Abstract
Carbohydrate regulatory element-binding protein (ChREBP), MAX-like factor X(MLX), and hepatic nuclear factor-4α (HNF-4α)are key transcription factors involved in the glucose-mediated induction of hepatic L-type pyruvate kinase (L-PK) gene transcription. n-3 polyunsaturated fatty acids (PUFA) and WY14643 (peroxisome proliferator-activated receptor α (PPARα) agonist) interfere with glucose-stimulated L-PK gene transcription in vivo and in rat primary hepatocytes. Feeding rats a diet containing n-3 PUFA or WY14643 suppressed hepatic mRNAL-PK but did not suppress hepatic ChREBP or HNF-4α nuclear abundance. Hepatic MLX nuclear abundance, however, was suppressed by n-3 PUFA but not WY14643. In rat primary hepatocytes, glucose-stimulated accumulation of mRNALPK and L-PK promoter activity correlated with increased ChREBP nuclear abundance. This treatment also increased L-PK promoter occupancy by RNA polymerase II (RNA pol II), acetylated histone H3 (Ac-H3), and acetylated histone H4 (Ac-H4) but did not significantly impact L-PK promoter occupancy by ChREBP or HNF-4α. Inhibition of L-PK promoter activity by n-3 PUFA correlated with suppressed RNA pol II, Ac-H3, and Ac-H4 occupancy on the L-PK promoter. Although n-3 PUFA transiently suppressed ChREBP and MLX nuclear abundance, this treatment did not impact ChREBP-LPK promoter interaction. HNF4α-LPK promoter interaction was transiently suppressed by n-3 PUFA. Inhibition of L-PK promoter activity by WY14643 correlated with a transient decline in ChREBP nuclear abundance and decreased Ac-H4 interaction with the L-PK promoter. WY14643, however, had no impact on MLX nuclear abundance or HNF4α-LPK promoter interaction. Although overexpressed ChREBP or HNF-4α did not relieve n-3 PUFA suppression of L-PK gene expression, overexpressed MLX fully abrogated n-3 PUFA suppression of L-PK promoter activity and mRNAL-PK abundance. Overexpressed ChREBP, but not MLX, relieved the WY14643 inhibition of L-PK. In conclusion, n-3 PUFA and WY14643/PPARα target different transcription factors to control L-PK gene transcription. MLX, the heterodimer partner for ChREBP, has emerged as a novel target for n-3 PUFA regulation.
The glycolytic enzyme, liver-type pyruvate kinase (L-PK),2 plays a key role in hepatic glucose and lipid metabolism (1). Phosphorylation and allosteric factors acutely regulate L-PK enzyme activity, whereas hormones and nutrients chronically control the abundance of L-PK protein by regulating L-PK gene transcription. Excess glucose consumption induces glycolysis, L-PK activity, de novo lipogenesis, and lipid storage, whereas n-3 polyunsaturated fatty acids (n-3 PUFA) inhibit these metabolic events (2, 3). L-PK gene transcription is induced by insulin-stimulated glucose metabolism (4) and inhibited by n-3 PUFA and activators of PPARα (WY14643), protein kinase A, and AMP kinase (AMPK) (5–8).
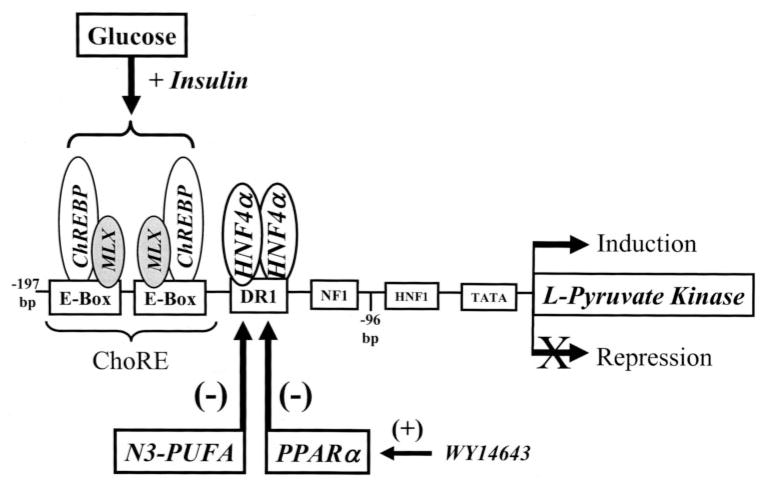
Key cis-regulatory elements controlling L-PK gene transcription are located between −197 and −125 bp upstream of the transcription start site (Fig. 1). Two basic helix-loop-helix transcription factors, carbohydrate regulatory element-binding protein (ChREBP), and MAX-like factor X (MLX) bind as heterodimers to carbohydrate regulatory elements (ChoRE) located between −197 and −145 bp from the transcription start site. The nuclear receptor, hepatic nuclear factor-4 (HNF-4), binds an imperfect direct repeat (DR-1) between −143 and −130 bp from the transcription start site. Although ChREBP and MLX are required for glucose-regulated transcription of L-PK (9–15), acetyl-CoA carboxylase (16), fatty-acid synthase (17), S14 (18), stearoyl-CoA desaturase-1, malic enzyme, and GLUT2 (15, 19), only glucose-regulated L-PK gene transcription requires a DR-1 element binding HNF-4 (5, 13).
FIGURE 1. Schematic of the L-PK promoter.
The diagram illustrates the location of key transcription factors and their binding sites on the L-PK promoter. Factors regulated by glucose, fatty acids, and the PPARα agonist WY14643 are identified. Linker scanning studies established that the ChoRE was required for glucose control, whereas the DR-1 element was required for PUFA and PPARα control of L-PK gene transcription (5, 6, 13).
Metabolism of glucose through the pentose pathway is required for glucose-regulated gene transcription (20). Glucose-derived metabolites, i.e. xylulose 5-phosphate, induce dephosphorylation of ChREBP by activating the phosphatase PP2A (21, 22). Dephosphorylated ChREBP moves to the nucleus where ChREBP/MLX heterodimers bind ChoREs in target genes (4, 23). Phosphorylation of ChREBP by AMP kinase or protein kinase A inhibits ChREBP nuclear translocation, thus suppressing transcription of L-PK and other glucose-regulated genes (21, 24). MLX movement into the nucleus is not known to be controlled by glucose.
HNF-4α, the predominant hepatic HNF-4 subtype (25–27), binds the L-PK DR1 element adjacent to the ChoRE. Although this element is required for full glucose activation of L-PK gene transcription (13), other glucose-regulated genes are not known to require HNF-4α. Thus, the role of HNF-4α in glucose-regulated gene expression appears unique to L-PK. HNF-4α, however, has been implicated in n-3 PUFA and WY14643 control of L-PK gene transcription because the L-PK DR1 element is required for both n-3 PUFA and WY14643 suppression of LPK gene transcription (5, 6). Unlike WY14643, n-3 PUFA suppression of L-PK gene transcription does not require functional PPARα. Thus, n-3 PUFA and WY14643 utilize separate mechanisms to control L-PK gene expression.
Despite the progress in defining the transcription factors that control L-PK gene transcription, there remains a poor understanding of how these factors interact on the L-PK promoter in cells. Moreover, the molecular basis of n-3 PUFA or WY14643 antagonism of glucose-mediated induction of L-PK gene transcription remains unknown. To address these issues, we examined the glucose, n-3 PUFA, and WY14643 regulation of hepatic nuclear abundance of ChREBP, MLX, and HNF-4α in vivo and in cultured rat primary hepatocytes. The chromatin immunoprecipitation (ChIP) assay was used for a detailed analysis of L-PK promoter composition in response to glucose, n-3 PUFA, and WY14643 challenge of rat primary hepatocytes. These studies coupled with gene expression analyses have clarified the effects of glucose, n-3 PUFA, and WY14643 on L-PK promoter activity and composition. More importantly, these studies have identified a new n-3 PUFA-regulated transcription factor that is involved in the control of L-PK gene transcription.
MATERIALS AND METHODS
Animals and Primary Hepatocytes
All procedures for the use and care of animals for laboratory research have been approved by the All University Committee for Animal Use and Care at Michigan State University. Male Sprague-Dawley rats (Charles River Breeding Laboratories, Kalamazoo, MI) were maintained on Harlan-Teklad laboratory chow (catalog number 8640) and water ad libitum. Protocols for meal-feeding rats high carbohydrate diets supplemented with olive oil (10% w/w), fish oil (10% w/w), or olive oil (10% w/w) + WY14643 (0.1% w/w) were described previously (28).
Rat primary hepatocytes were prepared from male Sprague-Dawley rats fed Teklad chow (ad libitum), cultured on BioCoat (collagen type I) plates (BD Biosciences), and treated with insulin (Invitrogen), fatty acids (Nu-Chek Prep, Elysian, MN), lactate, glucose, or PPARα agonist WY14643 (Chemsyn Labs, KS) as described previously (28). For the ChIP assay and RNA and protein extraction, cells were plated onto 10-cm type I collagen-coated plates (BD Biosciences) at 107 cells/plate in Williams E (Invitrogen), 10 mM lactate, 10 nM dexamethasone (Sigma), 1 μM insulin (Invitrogen), and 10% fetal bovine serum (Invitrogen). For transfection studies, cells were plated in the same media onto 6-well type 1 collagen-coated plates at 1.5 × 106 cells/well. The ratio of culture media to cell number was maintained constant for the different plating conditions. For treatments, hepatocytes were incubated in medium (Williams E + 25 mM glucose, 10 nM dexamethasone, 1 μM insulin, 50 μM bovine serum albumin, and no serum) in the absence and presence of 250 μM fatty acids (18:1, n-9 or 20:5, n-3) or 100 μM WY14643. Bovine serum albumin used in these studies is endotoxin-and fatty acid-free (Serological Proteins, Inc., Kankakee, IL).
Reporter Plasmids
The reporter plasmid TK-MH100X4-LUC contains four copies of the Gal4-binding element upstream of the thymidine kinase promoter driving luciferase expression (28). phRG-Luc expressing Renilla luciferase was obtained from Promega (Madison, WI); phRG-Luc serves as an internal control for transfection efficiency. The pM-HNF4α1-LBD expression vector was constructed by PCR amplification of the E region, i.e. the ligand binding domain (LBD), of HNF-4α1 (forward primer AATTCATCAGCACGCGGAGGTCAAC-TAC; reverse primer GGGGATCCCGGCAGACCCCAAGCAG-CATCTC) using pBS-HNF-4α1 (obtained from F. Sladek, University of California, Riverside) as template. The HNF-4α1 LBD was ligated in-frame with the Gal4 DNA binding domain of the pM vector (Clontech). Transcription of the Gal4-HNF4α fusion protein is driven by the SV40 early promoter. The sequence of the construct was verified by DNA sequencing. L-PK-Luc was constructed by excising the −196- to +12-bp region from LPK-CAT (5) with BamHI and XhoI and subcloning into TOPOII (Invitrogen). After amplification, the TOPO-PK plasmid was digested with XhoI and SacI to release the L-PK promoter; the fragment was ligated into pGL3-basic vector (Promega).
