Abstract
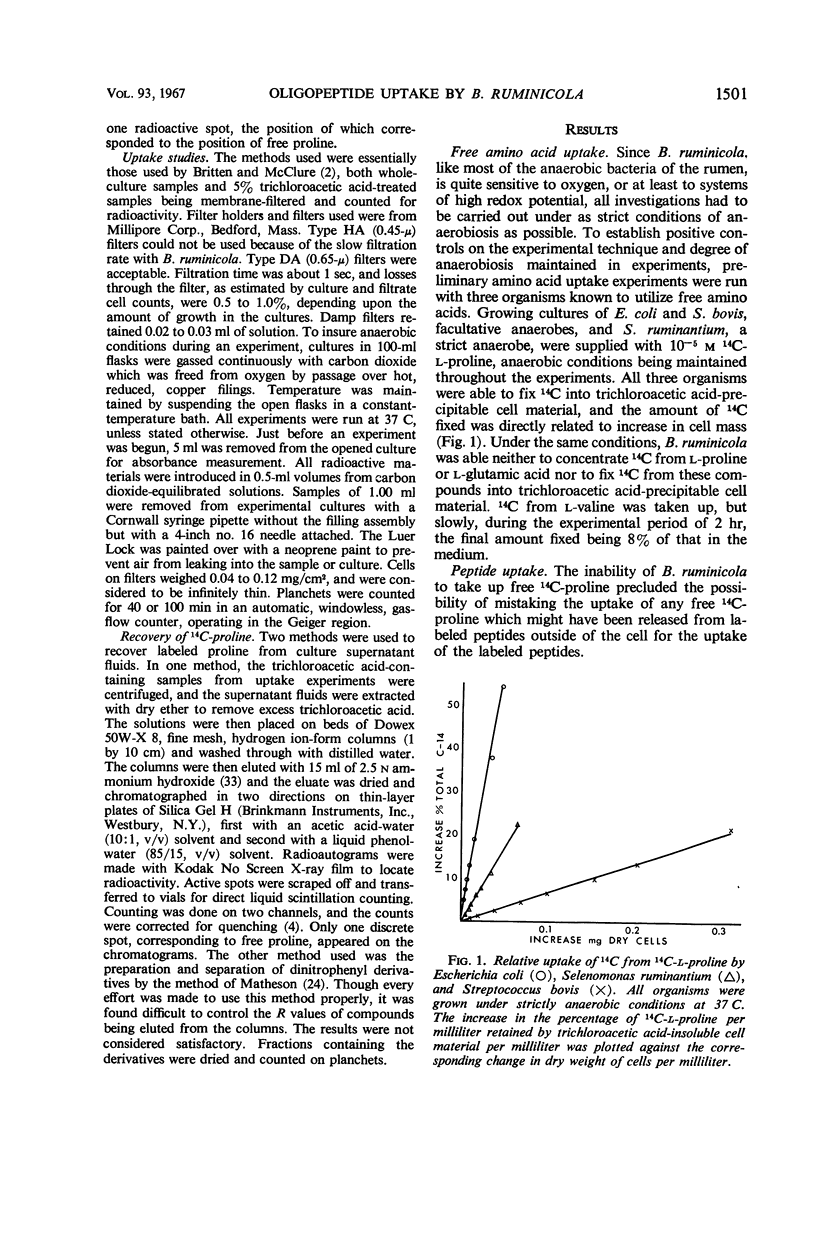
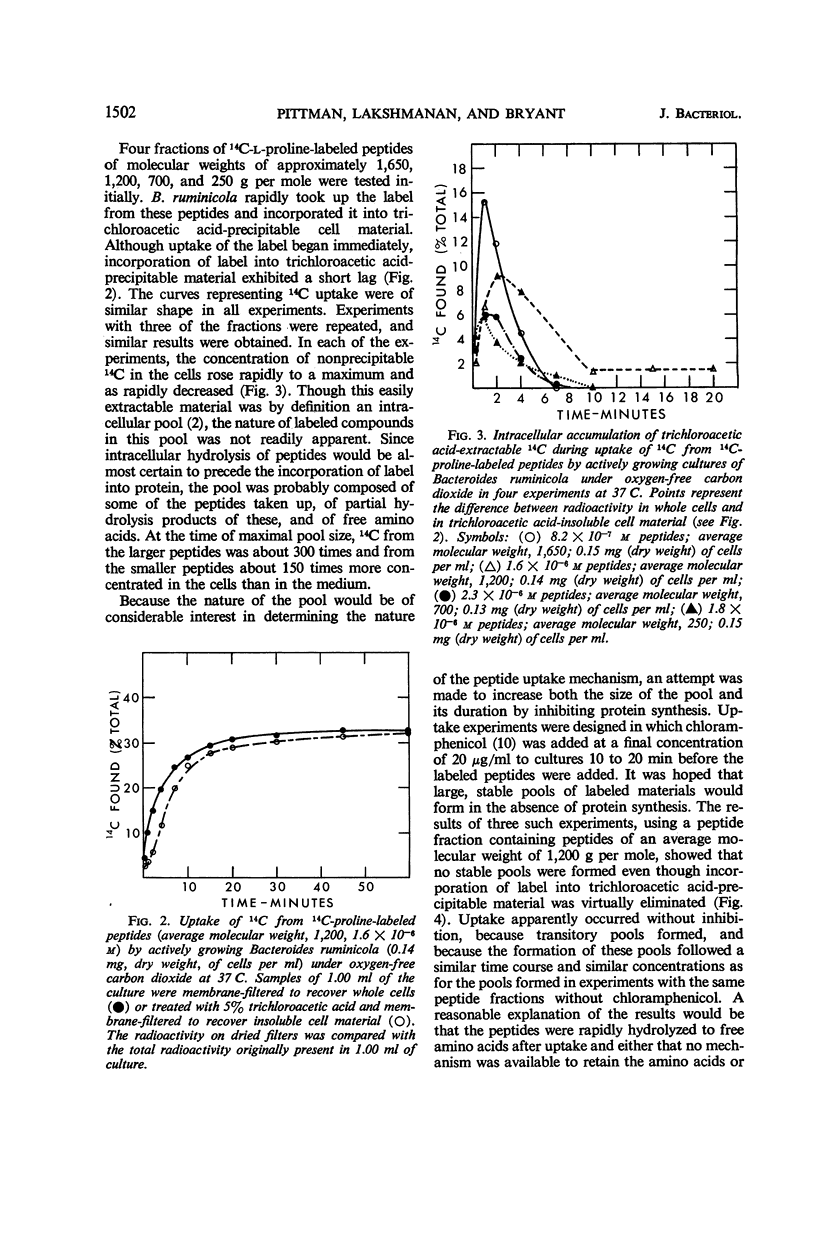
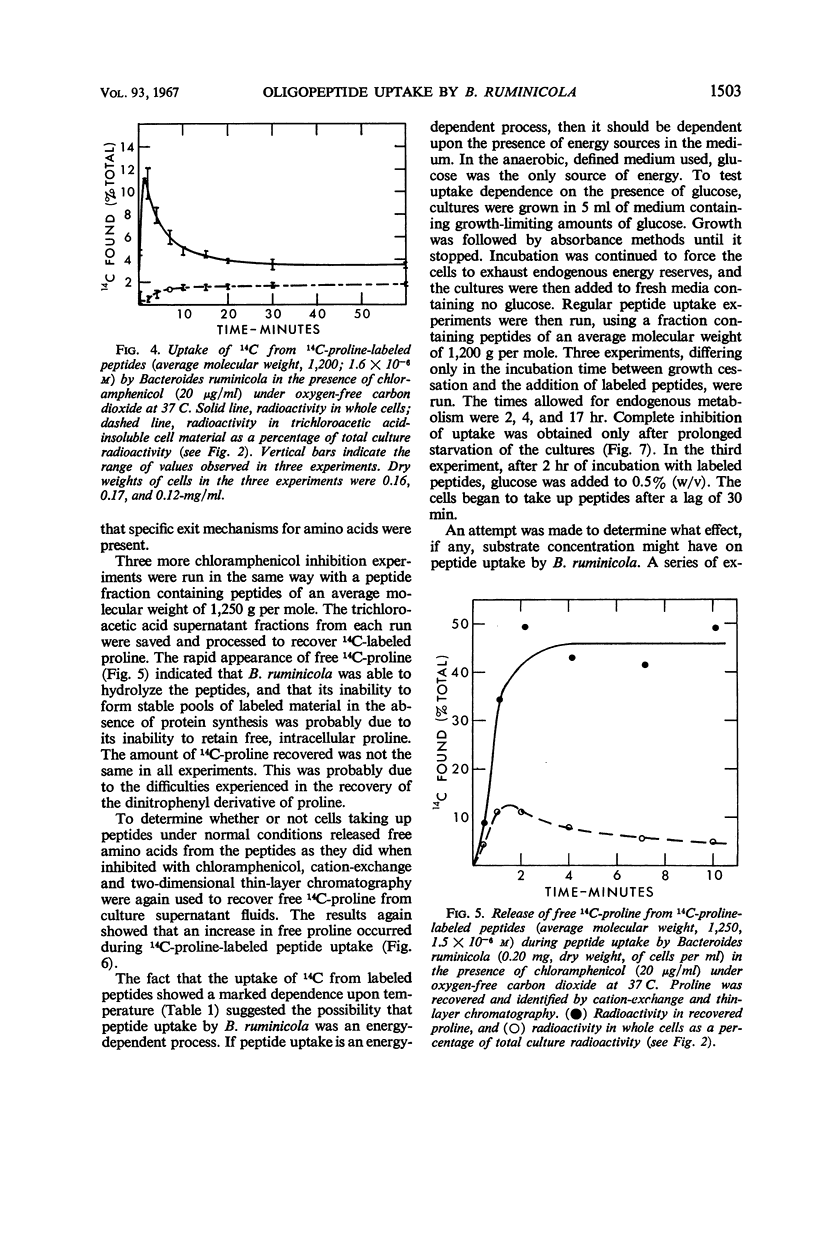
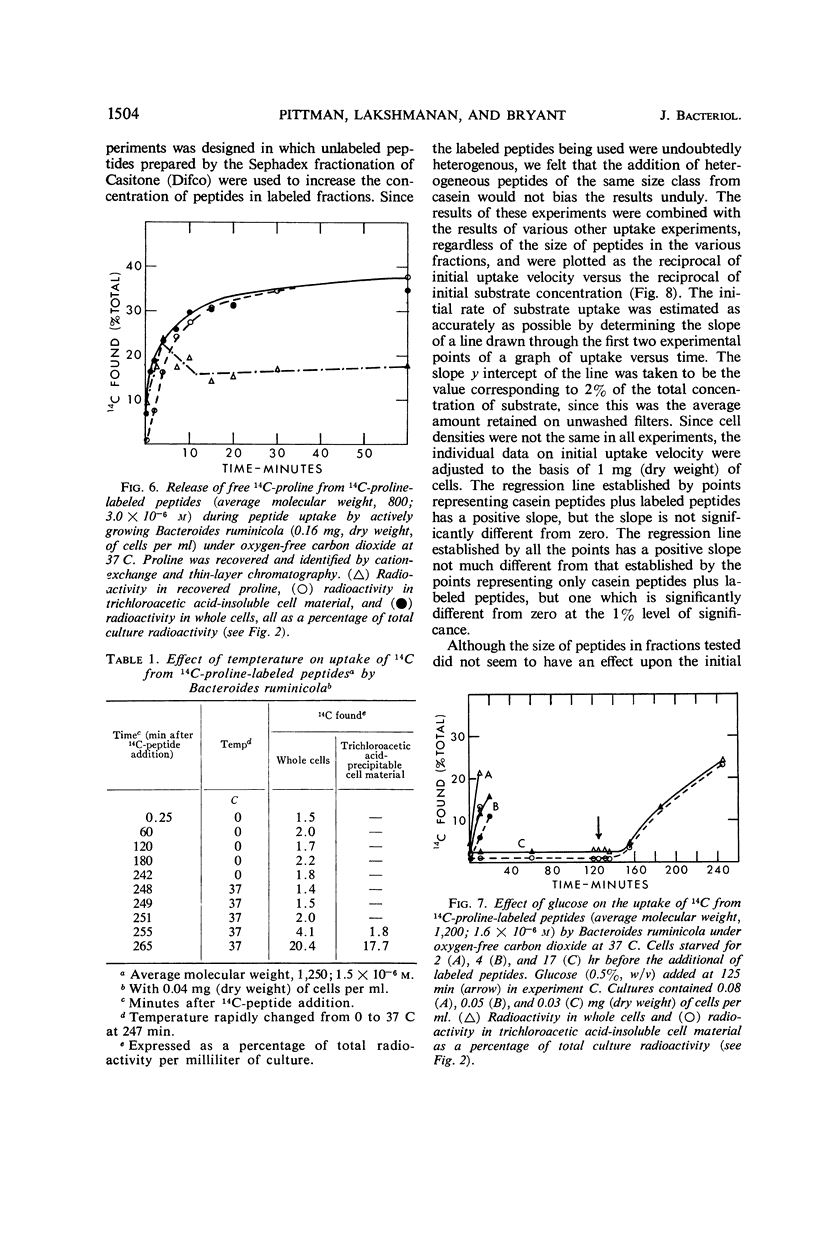
Bacteroides ruminicola did not take up 14C from exogenous 14C-labeled l-proline or 14C-labeled l-glutamic acid and took up very little 14C from exogenous 14C-labeled l-valine. Growing cultures of B. ruminicola rapidly took up 14C from 14C-proline-labeled peptides of molecular weights up to 2,000 and incorporated it into trichloroacetic acid-insoluble cell material. Uptake and incorporation did not occur at 0 C and were reduced or eliminated in glucose-starved cells, depending upon the length of time the cells were starved. The initial rate of uptake of peptides seemed to exhibit saturation kinetics, but it was impossible to establish this conclusively. The initial uptake of 14C from peptides was not affected by chloramphenicol but the incorporation of it into trichloroacetic acid-insoluble cell material was virtually eliminated. Only moderate amounts of trichloroacetic acid-extractable, labeled material were present in cells during peptide uptake, whether or not chloramphenicol was present. 