Abstract
The ability of tumor cells to adhere to, migrate on and remodel extracellular matrices is mediated by cell surface receptors such as β1-integrins. Here, we conducted functional live-cell imaging in real-time to investigate the effects of modulating β1-integrin expression and function on proteolytic remodeling of the extracellular matrix. Human breast and prostate cancer cells were grown on reconstituted basement membrane containing a quenched fluorescent form of collagen IV. Generation of cleavage products and the resulting increases in fluorescence were imaged and quantified. Decreases in the expression and activity of β1-integrin reduced digestion of quenched fluorescent-collagen IV by the breast and prostate cancer cells and correspondingly their invasion through and migration on reconstituted basement membrane. Decreased extracellular matrix degradation also was associated with changes in constituents of proteolytic pathways: decreases in secretion of the cysteine protease cathepsin B, the matrix metalloproteinase-13 and tissue inhibitors of metalloproteinases-1 and -2; a decrease in expression of matrix metalloproteinase-14 or membrane type-1 matrix metalloproteinase; and an increase in secretion of tissue inhibitor of metalloproteinases-3. This is the first study to demonstrate through functional live-cell imaging that downregulation of β1-integrin expression and function reduces proteolysis of collagen IV by breast and prostate cancer cells.
Keywords: β1-integrin, cancer, proteases, proteolysis, functional imaging
Introduction
Integrins, including the β1-integrins, have been linked to tumor progression and the remodeling of extracellular matrix (ECM) associated with this progression [for review, see [1–4]]. Alterations in expression and function of β1-integrin occur during prostate cancer progression [for review see [5, 6]]. For example, an invasive phenotype is associated with a shift to expression of α6β1 [7]. In human breast carcinomas, expression of β1-integrin has been reported to be unchanged [8], but also to be increased and predictive of poor survival [9]. In vitro, a function-blocking antibody to β1-integrin reverses the malignant phenotype of breast cancer cells grown in three-dimensional (3D) cultures [10–12]. In vivo, the antibody-treated tumor cells form fewer and smaller tumors upon subcutaneous injection into nude mice [10]. In another in vivo study, antibody-treated breast cancer cells injected into the tail vein of nude mice formed fewer lung metastases [13]. Further evidence that β1-integrin is critical for proliferation of breast tumor cells has been shown by its targeted disruption in the mammary epithelium of mice predisposed to develop mammary carcinomas [14]. The ability of the mammary epithelial cells to proliferate both in vitro and in vivo is compromised, as is the initiation of tumorigenesis. Thus, β1-integrin functions in the maintenance of a polarized cellular architecture and cell proliferation.
Physical associations between β1-integrin and several classes of proteases, endogenous inhibitors of those proteases or protease binding partners have been identified, yet a mechanism for β1-integrin involvement in the proteolytic remodeling of ECM has not been established. β1-integrin forms a stable complex with the urokinase plasminogen activator receptor (uPAR), linking β1-integrin to serine proteases [15]. Downregulation of uPAR in colon cancer cells disrupts the uPAR:β1-integrin complex without changing expression of β1-integrin [16]. Thus, the associated reduction in degradation of radiolabeled collagen IV is not directly linked to β1-integrin. On the other hand, β1-integrin may play a direct role in adhesion: maspin, a putative serine protease inhibitor and tumor suppressor, associates with β1-integrin in mammary epithelial cells where its function is not inhibition, but rather regulation of adhesion to ECM secreted by these cells [17]. β1-integrin is also linked to matrix metalloproteinases (MMPs) via interactions with tissue inhibitors of metalloproteinases (TIMPs) and with TIMPs through tetraspanins. These interactions play roles in cell survival, apoptosis, angiogenesis, but are independent of MMP activity [18]. Direct interactions between cysteine proteases of the cysteine cathepsin family and β1-integrin have not been identified [for review, see [19]]; however, cysteine proteases, β1-integrin and tetraspanins are present in the same membrane microdomains. Furthermore, these microdomains also contain both serine proteases and MMPs, suggesting that cell-surface proteolytic pathways may be initiated from these membrane regions. In colon cancer cells, for example, cathepsin B has been localized to caveolar lipid rafts through its binding to S100A10, the light chain of the annexin II heterotetramer [20]. Downregulation of caveolin-1 reduces association of cathepsin B, S100A10, uPA, uPAR and β1-integrin with lipid raft fractions of these cells [21]. Furthermore, there is a reduction in invasion through reconstituted basement membrane (rBM) and in degradation of collagen IV. A direct role for β1-integrin in degradation of collagen IV has not been demonstrated.
In the present study, we performed functional live-cell imaging in real-time and used β1-integrin short hairpin RNA interference (shRNAi) and β1-integrin function-blocking antibodies to determine whether β1-integrins play a role in the degradation of ECM by breast and prostate carcinoma cells. We demonstrate that downregulating β1-integrin expression and function significantly reduces degradation of collagen IV as well as invasion and migration of the breast and prostate carcinoma cells. We further show that there are parallel changes in multiple constituents of proteolytic pathways that have been implicated in ECM degradation, cell migration and invasion.
Materials and Methods
Cell culture
BT-549 human breast and DU145 human prostate carcinoma cells were purchased from American Type Culture Collection (Rockville, MD) and cultured in RPMI 1640 supplemented with 10% FBS and Dulbecco’s minimal essential medium (DMEM) supplemented with 10% FBS, respectively (Sigma).
Construction of β1-integrin shRNA
The plasmid to transcribe β1-integrin shRNA contained the sequence for top strand (sense), 5'-GATCCAGCTTCTCTGCTGTTCCTTCTCAAGAGAAAGGAACAGCAGAGAAGCTCATTTTTTGGAAA-3' and for bottom strand (antisense), 5'-AGCTTTTCCAAAAAATGAGCTTCTCTGTTCCTTTCTCTTGAGAAGGAACAGCAGAGAAGCTG-3' (Integrated DNA Technologies). 2 µl of each oligonucleotide solution (1 µg/µl) were mixed in 46 µl 1XDNA annealing solution. The mixture was heated to 90 °C for 5 min, then cooled to 37 °C and incubated at room temperature for 1 hr. The annealed shRNA template insert was ligated into a pSilencer vector, pSilencer3.1-H1Puro using the standard cloning technique. The identity and orientation of the construct was confirmed by DNA sequencing. The negative control plasmid encodes an shRNA sequence not found in the mouse, human, or rat genome database (Ambion).
Establishment of stable β1-integrin shRNA BT-549 and DU145 cell lines
Parental BT-549 and DU145 cells grown to 60% confluency were transfected with β1-integrin shRNA using FuGENE 6 reagent according to the manufacturer’s instructions (Roche). Transfected cultures were selected with puromycin (0.5 mg/ml for BT549 cells and 1 µg/ml for DU145 cells) and antibiotic-resistant colonies grown under selection conditions.
Semi-quantitative reverse-transcription PCR
RNA was isolated from parental cells and cells transfected with β1-integrin shRNA using RNeasy Mini Kit (QIAGEN) and reverse transcribed. 1 mg total RNA was annealed with 0.5 mg oligo-dT15 and reverse transcribed in a 20 ml volume containing 1x Reverse-transcription buffer (Promega), 0.1mg/ml BSA, 40 U RNAs in, 1mM dNTPs and 200U mouse Moloney leukemia virus reverse transcriptase at 37 °C for 120 minutes. PCR Master Mix (Promega) was used for subsequent PCR reactions. For b2-microglobulin (150-bp product) and β1-integrin (210-bp product), the amplification conditions were as follows: 25 cycles of 94 °C for 30 seconds, 60 °C for 30 seconds, 72 °C for 1 minute, followed by a final 10-minute extension at 72 °C. The primer sequences were as follows: β2-microglobulin forward, 5'-TTAGCTGTGCTCGCGCTACTCTCTC-3'; b2-microglobulin reverse, 5'-GTCGGATGGATGAAACCCAGACACA-'; β1-integrin forward, 5'-ATCATTCCAATTGTAGCTGGT-3’; β1-integrin reverse, 5'-TTTTCCCTCATACTTCGGATT-3' (DNA Technologies).