Transfection, Fatty Acid Treatments, and the Luciferase Assay
Primary hepatocytes were plated in 6-well collagen-coated plates. Each well received 0.5 μg of reporter plasmid (L-PK-LUC or TKMH100X4-LUC) and 0.1 μg of internal control plasmid (phRG-LUC), plus Lipofectin (6.6 μl/μg DNA) or Lipofectamine 2000 (1.5 μl/μg DNA) (Invitrogen) in serum-free Williams E/lactate media (see above). After an overnight transfection, cells were treated with fatty acids (250 μM) for 24 h in serum-free media containing bovine serum albumin (50 μM). After treatment, cells were lysed and assayed for luciferase activity using the dual luciferase assay kit (Promega, Madison, WI) with a dual channel Turner Luminometer. Protein concentration of the cell lysate was measured using the Bio-Rad protein reagent with bovine serum albumin as standard. All experiments were run in triplicate and repeated at least one time.
Recombinant Adenovirus
Recombinant adenoviruses expressing ChREBP and MLXβ were obtained from H. Towle, University of Minnesota, Minneapolis, MN (19). The adenoviruses were constructed using the pAdenoVator-CMV5-IRES-GFP. As such, green fluorescent protein (GFP) is expressed from an internal ribosome entry site. Adenovirus was prepared from Hek293 cell lysates. Virus-containing lysates were titered using the Adeno-X rapid titer kit (Clontech). Confluent primary hepatocytes were infected (5–10 plaque-forming units/cell). After infection, cells were transfected with L-PK-Luc to monitor the effect of overexpressing recombinant proteins on the glucose, 20:5, n-3, or WY14643 control of L-PK-Luc.
RNA Extraction and Northern Blot
RNA was extracted from rat liver or primary hepatocytes and separated on denaturing (formaldehyde) agarose gels, transferred to nitrocellulose, and hybridized with 32P-labeled L-PK-cDNA (5, 29). Hybridization was visualized by Phosphor Imager analysis (Amersham Biosciences). Transcript abundance was also measured by the quantitative reverse transcriptase-PCR (qRT-PCR). Briefly, RNA was extracted from primary hepatocytes (29) and used as template for real time-PCR. Specific primers for each gene (see below) were designed using Primer Express software (Applied Biosystems, Foster City, CA). First strand cDNA was synthesized using the SuperScript II RNase H-reverse transcriptase (Invitrogen). Synthesized cDNA was mixed with 2× SYBR Green PCR Master Mix (Applied Biosystems) and various sets of gene-specific forward and reverse primers and subjected to real time-PCR quantification using the ABI PRISM 7900HT sequence detection system (Applied Biosystems). All reactions were performed in triplicate. The relative amounts of mRNAs were calculated by using the comparative CT method (User Bulletin 2, Applied Biosystems). Cyclophilin was used as a control, and all results were normalized to the abundance of cyclophilin mRNA.
Primers used for real time-quantitative PCR are as follows: L-PK (GenBank™ accession number X05684 (30)), 5′-AGGAGTCTTCCCCT-TGCTCT and 5′-ACCTGTCACCACAATCACCA; FAS (GenBank™ accession number X54671 (31)), 5′-GTGCACCCCATTGAAGGT-TCC and 5′-GGTTTGGAATGCTGTCCAGGG; S14 protein (S14) (GenBank™ accession number M33553 (32)), 5′-CAAGGTGGCAG-GCAATGAAG and 5′-ATGTGAGGAGGCTGGAGAAG; and cyclophilin (GenBank™ accession number NM_017101 (33)), 5′-TGGATGG-CAAGCATGTGGTCTTTG and 5′-CTTCTTGCTGGTCTTGCCAT-TCCT.
Nuclear Protein Extraction and Western Blot
Hepatic total, cytoplasmic, and nuclear proteins were extracted in the presence of protease inhibitors (P8340, Protease Inhibitor Mixture; Sigma), separated electrophoretically by SDS-PAGE (NuPAGE 4–12% polyacrylamide BisTris; Invitrogen), and transferred to nitrocellulose (BAS83; Schleicher & Schuell) as described (34). HNF-4α (C-19), MLX (N-17), anti-goat and anti-rabbit antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). ChREBP antibody was obtained from Novus Biologicals (Littleton, CO). AMPK and pAMPK antibodies were from Cell Signaling (Beverly, MA). The detection system used the Super Signal West Pico chemiluminescence kit (Pierce).
Chromatin Immunoprecipitation Assay
Rat primary hepatocytes were plated at 107 per 10-cm plate (type I collagen-coated) and treated with lactate, glucose, fatty acids, or WY14643. At the times indicated in the figures, cells were fixed by adding formaldehyde (1.0% final concentration) directly to the culture media and incubated at room temperature for 10 min (acetyl-histone-H4 and acetyl-histone-H3), 37 °C for 15 min (RNA pol II and ChREBP), or 37 °C for 30 min (HNF-4α). Cells were washed once with cold PBS containing protease inhibitors (P8340; Sigma), scraped in PBS, homogenized, and transferred into conical tubes. Cells were pelleted and resuspended in 2 ml of SDS lysis buffer (Upstate, Charlottesville, A) with protease inhibitors (P8340; Sigma). The cell lysate was placed on ice for 10 min. The lysate was sonicated with a Branson Sonifier 250 at power level 6, 60% output, 10 pulses for 10 times. Samples were kept on ice and cooled between pulses. After sonication, cell debris was removed by centrifugation, and the supernatant was retained. Sonicated chromatin was aliquoted and stored at −80 °C. Sonicated chromatin (300 μl) was used for each immunoprecipitation (IP) reaction. The IP reaction was performed with Upstate chromatin immunoprecipitation assay kit. The acetyl-histone H3, acetyl-histone H4, and RNA polymerase II antibodies were from Upstate. The HNF-4α (H-171) antibody was from Santa Cruz Biotechnology. 300 μl of sample was diluted into 2 ml with IP dilution buffer (Upstate); 20 μl was withdrawn for input DNA. After preincubation with protein A-agarose beads (Invitrogen) for 30 min and brief centrifugation, the supernatant was incubated with antibody (10 μl for Ac-H3, Ac-H4, or pol II, 20 μg for HNF4α, 5 μl for ChREBP) at 4 °C overnight. Antibody-protein-DNA complex was pulled down with protein A-agarose beads. The complex was washed sequentially with low salt, high salt, lithium washing buffers one time each and two times with 10 mM Tris-EDTA, pH 8.0. Protein-DNA complex was eluted by incubation in elution buffer (0.1 M NaHCO3, 1% SDS) at room temperature for 15 min twice. Supernatants were pooled and the DNA-protein cross-links reversed in 200 mM NaCl (final concentration) at 65 °C for 4 h. Proteins were digested by proteinase K (Roche Applied Science) at 45 °C for 1 h. DNA fragments were purified by phenol/chloroform extraction and ethanol precipitation. Glycogen was used to facilitate DNA precipitation. DNA pellets were resuspended in 50 μl of sterile Milli-Q water. DNA samples (5 μl) were used as template for the PCR. Primers for PCR amplification of the L-PK promoter are in Table 1: −288 to +12 bp (GenBank™ accession number X05684) (30); TAT enhancer, −3730 to −3431 bp (GenBank™ accession number x16379) (35); phosphoenolpyruvate carboxykinase (PepCk) promoter, −550 to +67 bp (GenBank™ accession number k03243) (36); fatty-acid synthase ChoRE, −7221 to −7125 bp (37); S14 ChoRE, −1485 to −1350 bp and the S14 proximal promoter, −290 to +16 bp (GenBank™ accession number M33553) (18, 32); β-actin coding region, +2383 to +3091 bp (GenBank™ accession number V01217) (38).
TABLE 1.
PCR primers used for the chromatin immunoprecipitation assay (CHIP)
| Gene | Region amplified | Primer pair | Primer sequences | Ref. |
|---|---|---|---|---|
| L-Pyruvate kinase | −288- and +12-bp promoter | 5′ | 5′-AGAGATGGAGGCCTTGTGGGG | 30 |
| 3′ | 5′-TACGTTGCTTACCTGCTGTGT | |||
| Tyrosine aminotransferase | −3730- and 3431-bp enhancer | 5′ | 5′-GATGAAGGTGTGTTCGTGGCA | 35 |
| 3′ | 5′-ACTGTGTTCTTGAAGCATCTG | |||
| Phosphoenolpyruvate carboxykinase | −550- and +67-bp promoter | 5′ | 5′-GATCCAGCAGACACCTAGTGG | 36 |
| 3′ | 5′-ATCTCAGAGCGTCTCGCC | |||
| Fatty-acid synthase | −7221- and −7125-bp ChoRE | 5′ | 5′-CTTCCTGCATGTGCCACAGGCGTGTCACCCTC | 37 |
| 3′ | 5′-GGAGGTTTGGCCAATGACCCCTTTGG | |||
| S14 | −1485- and −1350-bp ChoRE | 5′ | 5′-AGCTCTCCCAGCCCTGAC | 18, 32 |
| 3′ | 5′-CCTGGTTGTGTAACTCCCTTTG | |||
| S14 | −290- and +19-bp proximal promoter | 5′ | 5′-GCCTGCAGTCAAGTGTACTGGGT | 18, 32 |
| 3′ | 5′-GCTTCCTTTCTCAGAGACC | |||
| β-Actin | +2383- and +3091-bp coding region | 5′ | 5′-TACTCCTGCTTGCTGATCCAC | 38 |
| 3′ | 5′-GGCTACAGCTTCACCACCAC |
Statistical Analysis
Statistical analysis used Student’s t test and analysis of variance plus post hoc Tukey honestly significant difference test.
RESULTS
Regulation of Rat Hepatic Nuclear Content of ChREBP, MLX, and HNF-4α
ChREBP, MLX, and HNF-4α are key regulatory factors controlling L-PK gene transcription (Fig. 1). L-PK gene transcription is induced by insulin-stimulated glucose metabolism and inhibited by n-3 PUFA and PPARα agonists (4, 6). Fasting and refeeding rats control hepatic glycolysis, L-PK activity, and gene expression (1, 4), whereas feeding rats high carbohydrate diets supplemented with n-3 PUFA or WY14643 suppresses L-PK gene transcription (6). The effect of these dietary manipulations on hepatic L-PK gene expression and ChREBP, MLX, and HNF-4α nuclear abundance was examined (Figs. 2 and 3).
