Abstract
Background
Hypertension is more common among men at younger ages and among women after age 60, suggesting a possible link between endogenous estrogens and systolic blood pressure (SBP). We tested whether serum 17β-estradiol (E2) or any of its metabolites were associated with SBP among middle-aged and older adults.
Methods
Using a cross-sectional study design, we examined data from a population-based sample of 98 adults living in Cook County, Illinois. Age ranged between 55 and 69 years and body mass index (BMI) ranged between 19.8 and 50.6 kg/m2. Serum was analyzed for 17β-E2 and 14 estrogen metabolites (EMs) using mass spectrometry. SBP was measured using a tonometric device that records a pulse wave at the radial artery. Demographic and health history information were obtained via questionnaires.
Results
Univariate analysis revealed an inverse relationship between SBP and both natural log (ln) 16α-hydroxyestrone (OHE1) (r = −0.360, P < 0.05) and ln 16-ketoestradiol (ketoE2) (r = −0.360, P < 0.05) among women but not men. No significant correlations were found between SBP and 17β-E2 in either sex. In multivariate analysis which adjusted for age, race, ethnicity, BMI, and use of cardiovascular medications, ln 16α-hydroxyestrone (16α-OHE1) (B = −5.3, s.e. = 2.1, P < 0.05) and ln 16-ketoE2 (B = −4.7, s.e. = 1.9, P < 0.05) continued to be negatively associated with SBP among postmenopausal women.
Conclusions
These data suggest that serum 16α-OHE1 or 16-ketoE2 may be important for vascular health among postmenopausal women but not among similarly aged men.
Systolic blood pressure (SBP) increases with age among both men and women. The slope of this increase is steeper among men before age 60 but this pattern reverses after age 60 (ref. 1). As a result, hypertension is more prevalent among men at younger ages2 and among women at older ages.3 This pattern is consistent with age- and sex-related differences in 17β-estradiol (E2) production, a hormone with known vasodilatory effects.4
The mechanisms by which E2 influences vascular tone include genomic and nongenomic effects on endothelial and vascular smooth muscle cells (VSMCs).5 Genomic effects on vascular endothelium include increased mitogen-activated protein kinase–induced endothelial nitric oxide (NO) synthase gene expression and NO production, as well as increased endothelial cell production.5 Nongenomic effects of E2 on endothelial cells include increased endothelial NO synthase activity, increased NO generation, enhanced prostacyclin production, and reduced free radical (superoxide) formation.5 NO and prostacyclin from the endothelium have vasodilatory effects on VSMCs. In VSMC, E2 inhibits growth factor-mediated activation of mitogen-activated protein kinase, impairs gene transcription, and reduces VSMC growth and proliferation.5 Nongenomically, E2 reduces Ca2+ influx into VSMCs, thereby inhibiting Ca2+-dependent myosin light chain phosporylation and VSMC contraction.5
Several estrogen metabolites (EMs), including D-ring metabolites such as 16α-hydroxyestrone (OHE1), bind to both α and β estrogen receptors.6 This metabolite induces endothelial cell prostacyclin production to a greater extent that E27 and inhibits oxidation of low-density lipoproteins,8 suggesting it may have vasculoprotective effects. The purpose of the current study is to examine the relationship between endogenous serum estrogen concentrations and SBP in a population-based sample of middle-aged and older adults. Given the established vascular effects of 17β-E2 and the potential vasculoprotective effects of 16α-hydroxyestrone (16α-OHE1), we hypothesized significant inverse relationships between these estrogens and SBP in our study population.