Preparation of cell lysates and conditioned media
Cells were grown to approximately 80% confluency in 100 mm dishes and then serum-starved overnight. Cells were harvested using RIPA buffer containing protease inhibitor cocktail (Roche). The conditioned media were collected, centrifuged at 2000×g and concentrated using Millipore UltraFree 10K filters.
Immunoblotting
Cell lysates or conditioned media were normalized based on DNA determinations and subjected to SDS-PAGE using 12% gels. The protein was transferred to nitrocellulose and then immunoblotted using rabbit anti-human β1-integrin polyclonal antibody [22], monoclonal β-actin antibody (Sigma), monocloanal MMP-14 (R&D Systems), and rabbit anti-human cathepsin B polyclonal antibody [23] in 5% nonfat milk-T-TBS. Membranes were probed with a horseradish peroxidase-labeled secondary antibodies (Pierce) in 5% nonfat milk-T-TBS. Reactive proteins were detected using chemiluminescent kits (Perkin Elmer Life Sciences). Cell lysates or conditioned media were also analyzed by a human MMP antibody array (RayBiotech) according to the manufacturer’s instructions.
Immunocytochemistry
Cells were grown on rBM (BD Bioscience)-coated coverslips for 16–24 hours. Non-permeabilized cells were stained for surface β1-integrin at 4 °C and cells permeabilized with saponin were stained for intracellular β1-integrin at room temperature according to our published procedures [24]. Cells were fixed and then blocked for 45 minutes by incubating with PBS containing 2 mg/ml BSA. The cells were incubated with primary antibody (rabbit anti-human β1-integrin or preimmune rabbit IgG) for 2 hours. After washing with PBS, the cells were incubated for 1 hour with Texas red-conjugated affinity-purified donkey anti-rabbit IgG containing 5% normal donkey serum (Jackson ImmunoResearch). Cells were then washed, mounted upside-down with Slow Fade anti-fade reagent (Invitrogen Life Technologies) on glass slides and observed with a Zeiss 510 LSM confocal microscope.
Live-cell proteolysis assay
Glass-bottom microwell 35 mm Petri dishes (MatTek Corporation) were coated with 100 ml of rBM containing 25 mg/ml of DQ-collagen IV (Invitrogen Life Technologies) and placed in a 37 °C incubator for 15 min to solidify. Cells (3–4 × 104) were plated on top of the rBM and incubated at 37 °C for 30–60 min until they attached. Culture medium with or without β1-integrin blocking antibody mAb 13 (20 µg/ml) was added and the cells cultured for 16–24 hours. Preimmune IgG, instead of β1-integrin blocking antibody, was used as one control and rBM-coated coverslips without cells as another control. Degradation products of DQ-collagen IV (green) were imaged with a Zeiss LSM 510 META NLO confocal microscope at 488 nm using a 40X water immersion objective. Z stack images were captured and used to make 3D reconstructions of the spheroids (Autovisualize software). Using both Metamorph 6.0 and Volocity 4.2.0 softwares (Perkin Elmer, Waltham, MA), the intensity of DQ-collagen IV degradation was measured, normalized to the number of nuclei [stained with Hoechst (Invitrogen Life Technologies) and pseudocolored red] and expressed as fluorescent intensity per cell. Using the Z-stack, the depth to which the cells invaded into the rBM was assessed by the presence of fluorescent cleavage products
Cathepsin B activity assays
Cathepsin B activity assays on cell lysates and overnight serum-free conditioned media were performed as previously described [25]. A 100 µM final concentration of benzyloxycarbonyl-L-arginyl-L-arginine-4-methyl-7-coumarylamide (Z-Arg-Arg-NHMec) (Bachem) substrate was used and fluorescence was measured in triplicate at 1 minute intervals over a 30 minute period in a Tecan SPECTRAFLUOR PLUS plate reader at an excitation of 360 nm and an emission of 465 nm. Procathepsin B in the media was activated with 0.4 mg/ml pepsin prior to conducting the assays. DNA assays were performed on each sample and cathepsin B activity was expressed as pmol/min/µg of DNA. DQ-collagen IV substrate (50 µg/ml) was also used as a substrate in this assay and incubated overnight with conditioned media at 37°C. Following overnight incubation, fluorescence was read at an excitation of 485 nm and emission of 535 nm and recorded as relative fluorescent units (RFU)/µg DNA. To inhibit cathepsin B activity we incubated samples in the presence of 10 µM CA074, a highly selective inhibitor of cathepsin B [26], for 60 minutes prior to performing the assays.
DNA assay
A 5 µl aliquot of cell lysate was incubated with a 1:10,000 dilution of SYBR Green in DNA assay buffer (100 mM NaCl, 10 mM EDTA, pH7.0, 10 mM Tris) for 15 min in the dark. Fluorescence intensities were read at an excitation of 485 nm and emission of 535 nm. DNA concentrations were determined from a standard curve of known concentrations of salmon sperm DNA (Invitrogen Life Technologies).
Cell adhesion assay
Cells were harvested using cell dissociation buffer (Invitrogen Life Technologies), suspended in serum-free media with 0.1% BSA and 5 × 104 cells seeded in triplicate on 24-well tissue culture plates coated with 5 µg/ml of either collagen I, collagen IV, laminin or fibronectin (BD Biosciences). The cells were allowed to attach for 30 min and unattached cells were removed by washing with PBS. Then 2 µM of calcein AM (Invitrogen Life Technologies) in PBS was added to the cells and incubated for 30 min at room temperature. Fluorescent intensities were measured at an excitation of 485 nm and emission of 535 nm.
Wound healing assay
Cells were grown as a monolayer to 100% confluency in 35 mm dishes. Medium was replaced 5–6 hours before wounding with RPMI/0.1% BSA for BT-549 cells and DMEM/0.1% BSA for DU145 cells. A scratch wound was created using rubber scrapper across the cellular monolayer. Detached cells were removed by washing and the remaining cells were incubated at 37 °C for 20 h. The cells were imaged at five minute intervals on a Zeiss LSM 510 META NLO microscope, equipped with a controlled environmental chamber that maintains a 5% CO2/humidified atmosphere at 37 °C, and using a 10X water immersion objective.
Invasion assays
Invasion assays were performed using Boyden chambers according to our published procedures [27]. Briefly, polycarbonate filters (8 µm pores for BT-549 and 12 µm pores for DU145) (Poretics) were coated with 1% gelatin followed by rBM (50 µg/filter) (BD Biosciences). Cells (2.5 × 104 cells in 200 µl) with 1% FBS in the presence and absence of 20 µg/ml of β1-integrin blocking antibody (mAb 13) [22] onto the rBM-coated filters and media with 5% FBS was used as the chemoattractant. Following overnight incubation, the filters were removed, air dried and stained with Diff-Quik (Dade Behring) and the cells that had invaded were counted and imaged using 10X and 40X objectives.