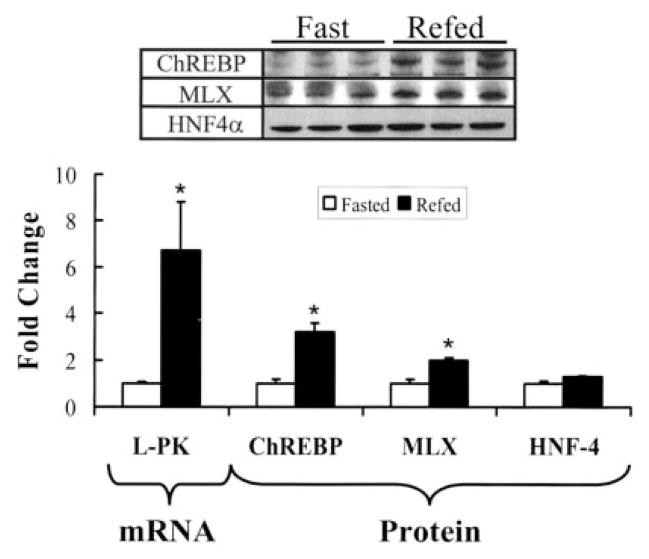
FIGURE 2. Effect of fasting and refeeding on hepatic content of ChREBP, MLX, and HNF-4α.
Rats were meal-fed a high carbohydrate diet supplemented with olive oil for 7 days (“Materials and Methods”). Olive oil-fed rats were fasted overnight (white bars) or meal-fed (black bars) the olive oil diet for 4 h. Fasted rats were euthanized at 8 a.m.; fed rats were euthanized 2 h after completion of the meal, i.e. at 2 p.m. Hepatic RNA and nuclear protein extracts were prepared for Northern and Western blotting. Upper panel, immunoblot of hepatic nuclear abundance of ChREBP, MLX, and HNF-4α from three separate rats per treatment. Lower panel, L-PK mRNA was measured by Northern analysis and quantified along with the abundance of nuclear ChREBP, MLX, and HNF-4 measured by immunoblot. Quantified results are expressed as fold change from the fasted level, mean ± S.D., n = 3. *, p < 0.005, Student’s t test.
FIGURE 3. Effect of dietary fat and WY14643 on hepatic content of ChREBP, MLX, and HNF-4α.
Rats were meal fed a high carbohydrate diet supplemented with olive oil, fish oil, or olive oil + WY14643 for 7 days (“Materials and Methods”). Hepatic RNA and cytoplasmic and nuclear protein were isolated from animals euthanized 2 h after completion of the meal. A, immunoblots for nuclear HNF-4α (HNF-4), ChREBP, and MLX abundance and cytoplasmic ChREBP and MLX. Results are from three separate animals per treatment. B, quantified levels of nuclear protein abundance of ChREBP, MLX, and HNF-4α as measured by immunoblot. C, mRNA abundance of L-PK, ChREBP, MLX, and HNF-4α as measured by qRT-PCR. Results are expressed as fold change from olive oil-fed rats. Results are the mean ± S.D. for seven different animals per treatment. *, p ≤ 0.01, analysis of variance.
When compared with rats fasted overnight, feeding rats a high carbohydrate diet supplemented with olive oil induced mRNAL-PK 6-fold. The nuclear abundance of ChREBP and MLX was induced 4- and 2-fold, respectively (Fig. 2). HNF-4α nuclear abundance remained unchanged by fasting or refeeding.
The effect of dietary fat and WY14643 on the nuclear abundance of ChREBP, MLX, and HNF-4α and L-PK gene expression was examined in rats meal-fed a high carbohydrate diet supplemented with olive oil, fish oil, or olive oil + WY14643 for 7 days (Fig. 3). Olive oil is enriched in n-9 monounsaturated fatty acids, and fish oil is enriched in C20–22 n-3 PUFA. Both fish oil and WY14643-supplemented diets suppressed L-PK mRNA (Fig. 3C). However, changes in dietary fat or WY14643 composition had no effect on hepatic nuclear or cytoplasmic ChREBP or HNF4α. Moreover, these diets did not affect hepatic mRNAChREBP or mRNAHNF4α abundance.
Rats fed the high carbohydrate diet supplemented with fish oil, however, had 50% less nuclear MLX when compared with the other diets. The fish oil diet had no effect on mRNAMLX abundance. Cytoplasmic MLX levels were higher in fish oil-fed animals when compared with olive or olive + WY14643-fed animals (Fig. 3A, lower panel) suggesting fish oil treatment may act at a post-translational level to inhibit MLX translocation to the nucleus.
These results indicate that in vivo changes in MLX, and not ChREBP or HNF-4α nuclear abundance, correlate with n-3 PUFA suppression of L-PK gene expression. In contrast, WY14643 has no effect on the nuclear abundance of the three main transcription factors controlling glucose-regulated L-PK gene expression.
Glucose, Fatty Acid, and WY14643 Effects on L-PK Expression and ChREBP, MLX, and HNF-4α Nuclear Abundance in Rat Primary Hepatocytes
Rat primary hepatocytes were used to examine the time course for glucose, fatty acid, and WY14643 control of mRNAL-PK (Fig. 4) and ChREBP and MLX nuclear abundance (Fig. 5). Primary rat hepatocytes maintained overnight in Williams E medium containing lactate (10 mM), insulin (1 μM), and dexamethasone (10 nM) were switched to Williams E medium containing glucose (25 mM), insulin (1 μM), dexamethasone (10 nM), and either fatty acids (oleic acid, 18:1, n-9, or eicosapentaenoic acid, 20:5, n-3) at 250 μM or WY14643 at 100 μM. Cells were then harvested at various times to examine hepatocyte abundance of mRNAL-PK (Fig. 4, A and B). A second group of cells were transfected with an L-PK-luciferase (L-PK-Luc) reporter plasmid and treated as above to monitor effects on L-PK promoter activity (Fig. 4C).
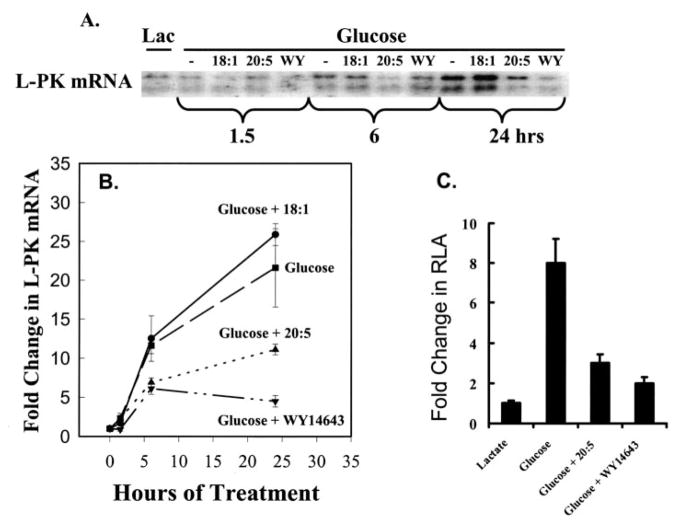
FIGURE 4. Regulation of L-PK mRNA in primary hepatocytes by glucose, 18:1, n-9, 20:5, n-3, and WY14643.
Rat primary hepatocytes were maintained in Williams E medium +10 mM lactate (Lac) + 10 nM insulin or switched to Williams E containing 25 mM glucose + 1 μM insulin in the absence or presence of 250 μM fatty acids (18:1, n-9 or 20:5, n-3) or 100 μM WY14643. Cells were harvested at the times indicated for total RNA extraction. L-PK mRNA was detected by Northern analysis (A) and quantified (B). Quantified results are expressed as fold change in L-PK mRNA from lactate-treated cells, mean + S.D., n = 4. C, primary hepatocytes in lactate-containing medium were transfected with L-PK-Luc (firefly) and phRG-Luc (Renilla) (see “Materials and Methods”) and treated as above with glucose, fatty acids, or WY14643 for 24 h. Cells were harvested for luciferase assays. Results are expressed as the fold change in relative luciferase activity (RLA) from lactate-treated cells, mean ± S.D., n = 4.
FIGURE 5. The effect of glucose, 20:5, n-3, and WY14643 on the nuclear abundance of ChREBP and MLX in rat primary hepatocytes.
Rat primary hepatocytes were maintained in lactate-containing Williams E + insulin or switched to Williams E + glucose + insulin without or with 20:5, n-3 (250 μM) or WY14643 (100 μM). Cells were harvested at the times indicated. Nuclear protein extracts were assayed for the amount of ChREBP or MLX by immunoblotting (A). The immunoblots were quantified for ChREBP(B) and MLX (C) nuclear abundance. HNF-4α remained unchanged by this treatment (not shown). Results are expressed as fold change from lactate (0-h)-treated cells; mean ± S.D., n = 3. *, p < 0.05 versus glucose treated cells, Student’s t test.
Glucose had no significant effect on mRNAL-PK abundance for the first 1.5 h after beginning treatment. By 6 h, however, mRNAL-PK was induced 12-fold by glucose and glucose +18:1, n-9 (Fig. 4). After 24 h of glucose treatment, mRNAL-PK was induced 20–25-fold by glucose or glucose + 18:1, n-9. Both 20:5, n-3 and WY14643 significantly attenuate the glucose induction of mRNAL-PK at 6 h (~50%). At 24 h, 20:5, n-3 and WY14643 suppressed the glucose-stimulated accumulation of mRNAL-PK by 60 and 80%, respectively.
Glucose induced L-PK-Luc activity 8-fold, and 18:1, n-9 did not significantly affect this response. 20:5, n-3 and WY14643, however, suppressed the glucose induction of L-PK-Luc activity by >75% (Fig. 4C). These studies confirmed previous studies (6) and provided key time course information on the response of the L-PK gene to glucose, fatty acid, and WY14643 challenge.
The effects of glucose, 20:5, n-3, and WY14643 on the nuclear abundance of ChREBP and MLX were examined (Fig. 5). Both ChREBP and MLX were detected in nuclei of hepatocytes maintained in lactate-containing medium (Fig. 5). Switching cells to glucose-containing medium induced nuclear ChREBP with no change in nuclear MLX. Particularly important is the robust (4-, 7-, and 12-fold) glucose-stimulated induction of hepatic nuclear ChREBP at 1.5, 6, and 24h. 20:5, n-3 and WY14643 had no effect on glucose-stimulated ChREBP nuclear abundance at 1.5 or 24 h but modestly attenuated (~50%) the response at 6 h. 18:1, n-9 also had no effect on glucose-stimulated translocation of ChREBP to the nucleus (not shown).
MLX nuclear abundance was transiently suppressed (70%) at 1.5 h by 20:5, n-3. By 6 and 24 h, MLX nuclear abundance was not different from cells receiving glucose alone. WY14643 had no effect on nuclear MLX abundance. As in vivo (Fig. 3), the nuclear abundance of HNF-4α in rat primary hepatocytes remained unaffected by 20:5, n-3 or WY14643 treatment (not shown). These results reveal a transient effect of PUFA and WY14643 on ChREBP nuclear abundance and PUFA on MLX nuclear abundance.