Methods
Study population
Data for this study were collected in 2005, the fifth year of the Chicago Health, Aging, and Social Relations Study, a longitudinal, population-based study of individuals born between 1935 and 1952. The Chicago Health, Aging, and Social Relations Study was designed to examine the social, psychological, and biological aspects of social isolation and health. The target population included Caucasian, African-American, and Latino-American subjects between the ages of 50 and 67 years living in Cook County, Illinois, who were English speaking and sufficiently ambulatory to come to the University of Chicago for a daylong visit to the laboratory. The sample was selected using a previously described multistage probability design.9 With attrition, the original sample size of 229 adults (120 females and 109 males) in year 1 declined to 163 adults (91 females and 72 males) in year 5. All procedures were approved by the University of Chicago Institutional Review Board and all participants provided informed consent.
After obtaining height and weight measurements, participants were seated in a comfortable padded chair and provided with a footrest if their legs were too short to rest on the floor. A Colin Vital Statistics monitor (model BP-508, Vital Signs, Minster, OH) was used to obtain systolic, diastolic, and mean arterial blood pressure readings from the nondominant arm, which was supported at the heart level by a cushion resting on the arm of the participant's chair. The Colin monitor records a pulse wave tonometrically by partial occlusion of the radial artery against the radius of the wrist, allowing for beat-to-beat measurement of blood pressure. The tonomometer was calibrated against an initial blood pressure reading using an oscillometric cuff and was periodically recalibrated either automatically or on experimenter initiation. As described in a previous report, our analysis used an average of 280 beat-to-beat blood pressure values obtained during 4 min in the seated posture.9 Participants listed their medications, and antihypertensive agents were categorized as either vasoactive (i.e., α2-agonists, α-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, and β-blockers) or volume active (i.e., diuretics) for inclusion in multiple linear regression models.
Following the blood pressure measurement, we obtained permission to collect venous blood samples from 52 women and 51 men. All women reported being postmenopausal at the time of collection. Serum aliquots were stored at −80 °C and batched for analysis. Samples were then shipped on dry ice to the Laboratory of Proteomics and Analytical Technologies, SAIC-Frederick, Maryland for analysis of EM concentrations.
EM assay
Reagents and materials
Fifteen EMs including estrone (E1), E2, estriol (E3), 16-epiestriol (16-epiE3), 17-epiE3, 16-ketoestradiol (16-ketoE2), 16α-OHE1, 2-methoxyestrone (2-MeOE1), 4-MeOE1, 2-OHE1-3-methyl ether (3-MeOE1), 2- methoxyestradiol (2-MeOE2), 4-MeOE2, 2-OHE1, 4-OHE1, and 2-hydroxyestradiol (2-OHE2) were obtained from Steraloids, (Newport, RI). Stable isotope-labeled estrogens (SI-EMs), including E2-13,14,15,16,17,18-13C6 (13C6-E2) and E1-13,14,15,16,17,18-13C6 (13C6-E1) were purchased from Cambridge Isotope Laboratories, (Andover, MA); E3-2,4,17-d3 (d3-E3), 2-OHE2-1,4,16,16,17-d5 (d5-2-OHE2), and 2- MeOE2-1,4,16,16,17-d5 (d5-2-MeOE2), were obtained from C/D/N Isotopes, (Pointe-Claire, Quebec, Canada). 16-epiE3-2,4,16-d3 (d3-16-epiE3) was purchased from Medical Isotopes (Pelham, NH). All EM and SI-EM analytical standards have reported chemical and isotopic purity ≥98%, and were used without further purification. Dichloromethane, methanol, and formic acid were obtained from EM Science (Gibbstown, NJ). Glacial acetic acid, sodium bicarbonate, and L-ascorbic acid were purchased from J.T. Baker (Phillipsburg, NJ) whereas sodium hydroxide and sodium acetate were purchased from Fisher Scientific (Fair Lawn, NJ). β-Glucuronidase/sulfatase (Helix pomatia, Type HP-2) was obtained from Sigma Chemical (St Louis, MO). Dansyl chloride and acetone were purchased from Aldrich Chemical (Milwaukee, WI). All chemicals and solvents used in this study were high performance liquid chromatography or reagent grade unless otherwise noted.
