Abstract
Neuroprotective properties of the mood stabilizer valproic acid (VPA) are implicated in its therapeutic efficacy. Heat shock protein 70 (HSP70) is a molecular chaperone, neuroprotective and anti-inflammatory agent. The present study aimed to investigate underlying mechanisms and functional significance of HSP70 induction by VPA in rat cortical neurons. VPA treatment markedly upregulated HSP70 protein levels, and this was accompanied by increased HSP70 mRNA levels and promoter hyperacetylation and activity. Other HDAC inhibitors - sodium butyrate, trichostatin A and Class I HDAC-specific inhibitors MS-275 and apicidin, - all mimicked the ability of VPA to induce HSP70. Pretreatment with PI3-kinase inhibitors or an Akt inhibitor attenuated HSP70 induction by VPA and other HDAC inhibitors. VPA treatment increased Sp1 acetylation, and a Sp1 inhibitor, mithramycin, abolished the induction of HSP70 by HDAC inhibitors. Moreover, VPA promoted the association of Sp1 with the histone acetyltransferases p300 and recruitment of p300 to the HSP70 promoter. Further, VPA-induced neuroprotection against glutamate excitotoxicity was prevented by blocking HSP70 induction. Taken together, the data suggest that the PI3-kinase/Akt pathway and Sp1 are likely involved in HSP70 induction by HDAC inhibitors, and induction of HSP70 by VPA in cortical neurons may contribute to its neuroprotective and therapeutic effects.
Keywords: valproic acid, HSP70, HDAC, PI3-kinase, Sp1
Introduction
The heat shock response is an essential and conserved mechanism for cellular protection against a number of environmental stimuli that induce stress or apoptosis. Triggering the heat shock response leads to upregulated synthesis of the heat-shock proteins (HSPs) (reviewed in Pirkkala et al., 2001). HSPs play an important role as molecular chaperones by facilitating protein folding and degradation of abnormally folded proteins, and are also cytoprotective (Li and Werb, 1982, reviewed in Morimoto and Santoro, 1998). The widely-studied stress-inducible heat shock protein 70 (HSP70) is part of the HSP70 family, which also includes the constitutively expressed heat shock cognate protein HSC70.
Certain neuronal cell types, such as motor, cortical, and hippocampal neurons, have attenuated or delayed heat shock response, compared to glial cells, which may make these neurons more susceptible to neurodegenerative insults (Batulan et al., 2003; Marcuccilli et al., 1996; Tagawa et al., 2007). Exogenous or endogenously-induced HSP70 can protect neurons from a wide range of stressful stimuli. For example, HSP70 protects neurons against toxicity resulting from intracellularly expressed β-amyloid (Magrane et al., 2004), and SOD1 mutation (Batulan et al., 2006). Furthermore, HSP70 has neuroprotective effects in animal models of neurodegenerative diseases, including stroke, Parkinson's disease and amyotrophic lateral sclerosis (Ren et al., 2003; Shen et al, 2005; Gifondorwa et al., 2007). HSP70's neuroprotective effects may be mediated by its multiple anti-apoptotic actions; for instance, HSP70 upregulates Bcl-2, binds to antagonize the apoptosis-inducing factor, and interferes with Apaf-1 function, thereby preventing the formation of the apoptosome (reviewed in Yenari et al., 2005). HSP70 also plays an anti-inflammatory role in brain ischemia that may contribute to its protective effects (Zheng et al., 2008).
The mood stabilizing and anticonvulsant drug valproic acid (VPA) has neuroprotective properties in cellular and animal disease models (reviewed in Young et al., 2002; Bachmann et al., 2005; Chuang, 2005). VPA has been shown to inhibit histone deacetylases (HDACs) (Göttlicher et al., 2001; Phiel et al., 2001), which have a prominent role in the regulation of chromatin remodeling and gene expression. In neuronal cultures, VPA protects against glutamate-related excitotoxicity (Hashimoto et al., 2002), and this effect is mimicked by other HDAC inhibitors (Kanai et al., 2004; Leng and Chuang, 2006). Neuroprotection by HDAC inhibitors is associated with decreased excitotoxicity-induced nuclear accumulation of proapoptotic glyceraldehyde-3-phosphate dehydrogenase, and with an upregulation of antiapoptotic α-synuclein (Kanai et al., 2004, Leng and Chuang, 2006). HDAC inhibition also protects neurons from oxidative stress, which is associated with Sp1 hyperacetylation and p21 upregulation (Ryu et al., 2003; Langley et al., 2008), and from oxygen-glucose deprivation, accompanied by upregulation of gelsolin (Meisel et al., 2006). In a rat model of cerebral ischemia, post-insult treatment with VPA and other HDAC inhibitors such as sodium butyrate (SB) and trichostatin A (TSA) reduced brain infarct volume, improved neurological performance, and had anti-inflammatory effects (Ren et al., 2004; Kim et al., 2007). Furthermore, the neuroprotective effects of VPA in ischemia models are associated with increased HSP70 levels in the brain. The aim of the present study was to determine whether VPA can induce the expression of HSP70 in primary cortical neurons through HDAC inhibition and, if so, to elucidate the molecular mechanisms underlying this induction as well as to assess the functional significance of the HSP70 upregulation.
Materials and Methods
Rat cerebral cortical neuronal cultures
Cortical neurons were prepared from 18-day-old Sprague Dawley rat embryos as described previously (Liang and Chuang, 2006). Briefly, cortices from embryonic brain were dissected, and cells were dissociated by trypsinization, followed by DNase treatment and repeated triturations. The dissociated cells were resuspended in serum-free B27/neurobasal medium and plated onto poly-D-lysine pre-coated cell culture dishes or plates. After seven or eight days in vitro (DIV), cortical neurons were routinely used for drug treatment.