Glucose, Fatty Acid, and WY14643 Effects on L-PK Promoter Composition
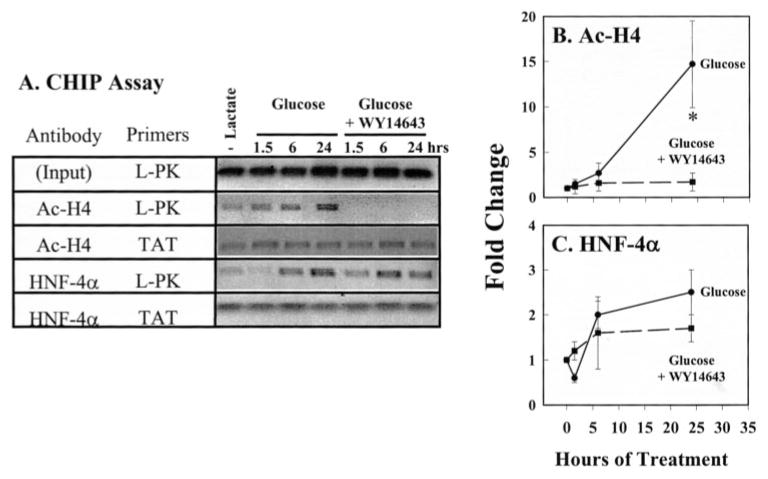
The ChIP assay was used in conjunction with the time course approach to examine changes in L-PK promoter composition during glucose, 20:5, n-3, and WY14643 control of L-PK gene expression (Fig. 6). In primary hepatocytes maintained in Williams E + insulin + lactate, L-PK promoter activity and mRNAL-PK is low (Fig. 4). Little RNA pol II, Ac-H3, and Ac-H4 is bound to the L-PK promoter (Fig. 6A). Despite the absence of RNA pol II, Ac-H3, and Ac-H4 from the L-PK promoter, both ChREBP and HNF-4α are bound to the promoter. Technical limitations precluded an accurate measure of MLX on the L-PK promoter. Because ChREBP binding to the L-PK ChoRE requires MLX in vitro (39), our analysis may indirectly reveal the presence of MLX on the L-PK promoter. As such, ChREBP/MLX heterodimers and HNF-4α homodimers are bound to the L-PK promoter in the absence of RNA pol II and acetylated histone H3 and H4.
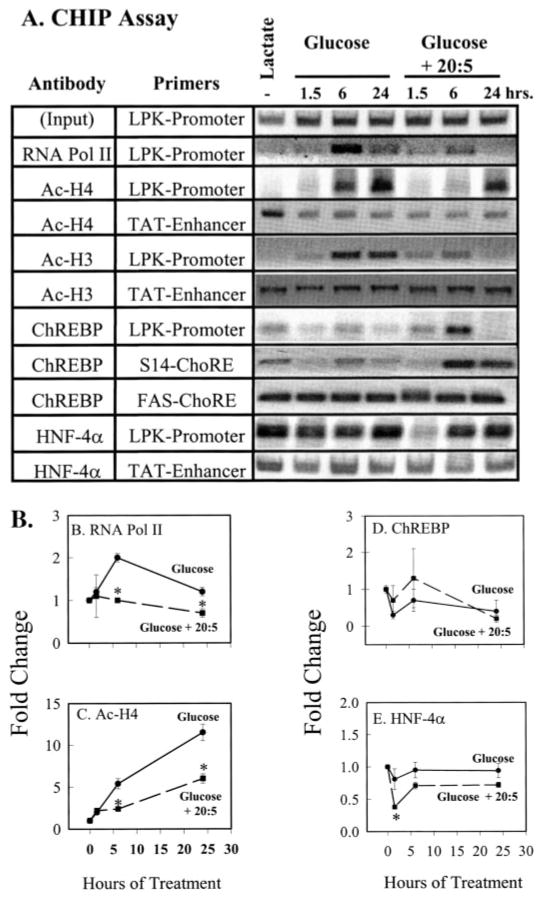
FIGURE 6. Effects of glucose and 20:5, n-3 on L-PK promoter composition.
The ChIP assay was used to examine the composition of the rat L-PK, TAT, FAS, and S14 promoters. HNF-4 binds the TAT enhancer (TAT-Enhancer) −3062 to −3579 bp upstream from the transcription start site. The S14 ChoRE is located between −1.4 and −1.35 kb from the transcription start site. The fatty-acid synthase ChoRE (FAS-ChoRE) is located between −7.2 and −7.1 kb from the transcription start site (Table 1). Rat primary hepatocytes were treated as described in Fig. 5. At the times indicated, cells were treated with formaldehyde for the ChIP assay. Fragmented chromatin was immunoprecipitated (IP) with antibodies against RNA polymerase II (RNA pol II), acetylated histone H3 (Ac-H3), acetylated histone H4 (Ac-H4), ChREBP or HNF-4α. DNA was extracted from the immunoprecipitated complex, and 5 μl of 50 μl of purified DNA was used as template for PCR to amplify the L-PK promoter (−288 to +12 bp) or the TAT enhancer (−3730 to −3431 bp), the S14 ChoRE (−1485 to −1350 bp) and the FAS-ChoRE (−7221 to −7125 bp). Primers for the S14 proximal promoter (−290 to +19 bp), the β-actin coding (+2383 to +3091 bp), and the PepCk promoter (−550 to + 67 bp) were also used (Table 1). The PCRs for the S14 promoter, β-actin coding region, and PepCk promoter did not yield PCR products from chromatin immunoprecipitated with ChREBP antibody. Input, 1% of the sample used for IP was taken out prior to IP to represent total DNA quantity. A, representative PCR products from the input DNA and DNA immunoprecipitated with RNA pol II, Ac-H3, Ac-H4, ChREBP, or HNF-4α antibodies. Results of the ChIP assay were quantified (B–E); mean ± S.D. for three independent studies. Glucose-treated cells (filled circles, solid line); glucose + 20:5-treated cells (filled box, dashed line). *, p < 0.01 versus glucose-treated cells, Student’s t test.
Glucose Effects
Switching primary hepatocytes from lactate to glucose-containing medium increased the abundance of RNA pol II, Ac-H3, and Ac-H4 on the L-PK promoter at 1.5 h. By 6 h, RNA pol II on the L-PK promoter increased to a maximum (2-fold) above basal values. By 24 h, however, RNA pol II levels on the L-PK promoter returned to near pretreatment levels (Fig. 6, A and B). Ac-H3 and Ac-H4 on the L-PK promoter increased significantly (6-fold) by 6 h and remained elevated 24 h after initiating glucose treatment (Fig. 6C). The effect of glucose on Ac-H3 and Ac-H4 promoter content was specific; the acetylation status of H3 and H4 on the TAT (Fig. 6) and PepCk (not shown) promoters was not affected by glucose. Neither TAT nor PepCk expression is induced by glucose.
In contrast to RNA pol II, Ac-H3, and Ac-H4, the abundance of ChREBP and HNF-4α on the L-PK promoter was not significantly affected by glucose treatment. Thus, glucose-stimulated recruitment of RNA pol II to the L-PK promoter and the increased acetylation of H3 and H4 are not accompanied by demonstrable changes in ChREBP or HNF-4α promoter occupancy.
Because glucose has no effect on HNF-4α nuclear content, we were not surprised to find no major effect of glucose on HNF-4α interaction with the L-PK promoter. In contrast, glucose stimulated a significant accumulation of nuclear ChREBP. As such, we expected to measure an increase ChREBP binding to the L-PK promoter using the ChIP assay. Instead, no major change in ChREBP-L-PK promoter interaction was detected. This outcome questioned the efficacy of the ChREBP-ChIP assay to accurately reflect ChREBP-ChoRE interaction. Several control studies were carried out to assess the efficacy of this assay.
β-Actin is not regulated by glucose or ChREBP; DNA encoding the β-actin structural gene was not detected in ChREBP immunoprecipitates (not shown). Transcription of both S14 and FAS is induced by glucose, and ChoREs have been identified in the promoters of both genes (17, 40). ChREBP immunoprecipitates contained both the S14 and FAS ChoRE DNA. Glucose did not consistently or significantly alter ChREBP interaction with the S14 or FAS ChoREs in primary hepatocytes (Fig. 6). ChREBP is not bound to the S14 proximal promoter (−290 to + 19 bp), and this DNA was not detected in the ChREBP immunoprecipitates (not shown). Taken together, these results indicate that the ChIP assay for ChREBP provides an accurate measure of ChREBP-ChoRE containing complexes.
20:5, n-3 Effects
Treating cells with glucose + 20:5, n-3 inhibits glucose-stimulated L-PK gene transcription (6), mRNAL-PK accumulation, and L-PK-Luc activity (Fig. 4). As expected, 20:5, n-3 attenuated recruitment of RNA pol II to the promoter and the acetylation of histones H3 and H4 (Fig. 6 A–C) at 1.5 and 6 h. This effect correlated with the 50% suppression of L-PK mRNA (Fig. 4) During this time, 20:5, n-3 treatment did not interfere with ChREBP interaction with the L-PK promoter. The effect of 20:5, n-3 on histone acetylation was specific to the L-PK promoter because histone H3 and H4 acetylation status remained unaffected on the TAT (Fig. 6) and PepCk promoters (not shown).
After 24 h of treatment, however, ChREBP, RNA pol II and acetylated H3 were not detected on the L-PK promoter (Fig. 6, A and D). ChREBP interaction with the ChoRE of the S14 and FAS promoter was not consistently affected by 20:5, n-3 treatment at any time point examined (Fig. 6A).
In contrast to ChREBP, 20:5, n-3 treatment induced a rapid (at 1.5 h) but transient (~60%) decline in HNF-4α interaction with the L-PK promoter (Fig. 6, A and E). The effect of 20:5, n-3 on HNF4α-L-PK promoter interaction is specific, because similar changes were not detected on the TAT (Fig. 6) or PepCk promoters (not shown).
WY14643 Effects
Treatment of cells with glucose plus WY14643 significantly suppressed mRNAL-PK and L-PK-Luc activity (Fig. 4) and completely suppressed acetylation of histone H4 on the L-PK promoter (Fig. 7, A and B). WY14643 had no effect on the interaction of HNF-4α with either the L-PK or TAT promoters (Fig. 7, A and C). Although RNA pol II abundance on the L-PK promoter was not examined, previous studies established that WY14643 strongly suppressed L-PK gene transcription (6).
FIGURE 7. Effects of glucose and WY14643 on L-PK promoter composition.
The ChIP assay was used to examine the promoter composition of the rat L-PK and TAT promoters following glucose and WY14643 treatment. Rat primary hepatocytes were treated as described in Fig. 4 and harvested for the ChIP assay. Fragmented chromatin was immunoprecipitated (IP) with antibodies against acetylated histone H4 (Ac-H4) and HNF-4α. DNA was extracted from the immunoprecipitates; 5 μl of 50 μl of purified DNA was used in a PCR to detect the L-PK promoter (−288 to +12 bp) and TAT promoter (−3730 to −3431 bp). Input, 1% of the samples to be used in IP were taken out prior to IP to represent total DNA quantity. A, representative PCR products form the input and products immunoprecipitated with Ac-H4 or HNF-4α antibodies. B and C, PCR products were quantified; mean ± S.D. for three independent studies. Glucose-treated cells (filled circles, solid line); Glucose + WY14643-treated cells (filled box, dashed line). Means ± S.D. for three independent studies. *, p < 0.01 versus glucose-treated cells, Student’s t test.
