytes, and melanoma cells. Biochem Cell Biol. 2005;83:196–211. doi: 10.1139/o04-130. [DOI] [PubMed] [Google Scholar]
- 25.Brigelius-Flohe R. Selenium compounds and selenoproteins in cancer. Chem Biodivers. 2008;5:389–95. doi: 10.1002/cbdv.200890039. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Jiang C, Li G, Lu J. PKB/AKT and ERK regulation of caspase-mediated apoptosis by methylseleninic acid in LNCaP prostate cancer cells. Carcinogenesis. 2005;26:1374–81. doi: 10.1093/carcin/bgi094. [DOI] [PubMed] [Google Scholar]
- 27.Reinhold U, Biltz H, Bayer W, Schmidt KH. Serum selenium levels in patients with malignant melanoma. Acta Derm Venereol. 1989;69:132–6. [PubMed] [Google Scholar]
- 28.Lee JH, Shin SH, Kang S, Lee YS, Bae S. A novel activation-induced suicidal degradation mechanism for Akt by selenium. Int J Mol Med. 2008;21:91–7. [PubMed] [Google Scholar]
- 29.Wu Y, Zu K, Warren MA, Wallace PK, Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–52. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- 30.Morse MA, Eklind KI, Hecht SS, et al. Structure-activity relationships for inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone lung tumorigenesis by arylalkyl isothiocyanates in A/J mice. Cancer Res. 1991;51:1846–50. [PubMed] [Google Scholar]
- 31.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–93. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Dan HC, Sun M, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–9. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 33.Lui P, Cashin R, Machado M, Hemels M, Corey-Lisle PK, Einarson TR. Treatments for metastatic melanoma: synthesis of evidence from randomized trials. Cancer Treat Rev. 2007;33:665–80. doi: 10.1016/j.ctrv.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 35.Mauro MJ, O’Dwyer M, Heinrich MC, Druker BJ. STI571: a paradigm of new agents for cancer therapeutics. J Clin Oncol. 2002;20:325–34. doi: 10.1200/JCO.2002.20.1.325. [DOI] [PubMed] [Google Scholar]
- 36.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 37.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 38.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 39.Zhu AX. Development of sorafenib and other molecularly targeted agents in hepatocellular carcinoma. Cancer. 2008;112:250–9. doi: 10.1002/cncr.23175. [DOI] [PubMed] [Google Scholar]
- 40.Madhunapantula SV, Robertson GP. Is B-Raf a good therapeutic target for melanoma and other malignancies? Cancer Res. 2008;68:5–8. doi: 10.1158/0008-5472.CAN-07-2038. [DOI] [PubMed] [Google Scholar]
- 41.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 42.Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 43.Eisen T, Ahmad T, Flaherty KT, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer. 2006;95:581–6. doi: 10.1038/sj.bjc.6603291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 45.Gullo VP, McAlpine J, Lam KS, Baker D, Petersen F. Drug discovery from natural products. J Ind Microbiol Biotechnol. 2006;33:523–31. doi: 10.1007/s10295-006-0107-2. [DOI] [PubMed] [Google Scholar]
- 46.Efferth T, Li PC, Konkimalla VS, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353–61. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 47.Gallagher BM., Jr Microtubule-stabilizing natural products as promising cancer therapeutics. Curr Med Chem. 2007;14:2959–67. doi: 10.2174/092986707782794014. [DOI] [PubMed] [Google Scholar]
- 48.Chen KM, Spratt TE, Stanley BA, et al. Inhibition of nuclear factor-kappaB DNA binding by organoselenocyanates through covalent modification of the p50 subunit. Cancer Res. 2007;67:10475–83. doi: 10.1158/0008-5472.CAN-07-2510. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka T, Kohno H, Murakami M, Kagami S, El-Bayoumy K. Suppressing effects of dietary supplementation of the organoselenium 1,4-phenylenebis(methylene)selenocyanate and the Citrus antioxidant auraptene on lung metastasis of melanoma cells in mice. Cancer Res. 2000;60:3713–6. [PubMed] [Google Scholar]
- 50.Madhunapantula SV, Desai D, Sharma A, Huh S, Amin S, Robertson GP. PBIse, a novel selenium containing drug for the treatment of malignant melanoma. Mol Cancer Ther. 2008;7:1297–308. doi: 10.1158/1535-7163.MCT-07-2267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.