Plasmid DNA
Promoter constructs of the human HSP70 genes HSP70-1 and HSP70-2 inserted in the pGL3 basic luciferase reporter vector (Wu et al., 2005) were gifts from Drs. David Sidransky and Barry Trink (Johns Hopkins University School of Medicine, Baltimore, MD). pcDNA3.1 plasmids encoding wild type human heat shock factor 1 (HSF1wt), constitutively activated HSF1 (HSF1(+) or BH-S), or non-activable HSF1 (HSF1(-) or AV-ST) were gifts from Dr. Richard Voellmy (University of Miami, Miami, FL) (Zuo et al., 1995). Transfections of reporter constructs of HSP70 promoters and HSF1 expression vectors in cortical neurons were carried out using an Amaxa Nucleofector (Amaxa, Cologne, Germany) and a rat neuron Nucleofector kit (Amaxa) at the time of plating. According to the manufacturer's protocol, three μg DNA were transfected into 6×106 cells. Human neuroblastoma SH-SY5Y cells were cultured in Dulbecco's Modified Eagle Medium supplemented with 10% fetal bovine serum. They were transfected with reporter constructs of HSP70 promoters using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). In both cortical neuronal cultures and SH-SY5Y cells phRG-B promotorless Renilla vector was used as an internal control. Luciferase activities were determined on a plate-reading luminometer (PerkinElmer Life and Analytical Sciences, Waltham, MA), using a dual luciferase reporter assay system (Promega, Madison, WI).
Semiquantitative RT-PCR
RNA was extracted from cortical neurons with RNeasy plus mini kit (Qiagen, Valencia, CA). 2 μg RNA were used for reverse transcription with a First Strand Superscript II kit using random hexamers (Invitrogen). cDNA was amplified by PCR with the oligonucleotide primers CTA CAA GGC GGA GGA CGA and TAG GAC TCG AGC GCA TTC TT specific for rat HSPA1A(HSP70-1)/HSPA1B(HSP70-2) - genes encoding the inducible HSP70 protein. For semiquantitative PCR, mRNA expression levels of HSP70 were normalized to those of β-actin (primers CCA CAG CTG AGA GGG AAA TCG and AGT AAC AGT CCG CCT AGA AGC A). Amplification was carried out in a total reaction volume of 25 μl in the presence of ReactionReady Hotstart “Sweet” PCR mix (SA Biosciences, Frederick, MD) containing 400 nM of each primer and 10 ng of cDNA in triplicate. PCR products were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and visualized by UV illumination. No-reverse transcriptase and no-template controls were used to exclude the presence of genomic or contaminating DNA.
Immunoblotting analysis, preparation of nuclear protein, and immunoprecipitation
Cells were washed with phosphate-buffered saline (PBS) and harvested by scraping into cell lysis buffer (Cell Signaling Technology, Beverly, MA), which was supplemented with protease inhibitors (Roche Applied Science, Indianapolis, IN). After sonication, homogenates were centrifuged at 20,000 × g for 10 minutes at 4°C and the supernatant was used for immunoblotting. Protein concentration was determined with the BCA protein assay reagent kit (Pierce, Rockford, IL). Aliquots of extracts were resolved by sodium dodecyl sulfate-PAGE on a 4-12% NuPage Bis-Tris gel under reducing conditions. Proteins were transferred onto a polyvinylidene fluoride membrane, blocked, and probed with primary polyclonal antibody against HSP70 (provided as whole rabbit antiserum), HSF1 (Assay Designs, Ann Arbor, MI), acetylated histone H3 against both Lys9 and Lys14 acetylation (Millipore, Billerica, MA), trimethylated at lysine 4 histone H3 (Abcam, Cambridge, MA), p300 and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), and then with secondary horseradish peroxidase-conjugated antibody. ECL Plus Western blotting detection system (GE Healthcare, Piscataway, NJ) was used to obtain immunoblotting signals. For preparation of nuclear protein, cortical neurons at DIV-8 were washed with PBS, and lysed in buffer A (20 mM HEPES, pH 7.5, 10 mM KCl, 1 mM MgCl2, 0.02% Triton X-100, 1 mM EGTA, 0.5 mM DTT, supplemented with protease inhibitors). After 10 strokes using a Dounce homogenizer, cell lysates were collected by centrifugation (1000 × g for 10 minutes) at 4°C. Nuclei were then lysed in high-salt buffer (buffer A containing 5.0 M NaCl and protease inhibitors). For immunoprecipitation experiments, 200 μl cell lysates containing 200 μg protein were precleared with 25 μl protein G PLUS-agarose slurry (Santa Cruz Biotechnology) for 20 minutes, and incubated with 2μg Sp1 antibody overnight. The mixtures were then incubated with 30 μl protein G PLUS-agarose slurry for four hours, followed by three consecutive washes with lysis buffer, addition of loading buffer with 5% β-mercaptoethanol, and boiling of samples for four minutes.
Immunofluorescence
Poly-D-lysine-coated chambered coverglasses (Lab-Tek II No. 1.5 or 1.0 borosilicate coverglass) were used for cortical neuronal cultures. Cells were washed with ice-cold PBS, fixed with 4% paraformaldehyde in PBS for 15 minutes at room temperature, and washed three times with ice-cold PBS. They were then blocked in normal goat serum for 60 minutes, incubated overnight with HSP70 antibody (1:400) at 4°C, incubated with secondary fluorescently conjugated anti-rabbit antibody, washed with PBS, mounted using VECTA-SHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and viewed under a fluorescent microscope.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP-IT EXPRESS assay kit protocol (Active Motif, Carlsbad, CA). Briefly, 1.2 × 107 cortical neurons at DIV-7 were treated with 1 mM VPA or its vehicle for 72 hours. Cells were cross-linked with 1% formaldehyde at 37°C for 10 minutes, washed with PBS and lysed, followed by centrifugation. The nuclear pellet was resuspended in shearing buffer, and sonicated with 12 pulses of 15 seconds each, using a sonicator (Sonic and Materials, Newtown, CT) to shear DNA into 500–1000 bp fragments. Ten percent of the mixture of protein/DNA complex was taken for “input DNA” analysis. An equal amount of the protein/DNA complex was then incubated with magnetic beads and an antibody against acetylated histone H3, Sp1, p300 or without an antibody at 4°C overnight. Immunoprecipitated DNA was then eluted from the magnetic beads and the cross-linking was reversed. Input and ChIP DNA were analyzed using PCR or quantitative real-time PCR. The HSPA1B promoter region was PCR amplified using forward primer 5′-CCC AGC CCC TAA AGT TTG TT-3′ and reverse primer 5′-GGG GAT AGG GCT GAT TAA GATT-3′ for determination of histone H3 acetylation. The HSPA1B promoter region was PCR amplified using forward primer 5′-TAC CTC ATC ATG TTT GGT CC-3′ and reverse primer 5′-CGT TTG GCT TGC TAG GCA AG-3′ for assessment of Sp1 and p300 recruitment to the HSP70 promoter. Quantitative real-time PCR amplification was performed in a total reaction volume of 20 μl, including 3 μl of chromatin immunoprecipitated DNA, 200 nM primers, 100 nM FAM-labeled probe (Universal Probe Library, Roche Applied Science), and Platinum Quantitative PCR SuperMix-UDG (Invitrogen). All samples were run in triplicate, including a no-template control. For regular PCR, ChIP and input DNA were mixed with ReactionReady Hotstart “Sweet” PCR mix (SA Biosciences, Frederick, MD) and amplification was carried out for 33 cycles. PCR products were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and visualized by UV illumination.