Statistical analysis
The statistical significance between control and individual conditions was determined by t-test. * represents a p value less than 0.05 and ** represents a p value less than 0.01.
Results
Downregulation of β1-integrin in human breast and prostate carcinoma cells
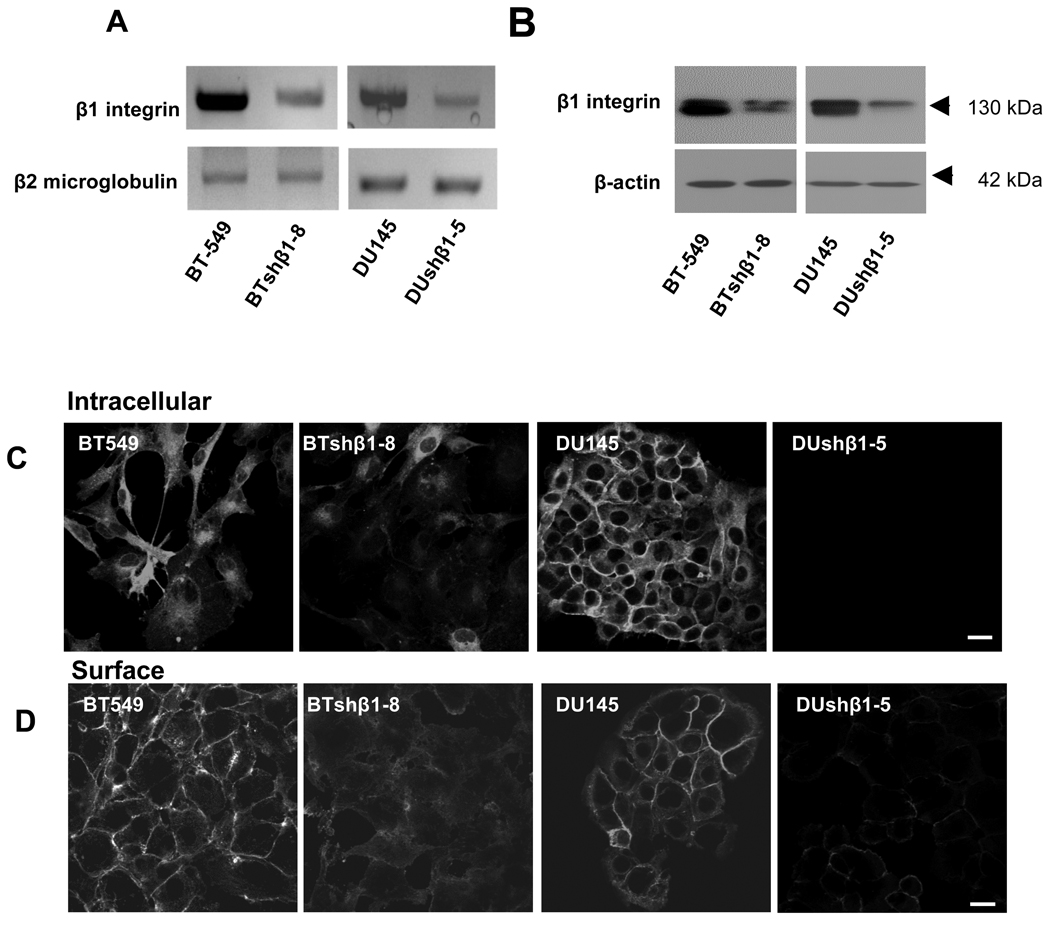
We investigated the consequences of β1-integrin downregulation in human breast (BT-549) and prostate (DU145) carcinoma cells that were stably transfected with shRNA encoding β1-integrin. Clones of each cell line were chosen and compared to parental cells and control cells that had been transfected with shRNA encoding a non-targeting sequence. We confirmed that levels of β1-integrin RNA and protein were reduced in the shRNA clones, illustrated here for clones BTshβ1-8 and DUshβ1-5 (Fig. 1A and B), but not in control cells transfected with non-targeting shRNA (data not shown). Clones in which β1-integrin expression was reduced more than in the BTshβ1-8 and DUshβ1-5 clones did not survive beyond 3–4 passages. Two other clones, BTshβ1-11 and DUshβ1-14, in which β1-integrin expression was reduced less than in the BTshβ1-8 and DUshβ1-5 clones were not evaluated further (data not shown). Staining of the BTshβ1-8 and DUshβ1-5 clones confirmed a reduction in protein levels of β1-integrin intracellularly (Fig. 1C) and on the cell surface (Fig. 1D). Downregulation of β1-integrin did not affect cell viability as determined by Live/Dead assays; both parental cells and clones were 99% viable (data not shown).
Figure 1. Downregulation of β1-integrin in breast and prostate carcinoma cells by shRNAi.
Breast (BT-549) and prostate (DU145) carcinoma cells were stably transfected with a plasmid containing β1-integrin shRNA. BT-549 and DU145 clones (BTshβ1-8 and DUshβ1-5 are illustrated here) were established and maintained in selection media as described in “Experimental Procedures”. A. Total RNA was isolated from parental cells and clones and subjected to reverse transcription followed by PCR using primer sequences to β1-integrin and β2-microglobulin (as a control for equal loading). PCR products were separated on a 1.2 % agarose gel and stained with ethidium bromide. B. Cells were solubilized in lysis buffer without reducing agents, equally loaded, subjected to SDS-PAGE and immunoblotted with antibodies against β1-integrin. Equal loading (normalized by DNA determination) was verified by probing with antibodies against β-actin. Immunoblots are representative of at least three experiments. C. Cells were immunolabeled for intracellular β1-integrin with polyclonal β1-integrin antibodies at room temperature in the presence of saponin. D. Surface labeling with polyclonal β1-integrin antibodies was performed at 4°C in the absence of saponin. Texas-Red-conjugated affinity-purified donkey anti-rabbit IgG was used as a secondary antibody. Bars, 10 µm.
Functional live-cell imaging illustrates that downregulation and blocking of β1-integrin reduces degradation of the ECM protein collagen IV
We have previously used a functional live-cell proteolysis assay [28] to demonstrate in real-time the ability of BT-549 human breast carcinoma and DU145 human prostate carcinoma cells to degrade a quenched fluorescent form of collagen IV (i.e., DQ-collagen IV) [24, 27, 29]. Fluorescent degradation products were observed both pericellularly and intracellularly. Here, we examined the degradation of DQ-collagen IV by live BT-549, DU145, BTshβ1-8 and DUshβ1-5 cells. The cells were grown overnight on rBM containing DQ-collagen IV in the presence or absence of the β1-integrin blocking antibody mAb 13 (Fig. 2A and B). Preimmune IgG was used as a control and showed no effect on DQ-collagen IV degradation (data not shown). Representative single optical sections at the equatorial plane are illustrated in Fig. 2A and C, and Fig. 3D reconstructions of the optical sections are shown in Fig. 2B and D (also see Videos 1 through 4 showing DU145 cells). Degradation products of DQ-collagen IV were observed pericellularly and intracellularly. Downregulation of β1-integrin expression and function resulted in smaller cellular spheroids and a reduction in proteolysis of DQ-collagen IV (Fig. 2). Due to the reduced size of tumor spheroids, we confirmed that proteolysis per cell was also reduced (Fig. 3B and E). Proteolysis was quantified using our established protocols [28] in which the total integrated intensity of fluorescence throughout an entire Z-stack is measured (Metamorph 6.0) and normalized to the total number of nuclei (Volocity 4.2.0) within that Z-stack [28] in order to determine proteolysis per cell. The reduction in both spheroid size and proteolysis was more extensive when BTshβ1-8 or DUshβ1-5 cells were also incubated with the β1-integrin blocking antibody mAb 13. The depth to which the cells invaded was assessed by measuring the Z-axis distance over which green fluorescence products of proteolysis were observed (Fig. 3 and Videos 5 through 8 showing DU145 cells). Reduced degradation of DQ-collagen IV corresponded to reduced invasion (Fig. 3C and F, respectively), suggesting a role for β1-integrin in invasion of tumor cells via degradation of the ECM.