14C-proline was rapidly released from labeled peptides during uptake, whether or not chloramphenicol was present. The amount of 14C fixed into trichloroacetic acid-insoluble cell material was directly related to the size of peptides originally supplied in the medium. It is concluded that B. ruminicola possesses a general system for the uptake of peptides, that peptides are rapidly hydrolyzed during or after uptake, and that oligopeptides function only to supply amino acids in a form available to the organism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D., WOOLEY S. O. GLYCYLGLYCINE UPTAKE IN STREPTOCOCCI AND A POSSIBLE ROLE OF PEPTIDES IN AMINO ACID TRANSPORT. Arch Biochem Biophys. 1964 Apr;105:51–57. doi: 10.1016/0003-9861(64)90234-6. [DOI] [PubMed] [Google Scholar]
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P., SMALL N., BOUMA C., CHU H. Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen. J Bacteriol. 1958 Jul;76(1):15–23. doi: 10.1128/jb.76.1.15-23.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P. The characteristics of strains of Selenomonas isolated from bovine rumen contents. J Bacteriol. 1956 Aug;72(2):162–167. doi: 10.1128/jb.72.2.162-167.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLORSHEIM H. A., MAKINENI S., SHANKMAN S. The isolation, identification and synthesis of a peptide growth factor for P. cerevisiae. Arch Biochem Biophys. 1962 May;97:243–249. doi: 10.1016/0003-9861(62)90076-0. [DOI] [PubMed] [Google Scholar]