Measurement of cell viability
For excitotoxicity experiments, cortical neurons were cultured in 96-well plates and treated with the indicated agent beginning at DIV-7. To determine cell viability, the mitochondrial dehydrogenase activity that reduces 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) was assayed in a quantitative colorimetric assay (Leng and Chuang, 2006). Cortical neurons were incubated with MTT for one hour at 37°C. The medium was then aspirated, and the formazan product was dissolved in dimethyl sulfoxide and quantified spectrophotometrically at 540 nm. The results are expressed relative to the viability of the control.
Analysis of chromatin condensation
Cortical neurons grown on 6-well plates were washed with ice-cold PBS and fixed with methanol. Cell nuclei were then stained with Hoechst 33258 (5 μg/ml) for five minutes. Nuclei were visualized under a fluorescence microscope at a wavelength of 360 nm.
Statistical analysis
All results are presented as mean ± SEM from three to four independent experiments. The results were analyzed for statistical significance using GraphPad Prism (GraphPad, San Diego, CA, USA) by using two-sample t-test or one-way analysis of variance.
Results
Characterization of HSP70 induction in rat cortical neurons
Previous studies of heat shock response in cortical neurons obtained disparate results depending on the various experimental paradigms used. Nonetheless, many of these results suggest that cortical and hippocampal neurons may have a delayed or partial heat shock response, compared to glial cells or cerebellar neurons (Marcuccilli et al., 1996; Tagawa et al., 2007). Therefore, we first characterized the induction of HSP70 in rat cortical cultures under our conditions. We tested the ability of HSF1 to induce HSP70 in rat cortical neuronal cultures. Transfection with the constitutively active HSF1 (HSF1(+)), but not the wild type HSF1 (HSF1wt) or dominant negative HSF1 (HSF1(-)), increased the protein levels of HSP70 by more than five-fold, as determined by immunoblotting in cortical neurons (Figure 1A). Next, we tested the effects of heat shock on the HSP70 expression levels under our culture conditions. Heat shock treatment at 42°C for one hour, followed by 24 hours of recovery at 37°C, robustly upregulated low basal HSP70 levels in cortical neurons (Figure 1B). The proteasome inhibitor MG-132 was also found to strongly induce HSP70 in cortical cultures (Figure 1C).
Figure 1. Characterization of HSP70 induction in cortical neurons.
A. Cortical neurons were electroporated with control vector, HSF1wt, HSF1(+) or HSF1(-) before plating. HSP70, HSF1 and β-actin protein levels were determined at DIV-7 by immunoblotting. Right panel shows quantified data. Data are mean ±SEM relative to control from three to four independent experiments. *p<0.05 compared with control. B. Cortical neurons at DIV-7 were heat-shocked for one hour at 42°C and recovered at 37°C for two or 24 hours. HSP70 and β-actin protein levels were determined by immunoblotting. C. Cortical neurons at DIV-7 were treated for 24 hours with vehicle or 0.25 μM MG-132 and HSP70 and β-actin were determined by immunoblotting.
VPA treatment increases the protein level of HSP70 in cortical neurons
We next tested whether VPA increases HSP70 protein levels in rat cortical cultures. Treatment with VPA increased the levels of HSP70 in a dose-dependent manner in the range of 0.25-1 mM (Figure 2A). The increase was approximately three-fold at 1 mM of VPA. VPA-induced upregulation of HSP70 was observed after 24 hours and reached a maximum after 72 hours of exposure (Figure 2B). Under these conditions, VPA treatment also increased the level of histone 3 acetylation at Lys 9 and 14, as previously described (Göttlicher et al., 2001; Phiel et al., 2001). Furthermore, VPA increased trimethylation at Lys 4 of histone H3, which is another mark of activated chromatin (Santos-Rosa et al., 2002). VPA treatment increased HSP70 levels in the nucleus as demonstrated by immunoblotting of nuclear proteins, with no change in the level of the nuclear protein HDAC1 (Figure 2C). Immunofluorescent staining also revealed that HSP70 was increased by VPA treatment in the cytoplasm, neurites and, to a lesser extent, nuclei of cortical neurons (Figure 2D).
Figure 2. Treatment with VPA increases HSP70 protein, mRNA and activity levels, as well as histone acetylation levels in the HSP70 promoter in cortical neurons.
A. Rat cortical neurons were treated with vehicle or VPA (0.25-1.0 mM) for 72 hours starting at DIV-7. HSP70, acetylated histone H3 (AcH3), trimethylated at lysine 4 histone H3 (H3K4Me3), and β-actin protein levels were determined by immunoblotting. Quantified data of the blots on the right panel are mean ± SEM relative to control from three independent experiments. *p<0.05 compared with the control. B. Cortical neurons were treated with 1 mM VPA for the indicated periods of time and HSP70 and β-actin protein levels were determined on DIV-11 by immunoblotting. C. Cortical neurons at DIV-8 were treated with 1 mM VPA or vehicle for 24 h. Protein levels of HSP70 and HDAC1, which was used as a control for a nuclear protein, were determined in nuclear fractions by immunoblotting. D. Immunostaining of HSP70 (red) in cortical neurons after treatment with 1 mM VPA for 72 hours. DAPI nuclear staining shown in blue. Scale bar = 20μm. E. Rat cortical neurons were treated with 1 mM VPA for the indicated periods of time. Levels of the mRNA transcripts of the rat HSP70-1/HSP70-2 genes were determined by semi-quantitative RT-PCR on DIV-11. The right panel shows mean ± SEM of relative HSP70 mRNA values quantified from three independent experiments. *p<0.05. F, G. Rat cortical neurons and SH-SY5Y cells were transfected with human HSP70-1 or HSP70-2 promoter constructs. Neurons were treated with the indicated VPA concentrations for 24 or 72 hours, and HSP70-1 and HSP70-2 promoter activities were determined by dual luciferase assay on DIV-9. HSP70-1 and HSP70-2 promoter activities were determined in SH-SY5Y cells after 24 hours treatment with VPA by dual luciferase assay. Data are mean ± SEM from three to four independent experiments of values relative to control. *p<0.05; **p<0.01 compared with the control. H. Cortical neurons at DIV-7 were treated with 1 mM VPA or vehicle for 72 hours and chromatin was prepared and sheared by sonication. The protein/DNA complex was then incubated with antibody against acetylated histone H3 or without antibody (No AB) for ChIP analysis. Representative gel electrophoresis and representative fluorescence graph of PCR amplification of HSP70-2 gene promoter are shown.