Taken together, these results indicate that glucose, 20:5, n-3, and WY14643 effects on L-PK gene transcription can be explained by regulation of RNA pol II and histone acetylation status on the L-PK promoter. Effects of these treatments on L-PK expression, however, cannot be explained by regulation of major changes in ChREBP or HNF-4α interaction with the L-PK promoter.
Mechanisms Controlling L-PK Promoter Activity
ChREBP, MLX, and HNF-4α are important for glucose-regulated L-PK gene transcription. The ChIP analysis, however, failed to provide clues as to how glucose, 20:5, n-3, or WY14643 control RNA pol II interaction with the L-PK promoter. More subtle mechanisms are likely involved. This section examines three mechanisms as possible explanations for the nutrient control of L-PK gene expression.
The Role of AMPK in the Control of L-PK Gene Expression
AMPK is an established regulator of L-PK gene expression (24, 41). Both ChREBP and HNF-4α are potential targets of AMPK. AMPK phosphorylation of ChREBP inhibits ChREBP translocation to the nucleus. AMPK phosphorylation of HNF-4α impairs HNF-4α dimer stability and DNA binding (42). Two groups have reported that fatty acids activate AMPK through phosphorylation of AMPK (24, 43). Because 20:5, n-3 and WY14643 transiently antagonized (at 6 h) glucose-mediated ChREBP translocation (Figs. 3 and 5) and 20:5, n-3 transiently antagonized HNF-4αinteraction with the L-PK promoter (at 1.5 h) (Fig. 6), we examined the effect of n-3 PUFA and WY14643 on AMPK phosphorylation.
As a control study, we first established that AMPK controls L-PK gene expression in primary rat hepatocytes. Metformin and 5-amino-4-imidazole carboxamide ribotide, two AMPK activators, strongly suppressed mRNAL-PK and L-PK promoter activity in rat primary hepatocytes (supplemental Fig. 1S). We next examined AMPK phosphorylation in vivo and in primary rat hepatocytes. Fasting rats overnight elevated cytosolic phospho-AMPK (pAMPK, active form) ~4-fold over levels seen in meal-fed rats (supplemental Fig. 2S). Feeding rats n-3 PUFA- or WY14643-supplemented diets did not significantly induce cytosolic pAMPK in liver (in vivo). Nuclear pAMPK levels were induced ~30% by fasting and 2-fold by WY14,643. Feeding a fish oil-supplemented diet, however, had no effect on nuclear pAMPK.
In primary rat hepatocytes, time course studies indicated that glucose, 20:5, n-3, or WY14643 did not significantly induce nuclear or cytosolic pAMPK or total AMPK (supplemental Fig. 2S). The results with primary hepatocytes differ from the in vivo studies in two ways. First, pAMPK was not elevated in lactate-treated cells. This result is likely due to the presence of insulin (1 μM) in the culture media. Second, WY14643 did not induce nuclear or cytosolic pAMPK in primary hepatocytes, as seen in vivo. This difference is likely due to experimental design. The effect on liver nuclear pAMPK was assessed after 7 days on the WY14643 diet. Livers from WY14643-fed rats were enlarged, i.e. hepatomegaly. Hepatomegaly is commonly seen in rodents fed robust PPARα activators, like WY14643 (44). Rat primary hepatocytes cultured in the absence or presence of WY14643 do not replicate within the 24-h time frame of the experiment. As such, the effect of WY14643 on nuclear pAMPK abundance in vivo is likely not a rapid effect of WY14643 on hepatic function. Taken together, the transient effects of n-3 PUFA and WY14643 on ChREBP and MLX nuclear abundance or HNF-4α interaction with the L-PK promoter cannot be explained by enhanced AMPK phosphorylation.
Effects of n-3 PUFA and WY14643 on HNF-4α1 Transactivation
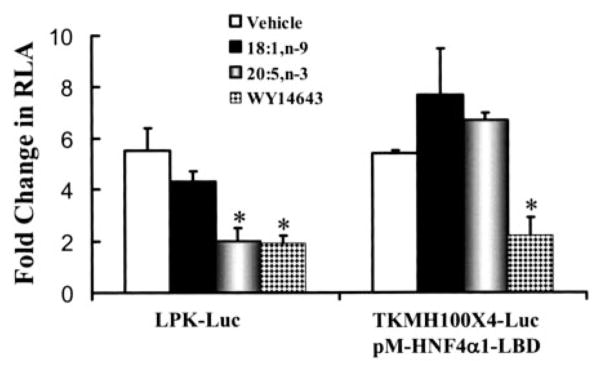
Treatment of primary hepatocytes with exogenous fatty acids leads to the rapid uptake of fatty acids, conversion to fatty acyl-CoA, and assimilation of fatty acids into complex lipids (45). HNF-4α and −4γ bind fatty acids and fatty acyl-CoA in vitro (46–48). HNF-4α is implicated as a target of fatty acid regulation because the DR-1 element, which binds HNF-4α, is required for the n-3 PUFA and WY14643 suppression of LPK gene transcription (6). Moreover, 20:5, n-3 but not WY14643 transiently antagonized HNF-4α interaction with the L-PK promoter (Fig. 6). A transfection approach was used to determine whetherHNF-4α1 transactivation was affected by either fatty acids or WY14643.
This study compared the effects of fatty acids (18:1, n-9 and 20:5, n-3) and WY14643 on two reporter systems as follows: 1) L-PK-Luc and 2) the Gal4-responsive reporter plasmid (TKMH100×4) activated by the Gal4-HNF4α1 expression vector (Fig. 8). The L-PK-luciferase reporter gene contains L-PK promoter elements extending to −197 bp (see Fig. 1 and Fig. 4). Treatment of transfected hepatocytes with glucose (vehicle) or glucose + 18:1, n-9 stimulated L-PK-Luc promoter activity 5–6-fold. Treatment with glucose + 20:5, n-3 or glucose + WY14643 attenuated this response by ~80%.
FIGURE 8. Effect of fatty acids and WY14643 on HNF-4α transactivation in rat primary hepatocytes.
Primary rat hepatocytes were transfected with L-PK-Luc reporter plasmid or co-transfected with the TKMH100×4-Luc reporter plasmid plus the pM-HNF4α1-LBD plasmid. pM-HNF-4α-LBD contains the SV40 early promoter driving expression of a fusion protein consisting of the Gal4 DNA binding domain fused to the HNF-4α1 ligand binding domain (E region). All transfections included phRG-Luc as an internal control (see “Materials and Methods”). All cells were transfected while in Williams E medium containing lactate +insulin. Cells were switched to Williams E medium + glucose + insulin in the absence and presence of 250 μM 18:1, n-9, 20:5, n-3, or 100 μM WY14643. Cells were harvested 24 h later for the luciferase assay. Results are expressed as fold induction of relative luciferase activity (RLA). L-PK-Luc activity was induced by glucose, whereas TKMH100×4-Luc activity was induced by co-transfection of pM-HNF4α-LBD. Lactate and glucose did not affect TKMH100×4-Luc activity in the presence or absence of pM-HNF4α-LBD. Means ± S.D., n = 6. Similar results were obtained using a pM-HNF-4α containing the full-length HNF-4α fused to the Gal4 DNA binding domain. *, p < 0.05 versus vehicle-treated cells, Student’s t test.
Transactivation of HNF-4α1 used an expression vector containing the HNF-4α1 ligand binding domain (E region) fused to the Gal4-DNA binding domain to create the pM-HNF4α1-LBD expression vector. The TKMH100×4-Luc reporter plasmid contains Gal4 regulatory elements that bind the Gal4-HNF4α1-LBD fusion protein. Co-transfection of pM-HNF4α1-LBD with MHTK100×4-Luc induces luciferase activity 6-fold (Fig. 8). Transfection of hepatocytes with pM-VP16 + MHTK100×4-Luc induced Luc activity 25-fold, whereas transfection of hepatocytes with the pM vector (contains only the Gal4-DBD) + MHTK100×4-Luc had no effect on luciferase activity (not shown).
Primary hepatocytes transfected with pM-HNF4α1-LBD plus THMH100×4-Luc and treated with glucose + 18:1, n-9 or glucose + 20:5, n-3 had no effect on luciferase activity (Fig. 8). In contrast, WY14643 treatment of transfected hepatocytes suppressed luciferase activity by 75%. Similar results were obtained with a Gal4 fusion protein containing the full-length (A–F regions) HNF-4α1 (not shown). Although co-transfection of pM-VP16 + TKMH100×4-Luc induced luciferase activity 25-fold, glucose, 20:5, n-3, or WY14643 treatment had no effect on this response. Thus, the inhibitory effect of WY14643 on HNF-4α1 is specific and not because of a generalized effect on gene transcription or luciferase activity. These results indicate that WY14643 interferes with HNF-4α1 transactivation. Fatty acids (18:1, n-9 or 20:5, n-3), however, have no effect on HNF-4α1 transactivation.
Effect of Overexpressed ChREBP and MLX on L-PK Gene Expression
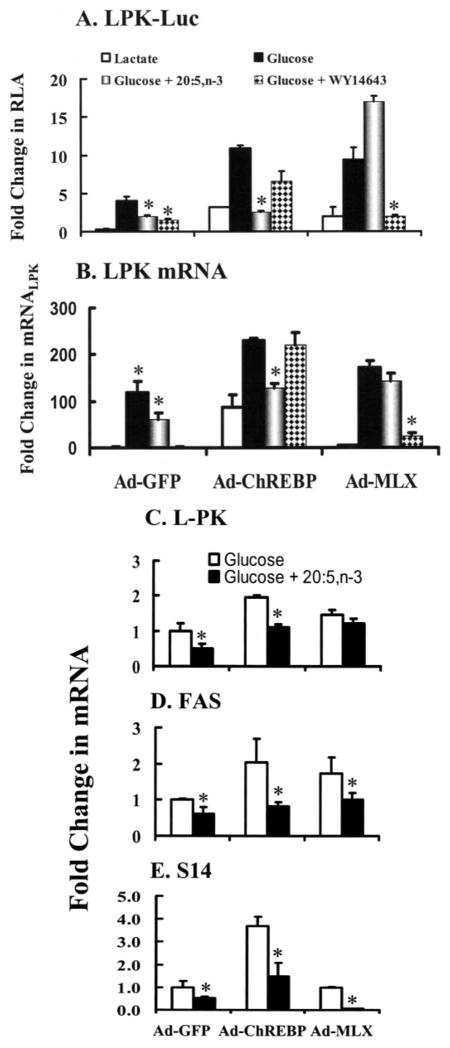
n-3 PUFA and WY14643 transiently suppressed ChREBP and MLX nuclear abundance in primary hepatocytes (Figs. 3 and 5). However, in vivo, n-3 PUFA but not WY14643 suppressed MLX nuclear abundance. Glucose, n-3 PUFA, and WY14643 had no effect on HNF-4αnuclear abundance in vivo or in rat primary hepatocytes. These studies suggest that transient control of ChREBP or MLX nuclear abundance might be important for the control of L-PK gene expression. To test this possibility, primary hepatocytes were infected with recombinant adenovirus-expressing green fluorescent protein (Ad-GFP) as a control, Ad-MLX, or Ad-ChREBP. After infection, hepatocytes were transfected with L-PK-Luc (Fig. 9A). A second group of cells was infected with recombinant adenovirus but not transfected with L-PK-Luc (Fig. 9, B–E).