- FOX E. N. Peptide requirements for the synthesis of streptococcal proteins. J Biol Chem. 1961 Jan;236:166–171. [PubMed] [Google Scholar]
- GILVARG C., KATCHALSKI E. PEPTIDE UTILIZATION IN ESCHERICHIA COLI. J Biol Chem. 1965 Jul;240:3093–3098. [PubMed] [Google Scholar]
- JONES K. M., WOOLLEY D. W. Peptides required for the growth of Lactobacillus bulgaricus. J Bacteriol. 1962 Apr;83:797–801. doi: 10.1128/jb.83.4.797-801.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL D., LUBIN M. On the distinction between peptidase activity and peptide transport. Biochim Biophys Acta. 1963 Jun 4;71:656–663. doi: 10.1016/0006-3002(63)91139-9. [DOI] [PubMed] [Google Scholar]
- KIHARA H., SNELL E. E. Peptides and bacterial growth. VII. Relation to inhibitions by thienylalanine, ethionine, and canavanine. J Biol Chem. 1955 Jan;212(1):83–94. [PubMed] [Google Scholar]
- KIHARA H., SNELL E. E. Peptides and bacterial growth. VIII. The nature of strepogenin. J Biol Chem. 1960 May;235:1409–1414. [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEACH F. R., SNELL E. E. The absorption of glycine and alanine and their peptides by Lactobacillus casei. J Biol Chem. 1960 Dec;235:3523–3531. [PubMed] [Google Scholar]