Figure 2. Reduced expression or function of β1-integrin in BT-549 and DU145 cells decreased degradation of DQ-collagen IV.
BT-549, BTshβ1-8, DU145 and DUshiβ1-5 cells were seeded in glass-bottom dishes coated with a mixture of rBM and DQ-collagen IV in the presence or absence of the β1-integrin blocking antibody mAb 13 (20 µg/ml). Following overnight incubation at 37°C, DQ-collagen IV cleavage products (green) were imaged with a Zeiss LSM 510 META NLO microscope, using a 40X water immersion objective, and superimposed on DIC images of cellular spheroids. A and C. Z stack images were captured and a representative optical section at the equatorial plane is shown for each cell line in the presence and absence of mAb 13. B and D. 3D reconstructions of the Z-stacks were created using Metamorph 6.0 and AutoVisualize softwares and an XY view of the DQ-collagen IV degradation is illustrated (also see Videos 1 through 4). Bars, 10 µm.
Figure 3. Reduced expression or function of β1-integrin in BT-549 and DU145 cells decreased the intensity and depth of degradation of DQ-collagen IV.
BT-549, BTshβ1-8, DU145 and DUshβ1-5 cells were seeded onto glass-bottom dishes coated with rBM-DQ-collagen IV mixture in the presence or absence of the β1-integrin blocking antibody mAb 13 (20 µg/ml). Following overnight incubation at 37°C, DQ-collagen IV cleavage products (green) were imaged with a Zeiss LSM 510 confocal microscope using a 40X water immersion objective. Nuclei were stained with Hoechst (pseudocolored red here). A and D. Z stack images were captured and used to make 3D reconstructions of the spheroids (also see Videos 5 through 8). B and E. Using both Metamorph 6.0 and Volocity 4.2.0 softwares (Perkin Elmer, Waltham, MA), the intensity of DQ-collagen IV degradation was measured, normalized to the number of nuclei and expressed as normalized integrated intensity per cell. C and F. The depth over which proteolysis occurs in the Z-stack of each cell line was recorded. Graphs are representative of at least three experiments and presented as mean ±standard deviation (S.D.). ** P < 0.01. S.D. bars are not depicted in graphs B and E because the values are negligible.
Downregulation and blocking of β1-integrin reduces tumor cell invasion
We further investigated whether β1-integrin is required for these cells to invade through rBM. Cells were grown on rBM-coated filters for 24 h, and the cells that invaded through the filters were stained (Fig. 4A and C) and counted (Fig. 4B and D). Since BT-549 cells migrate as single cells and DU145 migrate as sheets of cells (see Fig. 7), we assessed invasion through 8 mm and 12 mm pore filters, respectively. Downregulation of β1-integrin reduced invasion of BTshβ1-8 cells by 74% and DUshβ1-5 cells by 46%. The β1-integrin blocking antibody mAb 13 was more effective in reducing invasion of BT-549 and DU145 cells than was shRNA. Invasion of the BT-549 and DU145 was reduced by 95% and 63%, respectively. These data indicate that β1-integrin is involved in invasion of breast and prostate carcinoma cells through rBM in vitro.
Figure 4. Invasion of BT-549, DU145 and β1shRNA transfected cells through rBM.
rBM-coated filters were placed in Boyden chambers and media containing 5% FBS was used as a stimulus in the bottom compartment of the chambers. BT-549, DU145, BTshβ1-8 or DUshβ1-5 cells were seeded (2.5 × 104 cells in 200 µl) in the top compartment of the chamber with 1% FBS and incubated at 37 °C overnight. Invasion of BT-549 and DU145 cells was also assessed in the presence of 20 µg/ml of the β1-integrin blocking antibody mAb 13. A and C. The cells that had invaded were stained, counted and imaged using 10X and 40X (Fig. inserts) objectives. B and D. Graphs are representative of at least three experiments and presented as mean ± S.D. ** P < 0.01
Figure 7. Adhesion and migration of BT-549, DU145 and β1shRNA transfected cells.
Adhesion assays were performed by seeding 50,000 BT-549 (A, black bars), DU145 (B, black bars), BTshβ1-8 (A, white bars) or DUshβ1-5 (B, white bars) cells in 24-well tissue culture plates coated with collagen I, collagen IV, laminin or fibronectin (5 µg/ml). After 30 min, unattached cells were removed by washing with PBS. Two mM calcein AM in PBS was added to the adherent cells for 30 min at room temperature. Fluorescent intensities were measured at 485/535 nm and expressed as relative fluorescent units (RFU). Graphs are representative of at least three experiments and data are presented as mean ± S.D. * P < 0.05 and ** P < 0.01. C. Wound healing assays were performed on 100% confluent live cells in 35 mm dishes. Prior to wounding, cells were incubated in serum-free media containing 0.1% BSA for 5–6 h. After the wounds were made, detached cells were removed by washing, and adherent cells incubated at 37°C for 24 h and then imaged by phase contrast microscopy using a Zeiss LSM 510 META NLO microscope using a 10X immersion objective. Images are representative of at least three experiments. Bar, 50 µm.
Downregulation of β1-integrin decreases secretion of pro-cathepsin B
The reductions in ECM degradation by β1-integrin downregulated cells suggest that β1-integrin expression and function may regulate expression and activity of proteolytic enzymes. Our previous studies revealed that secretion of pro-cathepsin B from breast fibroblasts is reduced in the presence of β1-integrin blocking antibodies [30]. Here, we found that secretion of pro-cathepsin B (43/46 kDa) was decreased in BTshβ1-8 cells (Fig. 5A), an effect which was not observed in the prostate cancer cells stably transfected with β1-integrin shRNA (data not shown). There was a corresponding increase in intracellular mature cathepsin B (i.e., 31 kDa single chain and 25/26 plus 5 kDa double chain) in BTshβ1-8 cells. Cathepsin B activity assays confirm the immunoblotting data; there was an increase in active cathepsin B intracellularly and a decrease in secretion of pepsin-activatable pro-cathepsin B in the BTshβ1-8 cells (Fig. 5B). In addition, we analyzed the ability of the conditioned media to degrade DQ-collagen IV in vitro in the presence and absence of the highly selective cathepsin B inhibitor CA074 [26] (Fig. 5C). There was a reduction in degradation of DQ-collagen IV by the conditioned media of BTshβ1-8 cells as compared to BT-549 cells. The degradation of DQ-collagen IV was partially inhibited by CA074, confirming that cathepsin B participates in the extracellular degradation of DQ-collagen IV. Furthermore, the level of DQ-collagen IV degradation in the parental and β1-integrin downregulated cells in the presence of CA074 was comparable, implicating secreted cathepsin B in the extracellular degradation of this ECM protein.
Figure 5. Secretion of pro-cathepsin B was reduced in β1-integrin downregulated BT-549 cells.