FIGURE 9. Effect of overexpressed ChREBP and MLXβ on L-PK-Luc activity, mRNAL-PK mRNAS14, and mRNAFAS.
A, rat primary hepatocytes were infected with recombinant adenovirus expressing green fluorescent protein (GFP, Ad-GFP, control), ChREBP (Ad-ChREBP), or MLX (Ad-MLXβ) while in Williams E medium containing lactate and insulin. GFP is expressed from an internal ribosome entry site in the Ad-ChREBP and Ad-MLX adenovirus. Expression levels of GFP in primary hepatocytes were verified by fluorescence microscopy. Twenty four hours after infection, cells were transfected with L-PK-Luc and phRG-Luc overnight. The next day cells were maintained in Williams E containing lactate + insulin or switched to Williams E + glucose + insulin without or with 20:5, n-3 or WY14643. Cells were harvested 24 h later for luciferase activity. Results are expressed as fold change in relative luciferase activity (RLA); means ± S.D., n = 3. Results are representative of three separate studies with triplicate samples in each study. B and C, rat primary hepatocytes were infected with recombinant adenovirus as described above. Twenty four hours after infection, cells were switched from the lactate containing medium to the glucose containing medium in the absence or presence of 20:5, n-3 or WY14643. Cells were harvested 24 h later for RNA extraction and measurement of L-PK, FAS, S14, and cyclophilin mRNA abundance by qRT-PCR. The abundance of L-PK, FAS, or S14 mRNA relative to cyclophilin mRNA was calculated by the Ct method. B, results are quantified and expressed as fold change in mRNAL-PK-induced virus infection and by glucose, glucose + 20:5, or glucose + WY14643. Results are normalized to the level of mRNAL-PK in the lactate-treated cells infected with Ad-GFP. Results are the means of two separate studies. C–E, the effect of 20:5, n-3 on L-PK, FAS, and S14 mRNA in primary hepatocytes infected with Ad-GFP, Ad-ChREBP, or Ad-MLX was quantified. Cells were treated as above. Results are reported fold change in mRNA; results are normalized to the transcript level in glucose-treated-Ad-GFP-infected cells. Results are the means ± range of duplicate studies. Results are representative of two independent studies. *, p < 0.05 versus glucose-treated cells, Student’s t test.
In the transfection study, cells were treated with glucose or glucose + 20:5, n-3, or glucose +WY14643. In control (Ad-GFP)-infected cells, glucose induced L-PK-Luc activity 5-fold. Infection of cells with Ad-ChREBP induced L-PK-Luc activity 4-fold in hepatocytes cultured in lactate-containing medium. Changing the culture medium to glucose induced L-PK-Luc activity another 4-fold. Changing the culture medium to glucose + 20:5, n-3 suppressed L-PK-Luc activity by >50% and ~90% in control and Ad-ChREBP-infected cells, respectively. Overexpressed ChREBP partially relieved the inhibition of L-PK promoter activity by WY14643 (Fig. 9A).
Infection of cells with Ad-MLX induced L-PK-Luc activity 3-fold in hepatocytes maintained in lactate-containing medium. Glucose treatment induced L-PK-Luc activity another 5-fold. Switching the cells to glucose +20:5, n-3 induced Luc activity 8-fold, whereas switching hepatocytes to glucose + WY14643 did not induce L-PK-Luc activity.
Analysis of mRNAL-PK abundance indicated that the response of the endogenous L-PK gene was similar to transfected L-PK-Luc (Fig. 9B). These findings indicate that overexpressed ChREBP and MLX induce L-PK gene transcription. Overexpressed ChREBP relieves WY14643, but not 20:5, n-3, suppression of L-PK gene expression. Overexpressed MLX abrogates 20:5, n-3, but not WY14643, suppression of L-PK gene expression. Overexpressed HNF-4α has no effect on glucose, 20:5, n-3, or WY14643 control of L-PK-Luc activity (not shown).
Fatty-acid synthase and S14 are two well established glucose- and fatty acid-regulated genes (4, 19, 28). The effect of overexpressed ChREBP and MLX on glucose and fatty acid on control of mRNAFAS and mRNAS14 was compared with the effect on mRNALPK (Fig. 9, C–E). Overexpressed ChREBP induced mRNALPK, mRNAFAS, and mRNAS14 by 2- and 2–4-fold in hepatocytes cultured in glucose + insulin (not shown). Switching the medium to glucose + 20:5, n-3 suppressed mRNALPK, mRNAFAS, and mRNAS14 by ~50% in cells infected with Ad-GFP or Ad-ChREBP recombinant adenovirus. Overexpressed ChREBP did not relieve the 20:5, n-3 suppression of L-PK, FAS, or S14.
Overexpressed Ad-MLX abrogated the 20:5, n-3 suppression of mRNALPK but not 20:5, n-3 suppression of mRNAFAS or mRNAS14. In fact, overexpressed MLX accentuated the 20:5, n-3 suppression of S14.
These results indicate that MLX is a target for 20:5, n-3 control of L-PK gene expression. For genes like S14 and FAS that also utilize SREBP-1 as a key transcription factor (2, 49), changes in MLX abundance alone cannot relieve 20:5, n-3 suppression.
DISCUSSION
L-PK plays a central role in hepatic carbohydrate and lipid metabolism. Insulin-stimulated glucose metabolism induces L-PK gene expression, L-PK enzyme activity, and the flow of glucose metabolites toward fatty acid synthesis and storage. The inhibitory effect of dietary n-3 PUFA on L-PK gene transcription is part of a larger regulatory network that shifts hepatic metabolism away from lipid synthesis and storage and toward fatty acid oxidation (2). Glucose and n-3 PUFA manage these metabolic pathways by controlling the activity or nuclear abundance of key transcription factors, including ChREBP, MLX, SREBP-1, and PPARα. WY14643 and n-3 PUFA activate PPARα by direct binding (50). n-3 PUFA suppresses SREBP-1 nuclear abundance by multiple mechanisms, including enhanced 26 S proteasomal degradation (2, 51). Neither PPARα/RXRα nor SREBP-1 bind the L-PK promoter; neither transcription factor regulates L-PK expression directly (6, 49, 52). Until now, the mechanism by which n-3 PUFA and WY14643 controlled L-PK gene transcription remained elusive. This study presents new information to explain how glucose, n-3 PUFA, and WY14643 regulate hepatic L-PK gene transcription through effects on ChREBP and MLX nuclear abundance and L-PK promoter composition.
Our analysis has yielded both expected and unexpected results. As expected, fasting and refeeding rats or treating rat primary hepatocytes with glucose induced a robust accumulation of ChREBP in hepatic nuclei and the induction of mRNAL-PK (Figs. 2 and 5). Prior to glucose activation of L-PK gene transcription, the L-PK promoter has no detectable RNA pol II, Ac-H3, or Ac-H4. Surprisingly, however, both ChREBP and HNF-4α are bound to the L-PK promoter. Glucose treatment of rat primary hepatocytes stimulates ChREBP accumulation in nuclei (Fig. 5), recruitment of RNA pol II to the L-PK promoter, the acetylation of histones H3 and H4 (Fig. 6), and L-PK promoter activity (Fig. 4). During the time course of L-PK gene activation, ChREBP undergoes modest changes in occupancy on the L-PK promoter. HNF-4α interaction with the LPK promoter remains unchanged. These results indicate that factors other than the mere presence of ChREBP on the L-PK promoter are involved in glucose-mediated induction of L-PK gene expression.
20:5, n-3 and WY14643 suppressed L-PK promoter activity and inhibited RNA pol II recruitment to the L-PK promoter and the acetylation of histone H3 and H4 on the LPK promoter (Figs. 6 and 7). 20:5, n-3 and WY14643 transiently suppressed ChREBP and MLX nuclear abundance at 6 and 1.5 h but did not significantly affect ChREBP interaction for 6 h after initiating glucose treatment. Only after 24 h was a decline in ChREBP abundance on the L-PK promoter observed. HNF-4α interaction with the L-PK promoter was also transiently suppressed by 20:5, n-3 but not WY14,643. Clearly, the interaction of ChREBP and HNF-4α with the L-PK promoter is independent of the presence of RNA pol II, acetylated histones H3 and H4, or the transcriptional status of the L-PK gene.
The dissociation between glucose-stimulated ChREBP nuclear abundance and the lack of significant change in ChREBP occupancy on the L-PK promoter have at least two explanations. First, our measure of ChREBP and HNF-4α binding to the L-PK promoter is an artifact. Our control studies, however, validated the ChIP assay for ChREBP and HNF-4α (Fig. 6). Second, the ChIP assay when applied to the measure of endogenous proteins on chromatin does not reveal an exchange of one protein for another; it only detects the presence or absence of a protein on chromatin. During the 90-min lag preceding RNA pol II recruitment to the L-PK promoter, glucose stimulated a robust (5-fold) translocation of ChREBP to the nucleus (Fig. 5). This translocation is likely stimulated by glucose metabolism and the generation of xylulose 5-phosphate, a PP2A activator. Dephosphorylated ChREBP moves to the nucleus (22, 24). The influx of nascent ChREBP into the nucleus, coupled with its heterodimerization with MLX, likely remodels the L-PK promoter with nascent ChREBP/MLX heterodimers and HNF-4α homodimers. Binding of these nascent proteins to the L-PK promoter may induce recruitment of RNA pol II to the promoter and the acetylation of histones H3 and H4. Unfortunately, the differences in ChREBP abundance on the L-PK promoter over this time course did not reach significance.
Gene activation by ligand-activated nuclear receptors involves remodeling of promoter composition (53, 54). This process requires a change in the covalent modification status of chromatin-associated proteins. Ubiquitination of transcription factors and their 26 S proteasomal degradation has emerged as an important mechanism controlling promoter composition (53–55). Inhibitors of the 26 S proteasome significantly affect gene activation. In this regard, preliminary studies with MG132, a 26 S proteasome inhibitor, attenuated glucose activation of L-PK gene transcription (not shown). Whether any proteins on the L-PK promoter are ubiquitinated remains to be determined.
Glucose-stimulated exchange of proteins on the L-PK promoter may not fully explain why L-PK promoter activity is low despite the presence of ChREBP on the promoter. It is equally possible that ChREBP heterodimerization with other basic helix-loop-helix proteins yields a complex that fails to recruit RNA pol II and the associated co-regulators required for gene activation. Although ChREBP binding to ChoRE requires MLX (39), a role for other basic helix-loop-helix proteins forming heterodimer partners with ChREBP has not been excluded.
n-3 PUFA, like 20:5, n-3, inhibits glucose-stimulated L-PK promoter activity and the accumulation of mRNAL-PK by attenuating RNA pol II recruitment to the L-PK promoter and the acetylation of histone H3 and H4 bound to the L-PK promoter. 20:5, n-3 inhibition of RNA pol II recruitment to the L-PK promoter cannot be explained by a major suppression of ChREBP nuclear abundance or its interaction with the L-PK promoter (Figs. 5 and 6). 20:5, n-3 only transiently suppresses ChREBP nuclear abundance (at 6 h) and has no effect on hepatic ChREBP nuclear abundance in vivo (Figs. 3 and 5). Overexpression of ChREBP in rat hepatocytes stimulates L-PK gene expression but does not abrogate 20:5, n-3 suppression of L-PK expression.