- LEVINE E. M., SIMMONDS S. Metabolite uptake by serineglycine auxotrophs of Escherichia coli. J Biol Chem. 1960 Oct;235:2902–2909. [PubMed] [Google Scholar]
- Losick R., Gilvarg C. Effect of alpha-acetylation on utilization of lysine oligopeptides in Escherichia coli. J Biol Chem. 1966 May 25;241(10):2340–2346. [PubMed] [Google Scholar]
- MATHESON N. A. An improved method of separating amino acids as N-2,4-dinitrophenyl derivatives. Biochem J. 1963 Jul;88:146–151. doi: 10.1042/bj0880146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Group-translocation: a consequence of enzyme-catalysed group-transfer. Nature. 1958 Aug 9;182(4632):372–373. doi: 10.1038/182372a0. [DOI] [PubMed] [Google Scholar]
- PETERS V. J., PRESCOTT J. M., SNELL E. E. Peptides and bacterial growth. IV. Histidine peptides as growth factors for Lactobacillus delbrueckii 9649. J Biol Chem. 1953 Jun;202(2):521–532. [PubMed] [Google Scholar]
- PHILLIPS A. W., GIBBS P. A. Techniques for the fractionation of microbiologically active peptides derived from casein. Biochem J. 1961 Dec;81:551–556. doi: 10.1042/bj0810551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTMAN K. A., BRYANT M. P. PEPTIDES AND OTHER NITROGEN SOURCES FOR GROWTH OF BACTEROIDES RUMINICOLA. J Bacteriol. 1964 Aug;88:401–410. doi: 10.1128/jb.88.2.401-410.