A. BT-549 and BTshβ1-8 cell lysates and overnight serum-free conditioned media were equally loaded, analyzed by SDS-PAGE and immunoblotted with an anti-cathepsin B antibody; lysates were immunoblotted in addition with an anti-actin antibody as a loading control. Immunoblots are representative of at least three experiments. B and C. Cell lysates and media were assayed for cathepsin B activity against Z-Arg-Arg-NHMec substrate and activity was recorded as pmol/min/µg DNA. D. Media were assayed for cathepsin B activity against DQ-collagen IV substrate, in the absence (black bars) and presence (white bars) of the highly selective cathepsin B inhibitor CA074, and activity recorded as relative fluorescent units (RFU)/µg DNA. Graphs are representative of at least three experiments and presented as mean ± S.D. ** P < 0.01
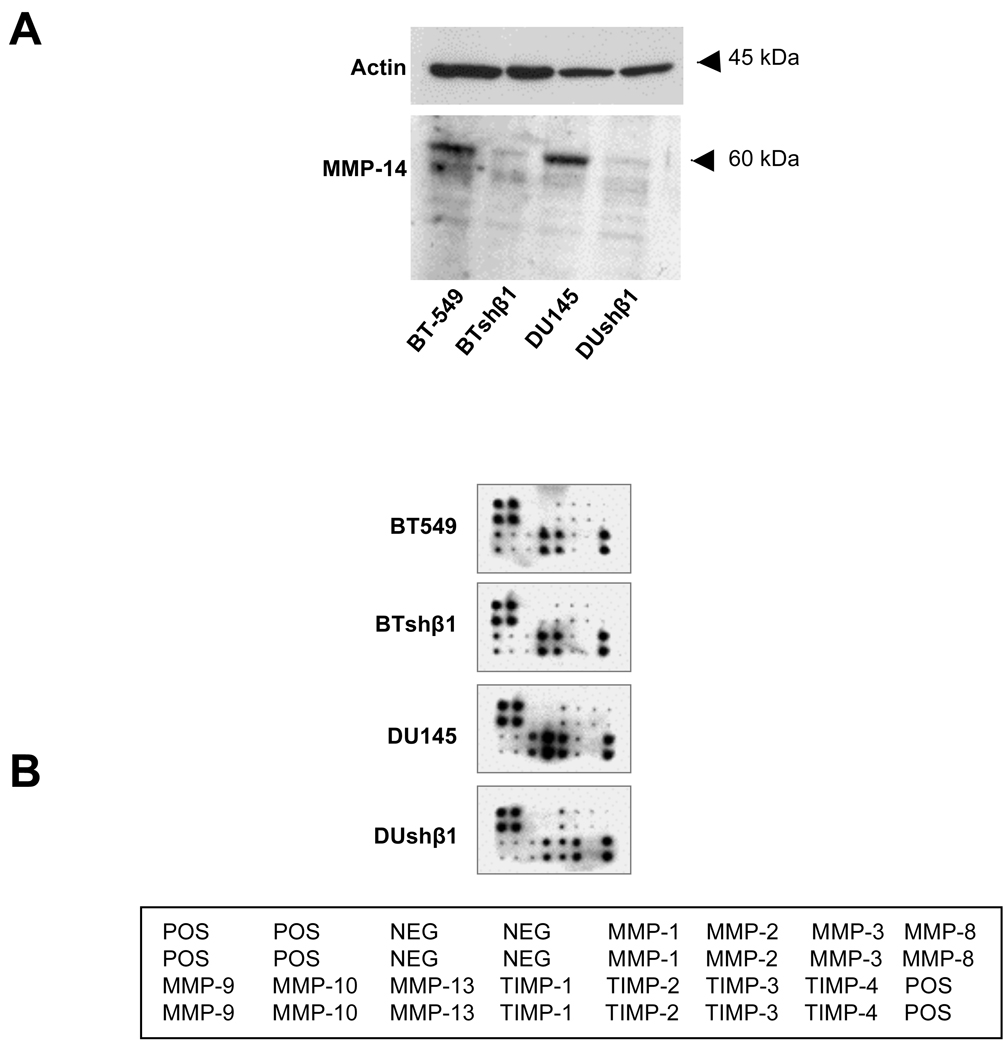
Downregulation of β1-integrin decreases MMP-14 expression and secretion of MMP-13, TIMP-1 and -2 and increases secretion of TIMP-3
Since inhibition of cathepsin B did not abolish the degradation of DQ-collagen IV, we also investigated the effects of β1-integrin downregulation on the expression and secretion of MMPs, the family of proteases most extensively linked to ECM degradation. We found that expression of MMP-14 was reduced in both β1-downregulated breast and prostate cancer cells (Fig. 6A). In addition, using antibody array analysis, we observed that the secretion of MMP-13 was reduced in β1-integrin downregulated prostate cancer cells but not in β1-integrin downregulated breast cancer cells (Fig. 6B). There was also a decrease in secretion of TIMP-1 and -2 and an increase in secretion of TIMP-3 from the prostate cancer cells. These data indicate differential roles for β1-integrin in the regulation of MMP and TIMP expression and secretion that is dependent upon the tumor cell type.
Figure 6. Expression of MMP-14 and secretion of MMP-13, TIMP-1 and -2 were reduced and secretion of TIMP-3 was increased in β1-integrin downregulated BT-549 and DU145 cells.
A. BT-549, DU145, BTshβ1-8 and DUshβ1-5 cell lysates were equally loaded, analyzed by SDS-PAGE and immunoblotted with an anti-MMP-14 and anti-actin antibody (loading control). Immunoblots are representative of at least three experiments. B. Conditioned media from these cells were analyzed on a human MMP antibody array (RayBiotech).
Downregulation of β1-integrin reduces adhesion to collagen I and IV and migration
We investigated the effects of β1-integrin on adhesion and migration of BT-549 and DU145 cells. Adhesion of BTshβ1-8 and DUshβ1-5 cells to collagen I and IV, but not to fibronectin and laminin, was reduced as compared to that of parental cells (Fig. 7A and B, respectively). We also investigated the effect of β1-integrin downregulation on cell migration in wound healing assays. During the twenty hours after wounding, migration of the cells was imaged at five minute intervals. Parental BT-549 and DU145 cells migrated faster than did BTshβ1-8 and DUshβ1-5 cells. Although both parental and β1-integrin downregulated BT-549 cells migrated as individual cells into the wounded (cell-free) areas, there was more migration of parental cells than BTshβ1-8 cells after 20 h (Fig. 7C). DU145 parental cells moved collectively as a sheet of cells and had nearly closed the wound after 20 h, whereas only a few DUshβ1-5 cells had moved into the wounded areas (Fig. 7C). These data indicate that β1-integrin is involved in the adhesion and migration of both breast and prostate cancer cells.
Discussion
β1-integrin facilitates signaling events that promote ECM adhesion, migration and degradation, thereby supporting tumorigenesis. Given the complexity of ECM, multiple families of proteases (serine, cysteine and metallo-) participate in ECM remodeling and degradation. Indeed there are numerous studies that have linked β1-integrin expression to alterations in proteases; however, this study is the first to use functional imaging on live tumor cells to show effects of downregulation and/or blocking the function of β1-integrin on collagen IV degradation by a network of proteases in both breast and prostate cancer cells. These findings are also accompanied by a decrease in cell adhesion to and invasion through collagen IV-containing matrix.