In contrast, MLX nuclear abundance is suppressed by n-3 PUFA in vivo (Fig. 3) and is transiently down-regulated at 1.5 h by 20:5, n-3 (Fig. 5). Overexpression of MLX fully abrogates 20:5, n-3 suppression of L-PK gene expression. This finding identifies MLX as a novel target for n-3 PUFA regulation.
Although HNF-4α interaction with the L-PK promoter is transiently regulated by 20:5, n-3, we consider this event to be independent of direct effect of fatty acids on HNF-4α, per se. We base this view on the absence of any effect of fatty acids on HNF-4α1 transactivation (Fig. 8) or HNF-4α interaction with other HNF4-regulated promoters (TAT or PepCk) (Fig. 6) and the fact that other HNF4-regulated genes (TAT and PepCk) are not affected by n-3 PUFA (2, 3). We suspect these transient changes in HNF-4α interaction with the L-PK promoter reflect remodeling of L-PK promoter composition during the course of glucose activation.
WY14643 also strongly inhibits glucose-stimulated L-PK gene transcription (6), the acetylation of histone H4 on the L-PK promoter (Fig. 7), and the accumulation of mRNAL-PK (Fig. 4). Like 20:5, n-3, WY14643 only modestly affects glucose-stimulated ChREBP translocation into the nucleus (Fig. 5). WY14643 had no effect on hepatic ChREBP nuclear abundance in vivo. WY14643 also had no effect on nuclear MLX levels in liver or primary hepatocytes or the interaction of HNF-4α with the L-PK promoter (Figs. 3, 5, and 6). WY14643, however, significantly antagonized HNF-4α1 transactivation (Fig. 8). Overexpressed ChREBP, but not overexpressed MLX, partially reversed the WY14643 suppression of L-PK promoter activity and mRNAL-PK (Fig. 9). HNF-4α regulates many hepatic genes, but only a few are inhibited by strong PPARα agonists (WY14643) like L-PK (6), apolipoprotein CIII (56), and Cyp7A (57). A likely explanation for this effect is that PPARα interferes with co-regulator interaction with HNF-4α on the L-PK promoter. A test of this hypothesis will first require identifying the co-regulators that interact with the L-PK promoter.
The outcome of these studies indicate that 20:5, n-3 promotes transient changes in ChREBP and MLX nuclear abundance and HNF4α-LPK promoter interaction (Figs. 5 and 6). Such transient effects of n-3 PUFA are not unusual. n-3 PUFA effects on PPARα-regulated transcripts and mRNASREBP-1c closely parallel changes in intracellular nonesterified 20:5, n-3 (45, 51). 20:5, n-3 is a minor fatty acid in both nonesterified and esterified lipid fractions of liver and hepatocytes (51). At any time point examined in this study, >95% of all intracellular 20:5, n-3 is esterified. Intracellular nonesterified 20:5, n-3 is highest at 1.5 and 6 h and lowest at 24 h. The transient effects of 20:5, n-3 on ChREBP, MLX, and HNF-4α may be sufficient to delay the onset of glucose-mediated induction of L-PK gene transcription (Figs. 4–6).
How can n-3 PUFA induce rapid but transient changes in ChREBP and MLX nuclear abundance or HNF-4α interaction with the L-PK promoter? A likely scenario is that n-3 PUFA regulates a signal transduction pathway(s). Two groups have reported that fatty acid activation of AMPK may account for fatty acid effects on gene expression (24, 43). AMPK regulates ChREBP entry into the nucleus. Once in cells, fatty acids are rapidly assimilated into complex lipids. A key event in this metabolic scheme is the conversion of fatty acids to fatty acyl-CoA, a reaction that requires ATP. Exogenous 20:5, n-3 is rapidly converted to 20:5-CoA in hepatocytes and rapidly assimilated into complex lipids, predominantly as triglycerides (45). We (see the Supplemental Material) and others (58, 59) found no evidence for fatty acid effects on AMPK phosphorylation in primary hepatocytes. Fatty acid treatment of primary hepatocytes, however, induces p38 MAPK (60) and ERK phosphorylation (51). We found no effect of p38 MAPK inhibitors on L-PK gene expression (not shown). 22:6, n-3, but not 20:5, n-3, induces ERK phosphorylation (51). We speculate that 20:5, n-3 regulates other signal transduction pathways that impact MLX and ChREBP nuclear abundance and HNF-4α interaction with the L-PK promoter. The identity of these other pathways remains to be established.
During the course of our studies, Dentin et al. (58) reported that PUFA interfered with ChREBP translocation to the nucleus in mouse liver and hepatocytes. Our in vivo and primary hepatocytes studies, however, show no effect of n-3 PUFA on ChREBP nuclear abundance in vivo and only a transient effect in primary hepatocytes (Figs. 3 and 5). Several factors can account for the different outcomes reported by Dentin et al. (58) and herein. First, rat primary hepatocytes were purified through Percoll (Amersham Biosciences), whereas Dentin et al. (58) used unfractionated mouse hepatocytes. Rats were meal-fed high carbohydrate diets supplemented with olive or fish oil for 7 days. Animals were euthanized 2 h after completion of the last meal. Dentin et al. (58) maintained mice on a high carbohydrate fat-free diet and switched the animals to a high carbohydrate diet supplemented with fatty acids; animals were euthanized 18 h later. Finally, Dentin et al. (58) did not examine hepatic MLX levels. Animal species or experimental design may account for these differences. Our results indicate that PUFA control of ChREBP is not the sole mechanism for fatty acid regulation of L-PK. MLX has emerged as a target for n-3 PUFA control.
As a result of these studies, we have revised our original interpretation for how n-3 PUFA and WY14643 control L-PK gene transcription. Our earlier studies were based on linker-scanning analysis and indicated that the DR-1 element was required for both WY14643 and n-3 PUFA control of L-PK gene transcription (5, 6) (Fig. 1). This interpretation, however, is confounded by fact that both n-3 PUFA and WY14643 only inhibit glucose-activated L-PK expression and not basal L-PK expression. HNF-4α is not a direct target of n-3 PUFA (Fig. 8). n-3 PUFA, however, only transiently affects HNF-4α interaction with the L-PK promoter (Fig. 6). In contrast, WY14643 interferes with HNF-4α1 transactivation (Fig. 8) but has no effect on HNF-4α interaction with the L-PK promoter (Fig. 7). A likely explanation for the WY14643 effect is that ligand-activated PPARα interferes with co-activator recruitment to the L-PK promoter. The robust inhibition of histone H4 acetylation on the L-PK promoter supports this concept (Fig. 7). In revision of our original model (Fig. 1), we now show that the PUFA and PPARα cis-regulatory targets should include the ChREBP/MLX and HNF-4α-binding sites.
The new information reported herein has raised many unanswered questions. First among these is how glucose treatment of hepatocytes induces RNA pol II and histone acetylation on the L-PK promoter. The lack of significant glucose-regulated ChREBP occupancy on the L-PK promoter suggests that something other than ChREBP accumulation in the nucleus is required to induce L-PK gene activation. Finding that the 26 S proteasome inhibitor, MG132, blocks glucose-activated L-PK gene transcription implicates the 26 S proteasome in this regulatory scheme. Analysis of the ubiquitination status of ChREBP, HNF-4α, and MLX will be important to define how nutrients control L-PK promoter composition and gene transcription. The suppressive effect of n-3 PUFA on MLX nuclear abundance without effects on MLX expression implicates mechanisms that control MLX nuclear import or export. Another heterodimer partner of MLX is MondoA. The subcellular distribution of MondoA: MLX heterodimers is determined by CRM1-dependent nuclear export signals in MondoA that interact with 14-3-3 family members. Leptomycin B, a nuclear export inhibitor, promotes MondoA: MLX accumulation in nuclei (61). Leptomycin B, however, does not prevent the n-3 PUFA suppression of MLX abundance.3 We speculate that n-3 PUFA transiently inhibits MLX import into the nucleus. The mechanism underlying this process requires further analysis.
In summary, glucose stimulates ChREBP translocation into hepatocyte nuclei, recruitment of RNA pol II to the L-PK promoter, and increased histone H3 and H4 acetylation on the L-PK promoter without major changes in promoter-bound ChREBP or HNF-4α. 20:5, n-3 interferes with this process by transiently suppressing nuclear MLX and ChREBP nuclear abundance. MLX is an obligate heterodimer partner of ChREBP that is required for ChREBP binding to the L-PK ChoRE. Over-expressed MLX eliminates the 20:5, n-3 but not the WY14643 control of L-PK gene expression. Overexpressed ChREBP relieves the WY14643 suppression of L-PK promoter activity but has no effect on n-3 PUFA inhibition of L-PK promoter activity. WY14643 encumbers HNF-4α transactivation but sn-3 PUFA does not. Overexpressed HNF-4α has no effect on n-3 PUFA or WY14643 suppression of L-PK gene expression. These studies have identified MLX as a key target for n-3 PUFA control of L-PK gene expression. n-3 PUFA and WY14643 clearly use distinct mechanisms to inhibit glucose-activated L-PK gene transcription.
Supplementary Material
Acknowledgments
We thank H. Towle (University of Minnesota, Minneapolis) for generously providing the recombinant adenovirus expressing ChREBP and MLX and critical review of this manuscript. We thank F. Sladek (University of California, Riverside) for the HNF-4α plasmid. T. Osborne (University of California, Irvine) and M-H. Kuo (Michigan State University) provided many helpful suggestions on the ChIP assay. We also thank J. Busik and L. K. Olson for their helpful suggestions during the course of our studies and critical review of the manuscript.
Footnotes
This work was supported by National Institutes of Health Grant DK43220, the National Research Initiative of the United States Department of Agriculture Cooperative State Research, Education, and Extension Service Grant 2003-35200-13400, and the Michigan Agriculture Experiment Station.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
The abbreviations used are: L-PK, L-type pyruvate kinase; Ac-H3, acetylated histone H3; Ac-H4 acetylated histone H4; Ad, adenovirus; AMPK, AMP kinase; ChIP, chromatin immunoprecipitation; ChREBP, carbohydrate regulatory element-binding protein; ChoRE, carbohydrate regulatory element; DR-1, direct repeat with 1 nucleotide spacer; FAS, fatty-acid synthase; GFP, green fluorescent protein; HNF-4, hepatic nuclear factor-4; MLX, MAX-like factor-X; PepCk, phosphoenolpyruvate kinase; PPARα, peroxisome proliferator-activated receptor α; PUFA, polyunsaturated fatty acid; qRT-PCR, quantitative real time-PCR; RNA pol II, RNA polymerase II; S14, S14 protein; SREBP-1, sterol regulatory element-binding protein-1; TAT, tyrosine aminotransferase; TK, thymidine kinase; BisTris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; LBD, ligand binding domain; IP, immunoprecipitation; ERK, extracellular signal-regulated kinase; MAPK, mitogen-activated protein kinase.