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957 Mar;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- SHANKMAN S., HIGA S., FLORSHEIM H. A., SCHVO Y., GOLD V. Peptide studies. II. Growth-promoting activity of peptides of L-leucine and L- and D-valine for lactic acid bacteria. Arch Biochem Biophys. 1960 Feb;86:204–209. doi: 10.1016/0003-9861(60)90405-7. [DOI] [PubMed] [Google Scholar]
- SHELTON D. C., NUTTER W. E. UPTAKE OF VALINE AND GLYCYLVALINE BY LEUCONOSTOC MESENTEROIDES. J Bacteriol. 1964 Oct;88:1175–1184. doi: 10.1128/jb.88.4.1175-1184.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLLEY D. W., MERRIFIELD R. B. Anomalies of the structural specificity of peptides. Ann N Y Acad Sci. 1963 Feb 4;104:161–171. doi: 10.1111/j.1749-6632.1963.tb17661.x. [DOI] [PubMed] [Google Scholar]
- YODER O. C., BEAMER K. C., CIPOLLONI P. B., Jr, SHELTON D. C. KINETIC STUDIES OF L-VALINE AND GLYCYL-L-VALINE UPTAKE BY LEUCONOSTOC MESENTEROIDES. Arch Biochem Biophys. 1965 May;110:336–340. doi: 10.1016/0003-9861(65)90129-3. [DOI] [PubMed] [Google Scholar]
- Young E. A., Bowen D. O., Diehl J. F. Transport studies with peptides containing unnatural amino acids. Biochem Biophys Res Commun. 1964;14:250–255. doi: 10.1016/0006-291x(64)90444-9. [DOI] [PubMed] [Google Scholar]