Preparation of stock and working standard solutions
Stock solutions of EM and SI-EM were each prepared at 80 μg/ml by dissolving 2 mg of the estrogen powders in methanol with 0.1% l-ascorbic acid to a final volume of 25 ml in a volumetric flask. The stock solutions are stable for at least 2 months while stored at −20 °C. Stock solutions were analyzed at the beginning of each analysis to verify no time-dependent degradation of the EM and SI-EM standards had occurred. Working standards of EM and SI-EM at 8 ng/ml were prepared by dilutions of the stock solutions using methanol with 0.1% l-ascorbic acid.
Calibration standards and quality control samples
Charcoal-stripped human serum (Golden West Biologicals, Temecula, CA) with no detectable levels of any EM and containing 0.1% (wt/vol) l-ascorbic acid, was employed for preparation of calibration standards and quality control samples. Calibration standards were prepared in charcoal-stripped human serum by adding 2 μl of the SI-EM working internal standard solution (16 pg of each SI-EM) to various volumes of the EM working standard solution. These calibration standards typically contain 0.2–200 pg of each EM in 0.4 ml of charcoal-stripped serum and were assayed in duplicate. The calibration standards cover three orders of magnitude. The quality control samples were prepared at three levels: 0.5, 20, and 250 pg/ml of each EM.
Sample preparation
To 400 μl of each serum sample, 500 μl of freshly prepared enzymatic hydrolysis buffer containing 2 mg of L-ascorbic acid, 5 μl of β-glucuronidase/sulfatase (Helix pomatia, Type HP-2), and 0.5 ml of 0.15 mol/l sodium acetate buffer (pH 4.6) was added. The samples were incubated for 20 h at 37 °C. Aliquots then underwent slow inverse extraction at 8 r.p.m. (RKVSD; ATR, Laurel, MD) with 6 ml dichloromethane for 30 min. After extraction, the aqueous layer was discarded and the organic solvent portion was transferred into a clean glass tube and evaporated to dryness at 60 °C under nitrogen gas (Reacti-Vap III; Pierce, Rockford, IL).
To each dried sample, 32 μl of 0.1 mol/l sodium acetate buffer (pH at 9.0) and 32 μl of dansyl chloride solution (1 mg/ml in acetone) were added. After vortexing, the sample was heated at 60 °C (Reacti-Therm III Heating Module; Pierce) for 5 min to form the EM and SI-EM dansyl derivatives (EM-Dansyl and SI-EM-Dansyl, respectively). Calibration standards and quality control samples were hydrolyzed, extracted, and derivatized following the same procedure as that used for unknown serum samples.
Capillary liquid chromatography-electrospray ionization-tandem mass spectrometry analysis
After derivatization, all samples were analyzed using capillary liquid chromatography-electrospray ionization-tandem mass spectrometry analysis.10 Capillary liquid chromatography-electrospray ionization-tandem mass spectrometry analysis was performed using an Agilent 1200 series nanoflow LC system (Agilent Technologies, Palo Alto, CA) coupled to a TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Electron, San Jose, CA). The LC separation was carried out on a 150 mm long × 300 μm internal diameter column packed with 4 μm Synergi Hydro-RP particles (Phenomenex, Torrance, CA) and maintained at 40 °C. A total of 8 μl of each sample was injected onto the column. The mobile phase, operating at a flow rate of 4 μl/min, consists of methanol as solvent A and 0.1% (vol/vol) formic acid in water as solvent B. A linear gradient from 72 to 85% solvent