VPA induces transcriptional activation of HSP70 in cortical neurons
Treatment with VPA time-dependently increased HSP70 mRNA levels (Figure 2E). The increase was observed after 6 hours and sustained for four days. Real-time RT-PCR demonstrated that after four days of VPA treatment HSP70 mRNA levels were increased by approximately five-fold (data not shown). To determine whether VPA treatment increases HSP70 promoter activity, cortical neurons were transfected with promoter constructs of the human HSP70-1 and HSP70-2 genes, which share structural homology with the rat genome, and code for the inducible form of HSP70 protein (Angeletti et al., 1996; Fiszer-Kierzkowska et al., 2003). Treatment with VPA (0.5-2 mM) for 24 or 72 hours increased the promoter activity of both constructs, as assessed by dual luciferase reporter assay (Figure 2F & G); this suggests that VPA increases HSP70 mRNA levels through transcriptional activation. When human neuroblastoma cells, which were studied for comparison, were transfected with HSP70-1 or HSP70-2 promoter constructs and treated with VPA for 24 hours, an even greater increase in HSP70 promoter activity was observed (Figure2F & G). ChIP assay revealed that VPA treatment markedly increased histone H3 acetylation in the HSP70 promoter, compared with the untreated control (Figure 2H).
HDAC inhibition participates in VPA-induced HSP70 upregulation
Because VPA is an HDAC inhibitor, we examined whether other HDAC inhibitors had similar effects on HSP70 expression. Treatment with SB, a structurally similar HDAC inhibitor, increased protein levels of HSP70 in a dose-dependent manner with a maximal effect at 1 mM (Figure 3A). TSA, a structurally dissimilar HDAC inhibitor, also increased HSP70 protein levels in the dose range tested (25-100 nM) (Figure 3B). Furthermore, treatment with MS-275 and apicidin – Class I HDAC-specific inhibitors (Khan et al., 2008), also increased HSP70 levels (Figure 3C & D), indicating that at least HDAC class I inhibition is involved. Treatment of cortical neurons with valpromide, a structural analog of VPA with no HDAC inhibitory activity (Phiel et al., 2001), did not change HSP70 levels (Figure 3E). Induction of HSP70 levels by HDAC inhibitors was associated with increased bulk histone H3 acetylation levels. Together, these results suggest that inhibition of HDACs, including HDAC Class I, is involved in VPA-induced HSP70 expression.
Figure 3. Treatment with HDAC inhibitors increases HSP70 levels in cortical neurons.
Rat cortical neurons were treated with the HDAC inhibitor SB (A) or TSA (B) at the indicated concentrations for 72 hours staring on DIV-7. Cortical neurons were also treated with the Class I specific HDAC inhibitor MS-275 (C) or apicidin (D) at the indicated concentration for 48 hours starting on DIV-8. E. Cortical neurons were treated for 72 hours starting at DIV-7 with the indicated concentrations of valpromide (VMD), which is a valproic acid structural analog lacking HDAC activity. Right panels in (A)-(E) show mean values ± SEM relative to control from three independent experiments with the indicated treatment conditions. *p<0.05; **p<0.01; ***p<0.001.
Phosphatidylinositol 3 (PI3)-kinase/Akt pathway activity is necessary for HSP70 upregulation by HDAC inhibitors
Under certain conditions, the synthesis of HSP70 is under the control of the PI3-kinase pathway (Zhou et al., 2004). Therefore, we tested the effect of PI3-kinase inhibitors on HSP70 induction by VPA. Pretreatment for one hour with LY294002 (1-10 μM) or wortmannin (100 nM-3μM), both of which are PI3-kinase inhibitors, dose-dependently diminished the induction of HSP70 by VPA (Figure 4A & B). Pretreatment of cortical neurons with LY294002 also decreased HSP70 induction by other HDAC inhibitors SB (Figure 4C) and TSA (Figure 4D), although relatively higher concentrations of LY294002 (20-30 μM) were required for the inhibition. These results suggest that there are differences in the mechanisms by which VPA, compared with SB and TSA, induce HSP70 levels.
Figure 4. PI3-kinase/Akt pathway activity is required for the induction of HSP70 by HDAC inhibitors.
A. Cortical neurons at DIV-7 were pretreated for one hour with the indicated concentrations of the PI3-kinase inhibitor, LY294002, before treatment with 1 mM VPA or vehicle for 72 hours. Right panel shows mean ± SEM of relative values of HSP70 levels between indicated groups quantified from three independent experiments. *p<0.05; ***p<0.001 between indicated groups. B. Cortical neurons at DIV-8 were pretreated for one hour with vehicle or the indicated concentrations of wortmannin, a PI3-kinase inhibitor, before treatment with 1 mM VPA for 48 hours. Treatment with wortmannin was limited to a 48 hour period due to its instability in culture medium. Cortical neurons at DIV-7 were treated with the indicated concentrations of LY294002 for one hour before treatment with 1 mM SB (C) or 50 nM TSA (D) for 72 hours. Immunoblots are representative of three independent experiments. Cortical neurons at DIV-8 were pretreated for one hour with vehicle or 0.5-1.0 μM of the Akt inhibitor, tricibine, before treatment for 72 hours with 1 mM VPA (E), 1 mM SB (F), or 50 nM TSA (G). Right panels show mean ± SEM of relative values quantified from three independent experiments with the indicated treatments. *p<0.05; **p<0.01.