Collagen IV, the structural backbone of the basement membrane, interacts with integrins and serves as scaffolding for the binding of other basement membrane components [31]. We have previously demonstrated that a network of proteases including MMPs, serine protease plasmin and cysteine protease cathepsin B participate in the degradation of collagen IV in breast, colon and prostate carcinoma cells [27, 32]. In the current study, downregulation of β1-integrin expression and/or function revealed differential effects on protease expression and secretion depending on the tumor cell type. For example, in β1-integrin downregulated breast cancer cells, secretion of procathepsin B was reduced, an effect not seen in prostate cancer cells. β1-integrin blocking antibodies also reduces secretion of procathepsin B by breast fibroblasts, and conversely, β1-integrin activating antibodies stimulates secretion of procathepsin B by these cells [30]. In highly invasive melanoma cells grown in collagen I, inhibition of β1-integrin activity by blocking antibodies reduces the secretion of both pro- and mature forms of cathepsin B [33]. A functional link between cathepsin B and β1-integrin is also seen in angiogenic signaling where anti-angiogenic endostatin, a fragment of collagen VIII that is generated by cathepsin B [34], blocks α5β1 integrin function [35]. Interestingly, we have recently found that cathepsin B and β1-integrin colocalize to caveolae of endothelial cells during differentiation of these cells into tube-like structures in vitro (unplished data), a process associated with degradation of collagen IV (unpublished data). Cathepsin B has also been found to colocalize with β1-integrin along with uPA and its receptor uPAR in caveolae of HCT 116 cells, an association mediated by caveolin-1 expression [21]. Since cathepsin B can activate pro-uPA to uPA [36], caveolae may serve as an initiating site for cell surface proteolysis.
Although β1-integrin downregulation did not affect cathepsin B in prostate cancer cells, there were effects on MMPs and TIMPs. We show that downregulation of β1-integrin reduces expression of MMP-14, also observed in breast cancer cells; reduces secretion of MMP-13, TIMP-1 and -2; and increases secretion of TIMP-3. In human chondrocytes, β1-integrin blocking antibodies inhibit the induction of MMP-13 expression in these cells by type I collagen [37]. Conversely, induction and activation of MMP-13 is augmented by β1-integrin activating antibodies in human skin fibroblasts grown on a collagen I matrix, [38], thus indicating that MMP-13 expression and activation in these cells is regulated by the interaction between β1-integrin and the ECM. The decrease in MMP-14 expression and TIMP-2 secretion in the β1-integrin downregulated prostate cancer cells is interesting since MMP-14 is an activator of MMP-2, and TIMP-2 acts as a linker protein for the activation of pro-MMP-2 by MMP-14 [39]. In ovarian carcinoma cells, β1-integrin was shown to stimulate the activation of pro-MMP-2 by MMP-14 [40]. Although we also observed a decrease in TIMP-1 secretion, TIMP-3 secretion was increased in β1-integrin downregulated prostate cancer cells. The association between TIMP-3 and β1-integrin is not clear; however, TIMP-3 is the only TIMP to completely inhibit the sphingosine-1-phosphate-induced and α2β1-dependent invasion of endothelial cells in collagen matrices [41]. A reduction in TIMP-1 expression was previously reported in β1-integrin downregulated DU145 cells [42] and TIMP-1 is hypothesized to interact with the CD63/β1-integrin signaling complex, which is required for cell survival and motility [18]. The association of β1-integrin with MMP-14 also involves cell migration. In endothelial cells, MMP-14 participates in cooperation with β1-integrin during migration of these cells on various ECMs [39]. MMP-14 was also found colocalize with β1-integrin in actin-rich, “collagenolysis-free” leading edges of migrating fibrosarcoma and breast carcinoma cells grown on a 3D collagen matrix [43]. It was suggested that at the leading edges of these migrating cells there is adhesion and remodeling of the ECM that facilitate forward movement [43]. Here, we show a perturbation in the migration of β1-integrin downregulated cells, an effect likely associated with the observed changes in protease and inhibitor expression and secretion.
Our data also revealed that a reduction of β1-integrin expression and activity in both breast and prostate carcinoma cell decreased the cell migration and adhesion to type I and IV collagen. These data complement previous studies that show that the interaction of human prostate cancer cells PC3 with the collagen matrix of bone is mediated by collagen-binding integrin α2β1 and is enhanced by the growth factor TGF-β1 [44, 45]. Moreover, bombesin-mediated activation of pro-MMP-9 in PC3 cells is facilitated by ligation of β1-integrin to collagen I, IV and fibronectin [46], which increases uPA expression, membrane-linked uPA activity and activation of Src and PI3K tyrosine kinases thereby augmenting invasion of these cells [46]. Our findings however revealed no effect of β1-integrin on the binding of breast and prostate cancer cells to fibronectin or laminin. Similar observations are reported in cell adhesion assays using squamous cell carcinoma cells [47]. On the other hand, there are several studies that show a role for β1-integrin adhesion to fibronectin and laminin including several cell signaling events [for review see [3]]. It is plausible, the lack of effect of β1-integrin downregulation on adhesion to fibronectin and laminin can be explained by integrin redundancy as reported for adhesion of breast cancer cells to fibronectin or vitronectin [48].
Thus, our study using functional imaging of live cells demonstrates an involvement for β1-integrin expression in the degradation of collagen IV via pro-cathepsin B secretion and activity, MMP-14 expression and MMP-13, TIMP-1, -2 and -3 secretion. Involvements of α integrins that interact with β1 integrin during collagen IV degradation are currently being investigated. In addition, other mechanisms that contribute to proteolysis of ECM such as the urokinase plasmin(ogen) cascade and signaling pathways involving p21-activated kinase [49] will be examined with respect to their roles in β1-integrin mediated ECM degradation and invasion of tumor cells.
Supplementary Material
Video 1. 3D confocal microscopy of DQ-collagen IV degradation by DU145 cells. DU145 cells were grown on rBM-DQ-collagen IV mixture. DQ-collagen IV cleavage products (green) were imaged, and superimposed on DIC images of cellular spheroids. Z stack images were captured and 3D reconstructions were created.
Video 2. 3D confocal microscopy of DQ-collagen IV degradation by DU145 cells plus β1-integrin blocking antibody. DU145 cells were grown on rBM-DQ-collagen IV mixture plus mAb 13. DQ-collagen IV cleavage products (green) were imaged, and superimposed on DIC images of cellular spheroids. Z stack images were captured and 3D reconstructions were created.
Video 3. 3D confocal microscopy of DQ-collagen IV degradation by DUshβ1-5 cells. DUshβ1-5 cells were grown on rBM-DQ-collagen IV mixture. DQ-collagen IV cleavage products (green) were imaged, and superimposed on DIC images of cellular spheroids. Z stack images were captured and 3D reconstructions were created.
Video 4. 3D confocal microscopy of DQ-collagen IV degradation by DUshβ1-5 cells plus β1-integrin blocking antibody. DUshβ1-5 cells were grown on rBM-DQ-collagen IV mixture plus mAb 13. DQ-collagen IV cleavage products (green) were imaged, and superimposed on DIC images of cellular spheroids. Z stack images were captured and 3D reconstructions were created.
Video 5. Intensity and depth of degradation of DQ-collagen IV by DU145 cells. DU145 cells were grown on rBM-DQ-collagen IV mixture. DQ-collagen IV cleavage products (green) were imaged, and nuclei were stained with Hoechst (pseudocolored red here). Z stack images were captured and used to make 3D reconstructions of the spheroids.
Video 6. Intensity and depth of degradation of DQ-collagen IV by DUshβ1-5 cells. DUshβ1-5 cells were grown on rBM-DQ-collagen IV mixture. DQ-collagen IV cleavage products (green) were imaged, and nuclei were stained with Hoechst (pseudocolored red here). Z stack images were used to make 3D reconstructions.