B in 75 min. was employed for separation of EM and SI-EM. The mass spectrometry conditions were: source: ESI; ion polarity: positive; spray voltage: 3,500 V; sheath and auxiliary gas: nitrogen; sheath gas pressure: seven arbitrary units; ion transfer capillary temperature, 270 °C; scan type: selected reaction monitoring; collision gas: argon; collision gas pressure: 1.5 mTorr; scan width: 0.7 u; scan time: 0.50 s; Q1 peak width: 0.70 u full-width half-maximum; Q3 peak width: 0.70 u full-width half-maximum. The optimized selected reaction monitoring conditions for the protonated molecules [MH]+ of EM-Dansyl and SI-EM-Dansyl were similar to those previously described.10
Quantitation of serum EMs
Quantitation of serum EM was carried out using Xcalibur Quan Browser (Thermo Electron, Waltham, MA). Briefly, calibration curves for the each EM were constructed by plotting EM-Dansyl/SI-EM-Dansyl peak area ratios obtained from calibration standards vs. amounts of EM injected on column and fitting these data using linear regression with 1/X weighting. The amounts of EM in sera were then interpolated using this linear function. Based on their similarity of structures and retention times, 13C6-E2 was used as the internal standard for E2; 13C6-E1 for E1; d3-E3 for E3, 16-ketoE2, and 16α-OHE1; d3-16-epiE3 for 16-epiE3 and 17-epiE3; d5-2-MeOE2 for 2-MeOE2, 4-MeOE2, 2-MeOE1, 4-MeOE1, and 3-MeOE1; d5-2-OHE2 for 2-OHE2, 2-OHE1, and 4-OHE1, respectively.
Statistical analysis
Statistical analysis focused on SBP because it is superior to diastolic blood pressure in predicting cardiovascular disease, especially in adults over the age of 50 years.11 For the purposes of this study, we utilized data from the 47 postmenopausal females who were not on hormone replacement therapy and from 51 men. Correlational analyses were conducted to examine the bivariate associations between serum EM concentrations and SBP in both women and men. Ordinary linear regression analyses were used to test the magnitude of effects of EMs on SBP independent of demographic characteristics (age, body mass index (BMI), and race/ethnicity), use of vasoactive blood pressure medications (0 = no; 1 = yes), and use of volume-active blood pressure medications (0 = no; 1 = yes). EM values exhibited a positively skewed distribution and were therefore subjected to a natural log (ln) transformation. Regression coefficients are therefore interpretable as the magnitude of change in SBP associated with one ln unit increase of the predictor metabolite. Regression analysis among women employed only those cases (N = 32–33) with no missing data on any of the variables used in the regression model. Statistical significance was set at P < 0.05. All analyses were conducted using SPSS 16.0 (SPSS, Chicago, IL).
Results
Among women in the study, 23 were white, 11 were African American, and 13 were Latino American. Among men, 22 were white, 15 were African American, and 14 were Latino American. Age, BMI, and SBP data, as well as EM concentrations for cases with detectable levels of metabolites are provided in Table 1. Concentrations of E1, E2, E3, and 2-OHE1 were at measurable levels in all participants; the remaining metabolites exhibited varying prevalence of undetectable levels. Consistent with previous research, the concentrations of several serum estrogens were higher among men compared to women in this age group.12
Table 1. Participant demographics and serum estrogen metabolite concentrations.