Furthermore, it is well established that Akt is a downstream target of PI3-kinase. Pretreatment of cortical neurons with the Akt inhibitor, tricibine, at 0.5 or 1 μM for one hour also decreased the induction of HSP70 by VPA, SB, or TSA (Figure 4E, F & G). These results suggest that PI3-kinase/Akt signaling activity is involved in the induction of HSP70 by HDAC inhibitors.
Sp1 is a target for HSP70 upregulation by HDAC inhibitors
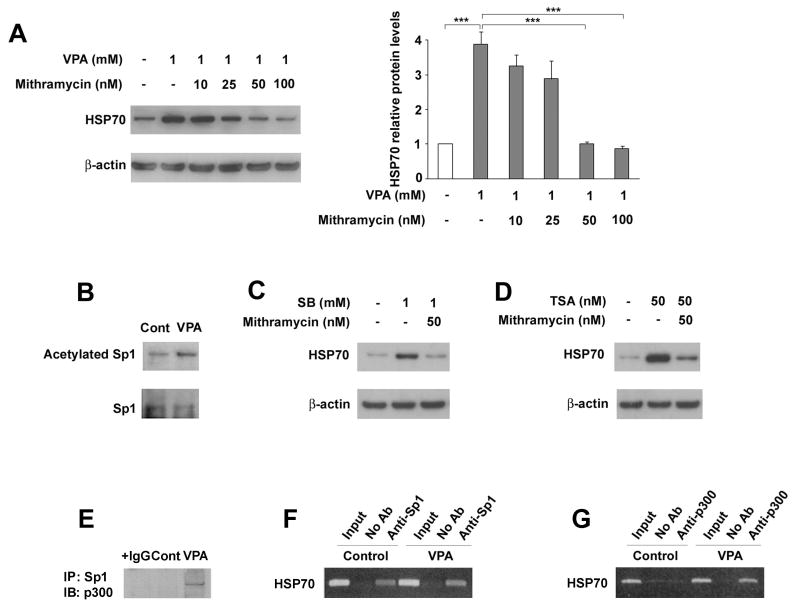
The transcription factor Sp1 can act downstream of Akt, because activated Akt phosphorylates Sp1 (Pore et al., 2004). We tested the effects of a Sp1 inhibitor, mithramycin, on HSP70 induction by VPA. Pretreatment for one hour with mithramycin (10-100 nM) dose-dependently and completely abolished the induction of HSP70 by VPA (Figure 5A). Mithramycin alone did not decrease basal levels of HSP70 in this concentration range (data not shown). Treatment of cortical neurons with 1 mM VPA for 24 hours increased Sp1 acetylation (Figure 5B), confirming that Sp1 is a target of HDAC inhibitors (Ryu et al., 2003). Pretreatment with mithramycin at 50 nM also abolished the induction of HSP70 by SB and TSA (Figure 5C & D). We next tested the association of Sp1 with the histone acetyltransferase p300. Immunoprecipitation analysis showed that VPA treatment increased the association between p300 and Sp1 (Figure 5E). ChIP assay showed that Sp1 was bound to the HSP70 promoter (Figure 5F) and VPA treatment enhanced p300 recruitment to the HSP70 promoter (Figure 5G).
Figure 5. Sp1 is a target for HSP70 induction by HDAC inhibitors.
A. Cortical neurons at DIV-7 were pretreated with the indicated concentrations of mithramycin before treatment with 1 mM VPA for 72 hours. Right panel shows mean ± SEM of relative values quantified from three independent experiments. ***p<0.001 between the indicated groups. B. Cortical neurons at DIV-7 were treated with vehicle or 1 mM VPA for 24 hours, and after immunoprecipitation with Sp1 antibody, acetylation of Sp1 was determined by immunoblotting with acetylated lysine antibody. Cortical neurons at DIV-7 were pretreated with 50 nM mithramycin before treatment with 1 mM SB (C) or 50 nM TSA (D) for 72 hours. Immunoblots are representative of three independent experiments. E. Cortical neurons at DIV-7 were treated with vehicle or 1 mM VPA for 72 h, and after immunoprecipitation with Sp1 antibody, the immunoblot was probed with p300 antibody. In F and G, cortical neurons at DIV-7 were treated with 1 mM VPA or vehicle for 72 hours and chromatin was prepared and sheared by sonication. The protein/DNA complex was then incubated without antibody (No AB), with antibody against Sp1 (F) or with antibody against p300 (G) for ChIP analysis. Representative gel electrophoresis images of PCR amplification of HSP70-2 gene promoter are shown.
VPA-induced neuroprotection against short-term glutamate exposure is diminished by HSP70 inhibition
Finally, we investigated whether HSP70 induction by VPA is neuroprotective. Immunoblotting revealed that VPA upregulated HSP70 to the same extent in the absence or presence of glutamate treatment. In addition, VPA-induced HSP70 upregulation was markedly attenuated by 1-hour pretreatment with KNK437, a HSP70 transcriptional inhibitor (Yokota et al., 2000) (Figure 6A). To test the functional significance of HSP70 induction, we treated cortical neurons with VPA or vehicle for 72 hours starting at DIV-7 with some conditions in which the culture was pretreated with KNK437 for one hour. The medium was removed from the culture, followed by exposure of cortical neurons to 100 μM glutamate in low chloride-containing buffer, which was selected because in this buffer glutamate toxicity is Ca2+-dependent (Rordorf et al., 1991). Cell viability was then determined by MTT assay. Morphological assessment and quantification of the results showed that VPA treatment significantly increased cell viability after short-term glutamate exposure, and that this neuroprotective effect was suppressed by pretreatment with KNK437 (Figure 6B & D). Treatment with KNK437 alone did not significantly affect cell viability. Glutamate treatment also induced chromatin condensation (a hallmark of apoptosis), as detected by staining with Hoechst dye 33258. VPA attenuated chromatin condensation induced by glutamate, and this protective effect was also prevented by pretreatment with KNK437 (Figure 6C).
Figure 6. HSP70 inhibition attenuates neuroprotection by VPA against glutamate-induced excitotoxicity in cortical neurons.