Video 7. Intensity and depth of degradation of DQ-collagen IV by DU145 cells plus β1-integrin blocking antibody. DU145 cells grown on rBM-DQ-collagen IV mixture plus mAb 13. DQ-collagen IV cleavage products (green) were imaged, and nuclei were stained with Hoechst (pseudocolored red here). Z stack images were used to make 3D reconstructions.
Video 8. Intensity and depth of degradation of DQ-collagen IV by DUshβ1-5 cells plus β1-integrin blocking antibody. DUshβ1-5 cells were grown on rBM-DQ-collagen IV mixture plus mAb 13. DQ-collagen IV cleavage products (green) were imaged, and nuclei were stained with Hoechst (pseudocolored red here). Z stack images were used to make 3D reconstructions.
Acknowledgments
We would like to thank Dr. Jianxin Mai for his technical assistance. This work was supported by National Institutes of Health (NIH) grant CA 56586 and DOD Award PC991261. The Microscopy and Imaging Resource Center is supported, in part, by NIH Center Grants P30 CA 22453, P30 ES 06639 and U54 RR02084330. KMY was supported in part by the Intramural Research Program of the NIH, NIDCR.
Nonstandard abbreviations used are
- 3D
three dimensional
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- rBM
reconstituted basement membrane
- RFU
relative fluorescent unit
- shRNAi
short hairpin RNA interference
- uPA
urokinase plasminogen activator
- uPAR
urokinase plasminogen activator receptor
- TIMP
tissue inhibitor of metalloproteinase
- Z-Arg-Arg-NHMec
benzyloxycarbonyl-L-arginyl-L-arginine-4-methyl-7-coumarylamide.
References
- 1.Akiyama SK, Olden K, Yamada KM. Fibronectin and integrins in invasion and metastasis. Cancer Metastasis Rev. 1995;14:173–189. doi: 10.1007/BF00690290. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LM. Integrin function in breast carcinoma progression. J Mammary Gland Biol Neoplasia. 1999;4:367–376. doi: 10.1023/a:1018766317055. [DOI] [PubMed] [Google Scholar]
- 3.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 4.Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Slack-Davis JK, Parsons JT. Emerging views of integrin signaling: implications for prostate cancer. J Cell Biochem. 2004;91:41–46. doi: 10.1002/jcb.10665. [DOI] [PubMed] [Google Scholar]
- 6.Knudsen BS, Miranti CK. The impact of cell adhesion changes on proliferation and survival during prostate cancer development and progression. J Cell Biochem. 2006;99:345–361. doi: 10.1002/jcb.20934. [DOI] [PubMed] [Google Scholar]
- 7.Cress AE, Rabinovitz I, Zhu W, Nagle RB. The alpha 6 beta 1 and alpha 6 beta 4 integrins in human prostate cancer progression. Cancer Metastasis Rev. 1995;14:219–228. doi: 10.1007/BF00690293. [DOI] [PubMed] [Google Scholar]
- 8.Berry MG, Gui GP, Wells CA, Carpenter R. Integrin expression and survival in human breast cancer. Eur J Surg Oncol. 2004;30:484–489. doi: 10.1016/j.ejso.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Yao ES, Zhang H, Chen YY, Lee B, Chew K, Moore D, Park C. Increased beta1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007;67:659–664. doi: 10.1158/0008-5472.CAN-06-2768. [DOI] [PubMed] [Google Scholar]
- 10.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, Turley EA, Bissell MJ. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J Natl Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, Bissell MJ. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton SA, Reeves EJ, Gralnick H, Mohla S, Yamada KM, Olden K, Akiyama SK. Inhibition of experimental metastasis of human breast carcinoma cells in athymic nude mice by anti-alpha 5 beta 1 fibronectin receptor integrin antibodies. Int J Oncol. 1995;6:1063–1070. doi: 10.3892/ijo.6.5.1063. [DOI] [PubMed] [Google Scholar]
- 14.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 15.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA. Regulation of integrin function by the urokinase receptor. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed N, Oliva K, Wang Y, Quinn M, Rice G. Downregulation of urokinase plasminogen activator receptor expression inhibits Erk signalling with concomitant suppression of invasiveness due to loss of uPAR-beta1 integrin complex in colon cancer cells. Br J Cancer. 2003;89:374–384. doi: 10.1038/sj.bjc.6601098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cella N, Contreras A, Latha K, Rosen JM, Zhang M. Maspin is physically associated with [beta]1 integrin regulating cell adhesion in mammary epithelial cells. Faseb J. 2006;20:1510–1512. doi: 10.1096/fj.05-5500fje. [DOI] [PubMed] [Google Scholar]
- 18.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 20.Cavallo-Medved D, Dosescu J, Linebaugh BE, Sameni M, Rudy D, Sloane BF. Mutant K-ras regulates cathepsin B localization on the surface of human colorectal carcinoma cells. Neoplasia. 2003;5:507–519. doi: 10.1016/s1476-5586(03)80035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallo-Medved D, Mai J, Dosescu J, Sameni M, Sloane BF. Caveolin-1 mediates the expression and localization of cathepsin B, pro-urokinase plasminogen activator and their cell-surface receptors in human colorectal carcinoma cells. J Cell Sci. 2005;118:1493–1503. doi: 10.1242/jcs.02278. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moin K, Day NA, Sameni M, Hasnain S, Hirama T, Sloane BF. Human tumour cathepsin B. Comparison with normal liver cathepsin B. Biochem J. 1992;285(Pt 2):427–434. doi: 10.1042/bj2850427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sameni M, Moin K, Sloane BF. Imaging proteolysis by living human breast cancer cells. Neoplasia. 2000;2:496–504. doi: 10.1038/sj.neo.7900116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linebaugh BE, Sameni M, Day NA, Sloane BF, Keppler D. Exocytosis of active cathepsin B enzyme activity at pH 7.0, inhibition and molecular mass. Eur J Biochem. 1999;264:100–109. doi: 10.1046/j.1432-1327.1999.00582.x. [DOI] [PubMed] [Google Scholar]
- 26.Murata M, Miyashita S, Yokoo C, Tamai M, Hanada K, Hatayama K, Towatari T, Nikawa T, Katunuma N. Novel epoxysuccinyl peptides. Selective inhibitors of cathepsin B, in vitro. FEBS Lett. 1991;280:307–310. doi: 10.1016/0014-5793(91)80318-w. [DOI] [PubMed] [Google Scholar]
- 27.Sameni M, Dosescu J, Moin K, Sloane BF. Functional imaging of proteolysis: stromal and inflammatory cells increase tumor proteolysis. Mol Imaging. 2003;2:159–175. doi: 10.1162/15353500200303136. [DOI] [PubMed] [Google Scholar]
- 28.Jedeszko C, Sameni M, Olive M, Moin K, Sloane BF. Visualizing Protease Activity in Living Cells: From 2D to 4D. Current Protocols in Cell Biology. 2008;39:4.20.1–4.20.15. doi: 10.1002/0471143030.cb0420s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Podgorski I, Linebaugh BE, Sameni M, Jedeszko C, Bhagat S, Cher ML, Sloane BF. Bone microenvironment modulates expression and activity of cathepsin B in prostate cancer. Neoplasia. 2005;7:207–223. doi: 10.1593/neo.04349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koblinski JE, Dosescu J, Sameni M, Moin K, Clark K, Sloane BF. Interaction of human breast fibroblasts with collagen I increases secretion of procathepsin B. J Biol Chem. 2002;277:32220–32227. doi: 10.1074/jbc.M204708200. [DOI] [PubMed] [Google Scholar]