| Variable | N | Female Mean (s.d.)a | N | Male Mean (s.d.)a | ANOVA P |
|---|---|---|---|---|---|
| Age | 47 | 61.1 (3.9) | 51 | 61.3 (4.3) | 0.823 |
| BMI (kg/m2) | 46 | 32.0 (7.3) | 50 | 31.0 (5.5) | 0.472 |
| SBP (mm Hg) | 46 | 131.5 (12.6) | 50 | 133.8 (16.6) | 0.432 |
| Serum estrogen metabolites (pg/ml) | |||||
| Estrone | 47 | 196.7 (226.0) | 51 | 409.2 (229.1) | 0.000 |
| 17β-estradiol | 47 | 31.6 (30.5) | 51 | 62.3 (33.3) | 0.000 |
| Estriol | 47 | 741.6 (437.2) | 51 | 878.4 (430.8) | 0.122 |
| 2-Hydroxyestrone | 47 | 136.4 (77.8) | 51 | 146.0 (111.6) | 0.624 |
| 2-Methoxyestrone | 45b | 32.8 (26.5) | 48b | 25.5 (18.2) | 0.199 |
| 2-Hydroxyestradiol | 46b | 15.9 (26.1) | 51 | 18.1 (22.0) | 0.598 |
| 2-Methoxyestradiol | 42b | 19.0 (17.6) | 46b | 16.1 (18.1) | 0.810 |
| 2-Hydroxyestrone-3-methyl ether | 36b | 8.9 (10.4) | 41b | 6.7 (6.7) | 0.958 |
| 4-Hydroxyestrone | 0b | ND | 0b | ND | NA |
| 4-Methoxyestrone | 38b | 16.6 (29.4) | 41b | 8.7 (11.4) | 0.267 |
| 4-Methoxyestradiol | 29b | 9.2 (9.9) | 27b | 4.1 (6.5) | 0.339 |
| 16α-Hydroxyestrone | 34b | 26.4 (22.5) | 43b | 45.3 (53.6) | 0.002 |
| 16-Ketoestradiol | 35b | 34.7 (38.8) | 43b | 55.4 (56.6) | 0.003 |
| 16-Epiestriol | 37b | 4.7 (6.3) | 47b | 7.0 (7.6) | 0.002 |
| 17-Epiestriol | 39b | 3.4 (4.6) | 38b | 3.1 (5.2) | 0.803 |
| Total estrogen concentration | 47 | 1,247.4 (603) | 51 | 1,686.1 (607.7) | 0.001 |
ND, analyte not detected.
Descriptive statistics for cases with detectable metabolite levels.
Number of cases for which metabolite levels were detectable.
Correlational analysis revealed significant inverse relationships between SBP and both 16α-OHE1 (r = −0.360; P = 0.031) and 16-ketoE2 (r = −0.360; P = 0.029) among women. Figures 1 and 2 demonstrate the scatterplots of the associations between SBP and 16α-OHE1 and between SBP and 16-ketoE2, respectively. None of the other EMs was significantly correlated with SBP in women. Among men, there were no significant correlations between SBP and E2 or any other serum estrogen. Although serum concentrations of certain EMs varied by sex, for cases with detectable levels, serum metabolite concentrations did not differ as a function of race or ethnicity, Ps > 0.05. Fifty percent of participants were taking vasoactive medications, 17% were taking volume-active medications, and the remainder were taking both vasoactive and volume-active medications.
Figure 1.
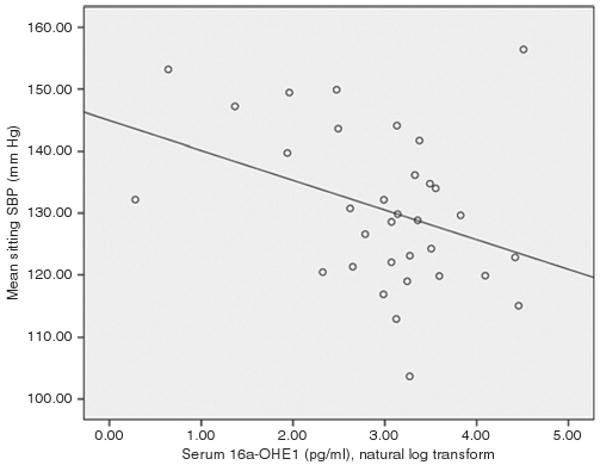
Scatterplot showing the bivariate relationships between natural log serum 16α-hydroxyestrone and systolic blood pressure among postmenopausal women not taking hormone replacement therapy. The regression line was fit using the least squares method.
Figure 2.