Cortical neurons at DIV-7 were pretreated with 25 μM KNK437 for one hour followed by treatment with 1 mM VPA or vehicle for 72 hours. They were then exposed to 100 μM glutamate in low chloride buffer for 10 minutes, followed by wash-out of glutamate and further incubation of the culture for four hours. A. HSP70 and β-actin protein levels were determined by immunoblotting. Cells were stained with MTT (B) or Hoechst dye 33258 (C) and then examined microscopically and photographed. Arrows indicate apoptotic neurons, undergoing chromatin condensation. Cell viability was quantified by MTT assay (D). Data are mean ± SEM of % of untreated control from three independent cultures. *p<0.05; ***p<0.001 between indicated groups.
Discussion
The present study had several salient findings. First, we showed that VPA and other HDAC inhibitors induce HSP70 in primary cortical neurons that have low basal levels of HSP70, and that are less resilient to various insults. Second, we found that HSP70 upregulation by VPA is associated with transcriptional induction, including increased HSP70 mRNA and promoter activity and histone acetylation. Third, we showed that inhibition of Class I HDACs is actively involved in HSP70 induction. Fourth, we established that PI3-kinase/Akt pathway activity is necessary for the induction of HSP70 by HDAC inhibitors, and that Sp1 is a target of HDACs for HSP70 induction. Finally, we found that HSP70 induction is involved in VPA neuroprotection against short-term glutamate-induced excitotoxicity.
Our results demonstrate that treatment with VPA in concentrations consistent with the therapeutic plasma levels of this drug increased protein levels of HSP70 in rat cortical neurons. The increase in HSP70 protein levels was accompanied by upregulation of HSP70 mRNA levels and promoter activity of the HSP70-1 and HSP70-2 genes that encode HSP70, as well as HSP70 promoter histone acetylation. Because the impaired or delayed heat shock response of neurons, compared to glial cells, may contribute to their sensitivity in neurodegenerative disorders, identifying treatments that can upregulate endogenous heat shock proteins in neurons is an important goal. Our results demonstrated that VPA and other HDAC inhibitors robustly increased HSP70 in primary cortical neurons, and these effects were accompanied by increased global histone acetylation levels. Valpromide, a structural analog of VPA, that does not inhibit HDAC activity, did not increase HSP70 levels. Taken together, these results support the role of HDAC inhibition in HSP70 induction. Furthermore, the ability of MS-275 and apicidin to upregulate HSP70 suggests that Class I HDACs are involved. However, we cannot exclude possible involvement of HDAC Class II in HSP70 upregulation.
Our study also demonstrates that the activity of PI3-kinase/Akt is critical for the induction of HSP70 by VPA, SB, and TSA. Specifically, HSP70 induction by HDAC inhibitors was diminished by selective inhibitors of PI3-kinase and Akt. Previous research had suggested that the PI3-kinase/Akt pathway was involved in the expression of heat shock proteins by increasing the heat shock response through disinhibition of glycogen synthase kinase-3 in cultured cell lines (Bijur et al. 2000; Xavier et al., 2000; Zhou et al, 2004). In addition, the role of PI3-kinase in the expression of basal HSP70 was suggested by a study using renal cell carcinoma RCC4 cells (Zhou et al, 2004), and VPA has been demonstrated to increase Akt phosphorylation in human neuroblastoma cells (De Sarno et al, 2002). Consistent with the role of HSF1 in the induction of HSP70, we found that VPA induced HSP70 in wild type, but not HSF1 null mouse embryonic fibroblasts (data not shown). However, the mechanisms underlying HDAC inhibition-mediated PI3-kinase and Akt activation, and the importance of this activation in HSP70 induction, remain elusive.
In the case of insulin-like growth factor-1 (IGF-1)-induced activation of PI3-kinase, an insulin receptor substrate (IRS) is required for the coupling of the IGF-1 receptor to PI3-kinase activation (Myers et al., 1993). Studies have found that TSA enhances IRS acetylation in human breast adenocarcinoma cell line MCF-7, thus facilitating the activation of Akt by insulin (Kaiser et al., 2004). We are now investigating whether IRS is acetylated in cortical neurons after treatment with HDAC inhibitors, resulting in PI3-kinase activation and HSP70 induction.
In addition to PI3-kinase and Akt, the work described here implicates transcription factor Sp1 as a target for HSP70 induction by HDAC inhibitors, because inhibition of Sp1 activity by mithramycin abolished the induction by HDAC inhibitors. Moreover, we showed that VPA treatment increased levels of Sp1 acetylation. Previous studies had shown that Sp1 can be an anti-apoptotic transcription factor in cortical neurons, and that Sp1 acetylation by HDAC inhibitors correlates with their neuroprotective effect against oxidative stress (Ryu et al, 2003). Therefore, the neuroprotective effects of Sp1 may partly involve induction of HSP70. Consistent with this view, the promoter region of HSP70 gene has previously been shown to contain an Sp1-responsive element in both rats and humans (Greene et al., 1987; Morgan, 1989). Sp1 also appears to be a downstream mediator of Akt through phosphorylation of Sp1 to increase its transcriptional activity (Pore et al., 2004). Thus, it is also conceivable that HSP70 induction by VPA and other HDAC inhibitors involves activation of the PI3-kinase-Akt-Sp1 signaling cascade. Alternatively, HDAC inhibitors may have a direct effect on Sp1. For example, treatment of pancreatic cancer cells with TSA activates the transforming growth factor- type II receptor promoter, via the recruitment of p300 and PCAF into an Sp1·NF-Y·HDAC complex (Huang et al., 2005). The recruitment of p300 and PCAF into the complex is associated with acetylation of Sp1 and a concomitant release of HDACs. Supporting this notion, we found in our study that VPA treatment increased the association between Sp1 and p300 and this effect correlated with an increased recruitment of p300 to the HSP70 promoter.