J. Xu, B. Christian, and D. B. Jump, unpublished observations.
References
- 1.Granner D, Pilkis S. J Biol Chem. 1990;265:10173–10176. [PubMed] [Google Scholar]
- 2.Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 3.Jump DB. CRC Crit Rev Clin Lab Sci. 2004;41:41–78. doi: 10.1080/10408360490278341. [DOI] [PubMed] [Google Scholar]
- 4.Towle HC. Trends Endocrinol Metab. 2005;16:489–494. doi: 10.1016/j.tem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Liimatta M, Towle HC, Clarke S, Jump DB. Mol Endocrinol. 1994;8:1147–1153. doi: 10.1210/mend.8.9.7838147. [DOI] [PubMed] [Google Scholar]
- 6.Pan DA, Mater MK, Thelen AP, Peters JM, Gonzalez FJ, Jump DB. J Lipid Res. 2000;41:742–751. [PubMed] [Google Scholar]
- 7.Large V, Beylot M. Diabetes. 1999;48:1251–1257. doi: 10.2337/diabetes.48.6.1251. [DOI] [PubMed] [Google Scholar]
- 8.Fulgencio JP, Kohl C, Girard J, Pegorier JP. Biochem Pharmacol. 2001;62:439–446. doi: 10.1016/s0006-2952(01)00679-7. [DOI] [PubMed] [Google Scholar]
- 9.Vaulont S, Vasseur-Cognet M, Kahn A. J Biol Chem. 2000;275:31555–31558. doi: 10.1074/jbc.R000016200. [DOI] [PubMed] [Google Scholar]
- 10.Towle HC. J Biol Chem. 1995;270:23235–23238. doi: 10.1074/jbc.270.40.23235. [DOI] [PubMed] [Google Scholar]
- 11.Vaulont S, Kahn A. FASEB J. 1994;8:28–35. doi: 10.1096/fasebj.8.1.8299888. [DOI] [PubMed] [Google Scholar]
- 12.Vaulont S, Puzenat N, Levrat F, Cognet M, Kahn A, Raymondjean M. J Mol Biol. 1989;209:205–219. doi: 10.1016/0022-2836(89)90273-8. [DOI] [PubMed] [Google Scholar]
- 13.Thompson KS, Towle HC. J Biol Chem. 1991;266:8679–8682. [PubMed] [Google Scholar]
- 14.Liu Z, Thompson KS, Towle HC. J Biol Chem. 1993;268:12787–12795. [PubMed] [Google Scholar]
- 15.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Proc Natl Acad Sci U S A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Callaghan BL, Koo SH, Wu Y, Freake HC, Towle HC. J Biol Chem. 2001;276:16033–16039. doi: 10.1074/jbc.M101557200. [DOI] [PubMed] [Google Scholar]
- 17.Rufo C, Teran-Garcia M, Nakamura MT, Koo SH, Towle HC, Clarke SD. J Biol Chem. 2001;276:21969–21975. doi: 10.1074/jbc.M100461200. [DOI] [PubMed] [Google Scholar]
- 18.Shih HM, Towle HC. J Biol Chem. 1992;267:13222–13228. [PubMed] [Google Scholar]
- 19.Ma L, Tsatsos NG, Towle HC. J Biol Chem. 2005;280:12019–12027. doi: 10.1074/jbc.M413063200. [DOI] [PubMed] [Google Scholar]
- 20.Doiron B, Cuif MH, Chen R, Kahn A. J Biol Chem. 1996;271:5321–5324. doi: 10.1074/jbc.271.10.5321. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Proc Natl Acad Sci U S A. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Proc Natl Acad Sci U S A. 2003;100:5107–5112. doi: 10.1073/pnas.0730817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uyeda K, Yamashita H, Kawaguchi T. Biochem Pharmacol. 2002;63:2075–2080. doi: 10.1016/s0006-2952(02)01012-2. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. J Biol Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 25.Sladek FM, Zhong WM, Lai E, Darnell JE., Jr Genes Dev. 1990;4:2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- 26.Miquerol L, Lopez S, Cartier N, Tulliez M, Raymondjean M, Kahn A. J Biol Chem. 1994;269:8944–8951. [PubMed] [Google Scholar]
- 27.Hadzopoulou-Cladaras M, Kistanova E, Evagelopoulou C, Zeng S, Cladaras C, Ladias JA. J Biol Chem. 1997;272:539–550. doi: 10.1074/jbc.272.1.539. [DOI] [PubMed] [Google Scholar]
- 28.Pawar A, Botolin D, Mangelsdorf DJ, Jump DB. J Biol Chem. 2003;278:40736–40743. doi: 10.1074/jbc.M307973200. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Botolin D, Christian B, Busik C, Xu J, Jump DB. J Lipid Res. 2005;46:706–715. doi: 10.1194/jlr.M400335-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cognet M, Lone YC, Vaulont S, Kahn A, Marie J. J Mol Biol. 1987;196:11–25. doi: 10.1016/0022-2836(87)90507-9. [DOI] [PubMed] [Google Scholar]
- 31.Amy CM, Williams-Ahlf B, Naggert J, Smith S. Biochem J. 1990;271:675–679. doi: 10.1042/bj2710675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jump DB, Bell A, Santiago V. J Biol Chem. 1990;265:3474–3478. [PubMed] [Google Scholar]
- 33.Danielson PE, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RJ, Sutcliffe JG. DNA (N Y) 1988;7:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- 34.Botolin D, Jump DB. J Biol Chem. 2003;278:6959–6962. doi: 10.1074/jbc.M212846200. [DOI] [PubMed] [Google Scholar]
- 35.Oddos J, Grange T, Carr KD, Matthews B, Roux J, Richard-Foy H, Pictet R. Nucleic Acids Res. 1989;17:8877–8878. doi: 10.1093/nar/17.21.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beale EG, Chrapkiewicz NB, Scoble HA, Metz RJ, Quick DP, Noble RL, Donelson JE, Biemann K, Granner DK. J Biol Chem. 1985;260:10748–10760. [PubMed] [Google Scholar]
- 37.Ishii S, Iizuka K, Miller BC, Uyeda K. Proc Natl Acad Sci U S A. 2004;101:15597–15602. doi: 10.1073/pnas.0405238101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epperson A, Hatton WJ, Callaghan B, Doherty P, Walker RL, Sanders KM, Ward SM, Horowitz B. Am J Physiol. 2000;279:C529–C539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- 39.Stoeckman AK, Ma L, Towle HC. J Biol Chem. 2004;279:15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- 40.Shih H, Towle HC. J Biol Chem. 1994;269:9380–9387. [PubMed] [Google Scholar]
- 41.da Silva Xavier G, Leclerc I, Salt IP, Doiron B, Hardie DG, Kahn A, Rutter GA. Proc Natl Acad Sci U S A. 2000;97:4023–4028. doi: 10.1073/pnas.97.8.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong YH, Varanasi US, Yang W, Leff T. J Biol Chem. 2003;278:27495–27501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- 43.Suchankova G, Tekle M, Saha AK, Ruderman NB, Clarke SD, Gettys TW. Biochem Biophys Res Commun. 2005;326:851–858. doi: 10.1016/j.bbrc.2004.11.114. [DOI] [PubMed] [Google Scholar]
- 44.Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pawar A, Jump DB. J Biol Chem. 2003;278:35931–35939. doi: 10.1074/jbc.M306238200. [DOI] [PubMed] [Google Scholar]
- 46.Dhe-Paganon S, Duda K, Iwamoto M, Chi YI, Shoelson SE. J Biol Chem. 2002;277:37973–37976. doi: 10.1074/jbc.C200420200. [DOI] [PubMed] [Google Scholar]
- 47.Wisely GB, Miller AB, Davis RG, Thornquest AD, Jr, Johnson R, Spitzer T, Sefler A, Shearer B, Moore JT, Willson TM, Williams SP. Structure (Camb) 2002;10:1225–1234. doi: 10.1016/s0969-2126(02)00829-8. [DOI] [PubMed] [Google Scholar]
- 48.Hertz R, Kalderon B, Byk T, Berman I, Za’tara G, Mayer R, Bar-Tana J. J Biol Chem. 2005;280:24451–24461. doi: 10.1074/jbc.M500732200. [DOI] [PubMed] [Google Scholar]
- 49.Mater MK, Thelen AP, Pan DA, Jump DB. J Biol Chem. 1999;274:32725–32732. doi: 10.1074/jbc.274.46.32725. [DOI] [PubMed] [Google Scholar]
- 50.Xu HE, Lambert MH, Montana VG, Parks DJ, Blanchard SG, Brown PJ, Sternbach DD, Lehmann JM, Wisely GB, Willson TM, Kliewer SA, Milburn MV. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 51.Botolin D, Wang Y, Christian B, Jump DB. J Lipid Res. 2006;47:181–192. doi: 10.1194/jlr.M500365-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoeckman AK, Towle HC. J Biol Chem. 2002;277:27029–27035. doi: 10.1074/jbc.M202638200. [DOI] [PubMed] [Google Scholar]
- 53.Dennis AP, O’Malley BW. J Steroid Biochem Mol Biol. 2005;93:139–151. doi: 10.1016/j.jsbmb.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 54.Nawaz Z, O’Malley BW. Mol Endocrinol. 2004;18:493–499. doi: 10.1210/me.2003-0388. [DOI] [PubMed] [Google Scholar]
- 55.Kang Z, Pirskanen A, Janne OA, Palvimo JJ. J Biol Chem. 2002;277:48366–48371. doi: 10.1074/jbc.M209074200. [DOI] [PubMed] [Google Scholar]
- 56.Hertz R, Bishara-Shieban J, Bar-Tana J. J Biol Chem. 1995;270:13470–13475. doi: 10.1074/jbc.270.22.13470. [DOI] [PubMed] [Google Scholar]
- 57.Marrapodi M, Chiang JY. J Lipid Res. 2000;41:514–520. [PubMed] [Google Scholar]
- 58.Dentin R, Benhamed F, Pegorier JP, Foufelle F, Viollet B, Vaulont S, Girard J, Postic C. J Clin Investig. 2005;115:2843–2854. doi: 10.1172/JCI25256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dobrzyn A, Dobrzyn P, Miyazaki M, Ntambi JM. Biochem Biophys Res Commun. 2005;332:892–896. doi: 10.1016/j.bbrc.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 60.Talukdar I, Szeszel-Fedorowicz W, Salati LM. J Biol Chem. 2005;280:40660–40667. doi: 10.1074/jbc.M505531200. [DOI] [PubMed] [Google Scholar]
- 61.Eilers AL, Sundwall E, Lin M, Sullivan AA, Ayer DE. Mol Cell Biol. 2002;22:8514–8526. doi: 10.1128/MCB.22.24.8514-8526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.