- 31.Laurie GW, Bing JT, Kleinman HK, Hassell JR, Aumailley M, Martin GR, Feldmann RJ. Localization of binding sites for laminin, heparan sulfate proteoglycan and fibronectin on basement membrane (type IV) collagen. J Mol Biol. 1986;189:205–216. doi: 10.1016/0022-2836(86)90391-8. [DOI] [PubMed] [Google Scholar]
- 32.Sloane BF, Sameni M, Podgorski I, Cavallo-Medved D, Moin K. Functional imaging of tumor proteolysis. Annu Rev Pharmacol Toxicol. 2006;46:301–315. doi: 10.1146/annurev.pharmtox.45.120403.095853. [DOI] [PubMed] [Google Scholar]
- 33.Klose A, Wilbrand-Hennes A, Zigrino P, Weber E, Krieg T, Mauch C, Hunzelmann N. Contact of high-invasive, but not low-invasive, melanoma cells to native collagen I induces the release of mature cathepsin B. Int J Cancer. 2006;118:2735–2743. doi: 10.1002/ijc.21700. [DOI] [PubMed] [Google Scholar]
- 34.Ferreras M, Felbor U, Lenhard T, Olsen BR, Delaisse J. Generation and degradation of human endostatin proteins by various proteinases. FEBS Lett. 2000;486:247–251. doi: 10.1016/s0014-5793(00)02249-3. [DOI] [PubMed] [Google Scholar]
- 35.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci U S A. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi H, Schmitt M, Goretzki L, Chucholowski N, Calvete J, Kramer M, Gunzler WA, Janicke F, Graeff H. Cathepsin B efficiently activates the soluble and the tumor cell receptor-bound form of the proenzyme urokinase-type plasminogen activator (Pro-uPA) J Biol Chem. 1991;266:5147–5152. [PubMed] [Google Scholar]
- 37.Ronziere MC, Aubert-Foucher E, Gouttenoire J, Bernaud J, Herbage D, Mallein-Gerin F. Integrin alpha1beta1 mediates collagen induction of MMP-13 expression in MC615 chondrocytes. Biochim Biophys Acta. 2005;1746:55–64. doi: 10.1016/j.bbamcr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Ravanti L, Heino J, Lopez-Otin C, Kahari VM. Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:2446–2455. doi: 10.1074/jbc.274.4.2446. [DOI] [PubMed] [Google Scholar]
- 39.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 40.Ellerbroek SM, Fishman DA, Kearns AS, Bafetti LM, Stack MS. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through beta1 integrin. Cancer Res. 1999;59:1635–1641. [PubMed] [Google Scholar]
- 41.Bayless KJ, Davis GE. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem Biophys Res Commun. 2003;312:903–913. doi: 10.1016/j.bbrc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Jung KK, Liu XW, Chirco R, Fridman R, Kim HR. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. Embo J. 2006;25:3934–3942. doi: 10.1038/sj.emboj.7601281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 44.Kostenuik PJ, Sanchez-Sweatman O, Orr FW, Singh G. Bone cell matrix promotes the adhesion of human prostatic carcinoma cells via the alpha 2 beta 1 integrin. Clin. Exp. Metastasis. 1996;14:19–26. doi: 10.1007/BF00157682. [DOI] [PubMed] [Google Scholar]
- 45.Goel HL, Breen M, Zhang J, Das I, Aznavoorian-Cheshire S, Greenberg NM, Elgavish A, Languino LR. beta1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005;65:6692–6700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- 46.Festuccia C, Angelucci A, Gravina G, Eleuterio E, Vicentini C, Bologna M. Bombesin-dependent pro-MMP-9 activation in prostatic cancer cells requires beta1 integrin engagement. Exp Cell Res. 2002;280:1–11. doi: 10.1006/excr.2002.5609. [DOI] [PubMed] [Google Scholar]
- 47.Brockbank EC, Bridges J, Marshall CJ, Sahai E. Integrin beta1 is required for the invasive behaviour but not proliferation of squamous cell carcinoma cells in vivo. Br J Cancer. 2005;92:102–112. doi: 10.1038/sj.bjc.6602255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartsch JE, Staren ED, Appert HE. Adhesion and migration of extracellular matrix-stimulated breast cancer. J Surg Res. 2003;110:287–294. doi: 10.1016/s0022-4804(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 49.Li Q, Mullins SR, Sloane BF, Mattingly RR. p21-Activated Kinase 1 Coordinates Aberrant Cell Survival and Pericellular Proteolysis in a Three-Dimensional Culture Model for Premalignant Progression of Human Breast Cancer. Neoplasia. 2008;10:314–328. doi: 10.1593/neo.07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. 3D confocal microscopy of DQ-collagen IV degradation by DU145 cells. DU145 cells were grown on rBM-DQ-collagen IV mixture. DQ-collagen IV cleavage products (green) were imaged, and superimposed on DIC images of cellular spheroids. Z stack images were captured and 3D reconstructions were created.
Video 2. 3D confocal microscopy of DQ-collagen IV degradation by DU145 cells plus β1-integrin blocking antibody. DU145 cells were grown on rBM-DQ-collagen IV mixture plus mAb 13. DQ-collagen IV cleavage products (green) were imaged, and superimposed on DIC images of cellular spheroids. Z stack images were captured and 3D reconstructions were created.
Video 3. 3D confocal microscopy of DQ-collagen IV degradation by DUshβ1-5 cells. DUshβ1-5 cells were grown on rBM-DQ-collagen IV mixture. DQ-collagen IV cleavage products (green) were imaged, and superimposed on DIC images of cellular spheroids. Z stack images were captured and 3D reconstructions were created.
Video 4. 3D confocal microscopy of DQ-collagen IV degradation by DUshβ1-5 cells plus β1-integrin blocking antibody. DUshβ1-5 cells were grown on rBM-DQ-collagen IV mixture plus mAb 13. DQ-collagen IV cleavage products (green) were imaged, and superimposed on DIC images of cellular spheroids. Z stack images were captured and 3D reconstructions were created.
Video 5. Intensity and depth of degradation of DQ-collagen IV by DU145 cells. DU145 cells were grown on rBM-DQ-collagen IV mixture. DQ-collagen IV cleavage products (green) were imaged, and nuclei were stained with Hoechst (pseudocolored red here). Z stack images were captured and used to make 3D reconstructions of the spheroids.
Video 6. Intensity and depth of degradation of DQ-collagen IV by DUshβ1-5 cells. DUshβ1-5 cells were grown on rBM-DQ-collagen IV mixture. DQ-collagen IV cleavage products (green) were imaged, and nuclei were stained with Hoechst (pseudocolored red here). Z stack images were used to make 3D reconstructions.
Video 7. Intensity and depth of degradation of DQ-collagen IV by DU145 cells plus β1-integrin blocking antibody. DU145 cells grown on rBM-DQ-collagen IV mixture plus mAb 13. DQ-collagen IV cleavage products (green) were imaged, and nuclei were stained with Hoechst (pseudocolored red here). Z stack images were used to make 3D reconstructions.
Video 8. Intensity and depth of degradation of DQ-collagen IV by DUshβ1-5 cells plus β1-integrin blocking antibody. DUshβ1-5 cells were grown on rBM-DQ-collagen IV mixture plus mAb 13. DQ-collagen IV cleavage products (green) were imaged, and nuclei were stained with Hoechst (pseudocolored red here). Z stack images were used to make 3D reconstructions.