The role of HSP70 induction in VPA-mediated protection against glutamate-induced, N-methyl-D-aspartate (NMDA)-receptor mediated excitotoxicity is supported by our data that pretreatment of cortical neurons with KNK437, a HSP70 synthesis inhibitor, attenuated VPA neuroprotection (Figure 6). Glutamate excitotoxicity has been implicated in the pathogenesis of a variety of neurodegenerative diseases including cerebral ischemia, traumatic brain and spinal cord injuries, Huntington's disease and cerebellar degeneration (reviewed in Chuang, 2005). Further, an increase in glutamate levels has been found in the frontal cortex of patients with bipolar disorder and major depression (Hashimoto et al., 2007), suggesting that excessive glutamate plays a role in the pathophysiology of mood disorders. In addition to being a molecular chaperone for unfolded or misfolded proteins, HSP70 is an anti-apoptotic, neuroprotective, and anti-inflammatory molecule. For instance, upregulation of HSP70 leads to increased expression of the major anti-apoptotic protein Bcl-2 (Kelly et al., 2002), inhibition of the pro-apoptotic Jun-N terminal kinase (Gabai et al, 1997) and suppression of proinflammatory NF-κB signaling by stabilizing the inhibitor IκB, thus preventing nuclear translocation of p65 (Guzhova et al., 1997; Zheng at al., 2008). Previous studies have shown that overexpression of HSP70 reduces brain damage in a mouse model of ischemic stroke (Rajdev et al., 2000; Hoehn et al., 2001). Additionally, in a rat model of cerebral ischemia, the neuroprotective and anti-inflammatory effects of VPA and other HDAC inhibitors are associated with HSP70 superinduction (Ren et al., 2004; Kim et al., 2007). Furthermore, VPA-induced HSP70 expression in neuroblastoma cells is correlated with neuroprotection against rotenone toxicity (Pan et al., 2005). Moreover, HSP70 deficiency has been reported in both mouse model and in vitro model of Huntington's disease (Tagawa et al. 2007; Hay et al., 2004; Yamanaka et al., 2008). Therefore, HDAC inhibitor-induced HSP70 overexpression is a rational strategy for therapeutic intervention in a number of neurodegenerative conditions.
Increasing data suggest that protein chaperones may play a role in the pathophysiology of mental disease. One DNA microarray study found increased levels of HSPB1 transcript, encoding HSP27, and HSPA1B and HSPA1A transcripts, encoding HSP70 - in the prefrontal cortex of individuals with schizophrenia (Arion et al., 2007). A microarray study confirmed by RT-PCR found increased mRNA levels of the HSPA6 and HSPB8 genes - coding for HSP70 and HSP22 - in the brains of individuals with autism (Garbett et al., 2008). Finally, HSP70 protein levels have been found to decrease significantly after acute immobilization or cold stress in rat hippocampus and cortex (Filipovic et al., 2005).
Together, our results demonstrate that HSP70 is upregulated by VPA and other HDAC inhibitors in cortical neurons through inhibition of Class I HDACs to induce hyperacetylation and activation of HSP70 promoter. This HSP70 transcriptional activation requires a pathway that involves the activity of Sp1 and PI3-kinase/Akt signaling. The induction of HSP70 by HDAC inhibitors is critical for protection against short-term glutamate excitotoxicity. Finally, HSP70 upregulation likely contributes to the neuroprotective effects of VPA in various models of neurodegenerative diseases and could be involved in the therapeutic effects of this drug in bipolar disorder.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health (NIMH), and a gift fund from the HSU Family Foundation. We thank Ioline Henter of the NIMH for the critical reading and comments on the manuscript. The authors declare no conflict of interest.
Abbreviations used
- HDAC
histone deacetylase
- HSF1
heat shock factor 1
- HSP70
heat shock protein 70
- PI3-kinase
phosphatidylinositol 3-kinase
- SB
sodium butyrate
- TSA
trichostatin A
- VPA
valproic acid
References
- Angeletti B, Pascale E, Verna R, Passarelli F, Butler RH, D'Ambrosio E. Differential expression of heat shock protein (HSP70) mRNAs in rat cells. Exp Cell Res. 1996;227:160–164. doi: 10.1006/excr.1996.0261. [DOI] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann RF, Schloesser RJ, Gould TD, Manji H. Mood stabilizers target cellular plasticity and resilience cascades: implications for the development of novel therapeutics. Mol Neurobiology. 2005;32:173–202. doi: 10.1385/MN:32:2:173. [DOI] [PubMed] [Google Scholar]
- Batulan Z, Shinder GA, Minotti S, He BH, Doroudchi MM, Nalbantoglu J, Strong MJ, Durham HD. High threshold for the activation of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batulan Z, Taylor DM, Aarons RJ, Minotti S, Doroudchi MM, Nalbantoglu J, Durham HD. Induction of multiple heat shock proteins and neuroprotection in a primary culture model of familial amyotrophic lateral sclerosis. Neurobiol Dis. 2006;24:213–225. doi: 10.1016/j.nbd.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3β in the regulation of HSF-1 activity. J Neurochem. 2000;75:2401–2408. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Filipovic D, Gavrilovic L, Dronjak S, Radojcic MB. Brain glucocorticoid receptor and heat shock protein 70 levels in rats exposed to acute, chronic or combined stress. Neuropsychobiology. 2005;51:107–114. doi: 10.1159/000084168. [DOI] [PubMed] [Google Scholar]
- Fiszer-Kierzkowska A, Wysocka A, Jarzab M, Lisowska K, Krawczyk Z. Structure of gene flanking regions and functional analysis of sequences upstream of the rat hsp70.1 stress gene. Biochim Biophys Acta. 2003;1625:77–87. doi: 10.1016/s0167-4781(02)00592-4. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Mosser DD, Caron AW, Rits S, Shifrin VI, Sherman MY. Hsp70 prevents activation of stress kinases. A novel pathway of cellular thermotolerance. J Biol Chem. 1997;272:18033–18037. doi: 10.1074/jbc.272.29.18033. [DOI] [PubMed] [Google Scholar]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis. 2008;30:303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifondorwa DJ, Robinson MB, Hayes CD, Taylor AR, Prevette DM, Oppenheim RW, Caress J, Milligan CE. Exogenous delivery of heat shock protein 70 increases lifespan in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2007;27:13173–13180. doi: 10.1523/JNEUROSCI.4057-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JM, Larin Z, Taylor IC, Prentice H, Gwinn KA, Kingston RE. Multiple basal elements of a human hsp70 promoter function differently in human and rodent cell lines. Mol Cell Biol. 1987;7:3646–3655. doi: 10.1128/mcb.7.10.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2:132–139. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J Neurochem. 2002;80:589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychi. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hay DG, Sathasivam K, Tobaben S, Stahl B, Marber M, Mestril R, Mahal A, Smith DL, Woodman B, Bates GP. Progressive decrease in chaperone protein levels in a mouse model of Huntington's disease and induction of stress proteins as a therapeutic approach. Hum Mol Genet. 2004;13:1389–1405. doi: 10.1093/hmg/ddh144. [DOI] [PubMed] [Google Scholar]
- Hoehn B, Ringer TM, Xu L, Giffard RG, Sapolsky RM, Steinberg GK, Yenari MA. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J Cereb Blood Flow Metab. 2001;21:1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhao S, Ammanamanchi S, Brattain M, Venkatasubbarao K, Freeman JW. Trichostatin A induces transforming growth factor beta type II receptor promoter activity and acetylation of Sp1 by recruitment of PCAF/p300 to a Sp1.NF-Y complex. J Biol Chem. 2005;280:10047–10054. doi: 10.1074/jbc.M408680200. [DOI] [PubMed] [Google Scholar]
- Kaiser C, James SR. Acetylation of insulin receptor substrate-1 is permissive for tyrosine phosphorylation. BMC Biol. 2004;2:23. doi: 10.1186/1741-7007-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai H, Sawa A, Chen RW, Leeds P, Chuang DM. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicity-induced GAPDH nuclear accumulation and apoptotic death in neurons. Pharmacogenomics J. 2004;4:336–344. doi: 10.1038/sj.tpj.6500269. [DOI] [PubMed] [Google Scholar]
- Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, Yenari MA, Steinberg GK. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol. 2002;52:160–167. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J. 2008;409:581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Langley B, D'Annibale MA, Suh K, Ayoub I, Tolhurst A, Bastan B, Yang L, Ko B, Fisher M, Cho S, Beal MF, Ratan RR. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21waf1/cip1 in cell cycle-independent neuroprotection. J Neurosci. 2008;28:163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Chuang DM. Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci USA. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MH, Chuang DM. Differential roles of glycogen synthase kinase-3 isoforms in the regulation of transcriptional activation. J Biol Chem. 2006;281:30479–30484. doi: 10.1074/jbc.M607468200. [DOI] [PubMed] [Google Scholar]
- Magrane J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J Neurosci. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuccilli CJ, Mathur SK, Morimoto RI, Miller RJ. Regulatory differences in the stress response of hippocampal neurons and glial cells after heat shock. J Neurosci. 1996;16:478–485. doi: 10.1523/JNEUROSCI.16-02-00478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel A, Harms C, Yildirim F, Bösel J, Kronenberg G, Harms U, Fink KB, Endres M. Inhibition of histone deacetylation protects wild-type but not gelsolin-deficient neurons from oxygen/glucose deprivation. J Neurochem. 2006;98:1019–1031. doi: 10.1111/j.1471-4159.2006.04016.x. [DOI] [PubMed] [Google Scholar]
- Morgan WD. Transcription factor Sp1 binds to and activates a human hsp70 gene promoter. Mol Cell Biol. 1989;9:4099–4104. doi: 10.1128/mcb.9.9.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacological targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Myers MG, Jr, Sun XJ, Cheatham B, Jachna BR, Glasheen EM, Backer JM, White MF. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3′-kinase. Endocrinology. 1993;132:1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- Pan T, Li X, Xie W, Jankovic J, Le W. Valproic acid-mediated Hsp70 induction and anti-apoptotic neuroprotection in SH-SY5Y cells. FEBS Lett. 2005;579:6716–6720. doi: 10.1016/j.febslet.2005.10.067. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Pirkkala L, Nykänen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–1131. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- Pore N, Liu S, Shu HK, Li B, Haas-Kogan D, Stokoe D, Milanini-Mongiat J, Pages G, O'Rourke DM, Bernhard E, Maity A. Sp1 is involved in Akt-mediated induction of VEGF expression through an HIF-1–independent mechanism. Mol Biol Cell. 2004;15:4841–4853. doi: 10.1091/mbc.E04-05-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci USA. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats:potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem. 2004;89:1358–1367. doi: 10.1111/j.1471-4159.2004.02406.x. [DOI] [PubMed] [Google Scholar]
- Rordorf G, Koroshetz WJ, Bonventre JV. Heat shock protects cultured neurons from glutamate toxicity. Neuron. 1991;7:1043–1051. doi: 10.1016/0896-6273(91)90348-4. [DOI] [PubMed] [Google Scholar]
- Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M, Flemington E, Azizkhan-Clifford J, Ferrante RJ, Ratan RR. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J Biol Chem. 2005;280:39962–39969. doi: 10.1074/jbc.M505524200. [DOI] [PubMed] [Google Scholar]
- Tagawa K, Marubuchi S, Qi ML, Enokido Y, Tamura T, Inagaki R, Murata M, Kanazawa I, Wanker EE, Okazawa H. The induction levels of heat shock protein 70 differentiate the vulnerabilities to mutant huntingtin among neuronal subtypes. J Neurosci. 2007;27:868–880. doi: 10.1523/JNEUROSCI.4522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Osada M, Guo Z, Fomenkov A, Begum S, Zhao M, Upadhyay S, Xing M, Wu F, Moon C, Westra WH, Koch WM, Mantovani R, Califano JA, Ratovitski E, Sidransky D, Trink B. ΔNp63α up-regulates the Hsp70 gene in human cancer. Cancer Res. 2005;65:758–766. [PubMed] [Google Scholar]
- Xavier IJ, Mercier PA, McLoughlin CM, Ali A, Woodgett JR, Ovsenek N. Glycogen synthase kinase 3β negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J Biol Chem. 2000;275:29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Miyazaki H, Oyama F, Kurosawa M, Washizu C, Doi H, Nukina N. Mutant Huntingtin reduces HSP70 expression through the sequestration of NF-Y transcription factor. EMBO J. 2008;27:827–839. doi: 10.1038/emboj.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000;60:2942–2948. [PubMed] [Google Scholar]
- Young LT, Bakish D, Beaulieu S. The neurobiology of treatment response to antidepressants and mood stabilizing medications. J Psychiatry Neurosci. 2002;27:260–265. [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- Zhou J, Schmid T, Frank R, Brüne B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1α from pVHL-independent degradation. J Biol Chem. 2004;279:13506–13513. doi: 10.1074/jbc.M310164200. [DOI] [PubMed] [Google Scholar]
- Zuo J, Rungger D, Voellmy R. Multiple layers of regulation of human heat shock transcription factor 1. Mol Cell Biol. 1995;15:4319–4330. doi: 10.1128/mcb.15.8.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]