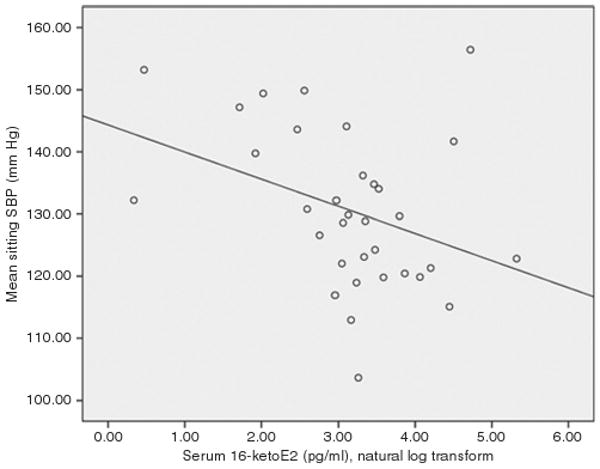
Scatterplot showing the bivariate relationships between natural log serum 16-ketoestradiol and systolic blood pressure among postmenopausal women not taking hormone replacement therapy. The regression line was fit using the least squares method.
Multivariate regression analysis of data from 32 to 33 women for whom we had both EM and covariate information showed that both 16α-OHE1 and 16-ketoE2 retained significant associations with SBP when age, BMI, race/ethnicity, and vasoactive and volume active hypertension medications were kept constant. Table 2 shows that a ln unit increase in 16α-OHE1 was associated with a 5.3 mm Hg decrease in SBP whereas a ln unit increase in 16-ketoE2 was associated with a 4.7 mm Hg decrease in SBP. A ln unit increase in the sum of 16α-OHE1 and 16-ketoE2 was associated with a 4.6 mm Hg decrease in SBP after adjusting for multiple covariates. As combining these metabolites provided no increase in effect magnitude, it is unlikely that 16α-OHE1 and 16-ketoE2 have additive vasodilatory effects. When both metabolites were simultaneously entered in the regression model, neither had a significant effect on SBP, Ps > 0.4, suggesting a high correlation between these variables. Ancillary analysis revealed the correlation coefficient between 16-OHE1 and 16-ketoE2 was 0.91. Therefore it is likely that 16-ketoE2 is not independently associated with SBP but serves as a marker for 16α-OHE1 (or vice versa).
Table 2. Regression coefficients predicting SBP in postmenopausal women not taking hormone replacement therapy.
|
B (s.e.) (N = 32) |
B (s.e.) (N = 33) |
B (s.e.) (N = 32) |
|
|---|---|---|---|
| Serum estrogen predictor | |||
| ln 16α-Hydroxyestrone | −5.3 (2.1)* | ||
| ln 16-Ketoestradiol | −4.7 (1.9)* | ||
| ln (16α-Hydroxyestrone + 16-ketoestradiol) | −4.6 (1.9)* | ||
| Covariates | |||
| Age | 0.7 (0.6) | 0.6 (0.6) | 0.7 (0.6) |
| BMI | 0.3 (0.3) | 0.3 (0.3) | 0.3 (0.3) |
| Race/ethnicity | |||
| African American | −2.0 (4.8) | −2.7 (4.9) | −2.4 (4.9) |
| Latino American | −6.9 (4.7) | −7.6 (4.7) | −7.3 (4.7) |
| Vasoactive medications | −0.7 (4.9) | −1.8 (5.0) | −1.6 (4.9) |
| Volume active medications | 3.1 (5.7) | 4.7 (5.5) | 4.1 (5.6) |
| Intercept | 97.6 (37.6) | 102.2 (38.8) | 103.0 (38.7) |
SBP, systolic blood pressure.
P < 0.05.
Discussion
The present study is the first to examine the relationship between serum EMs and blood pressure in a population-based sample of middle-aged and older adults. Consistent with prior studies, postmenopausal women had lower levels of serum E1 and 17β-E2 compared to similarly aged men.12 In addition, the abundance of E3 in the serum is consistent with studies showing it is the most abundant urinary estrogen.12 Among postmenopausal women, we found an inverse relationship between serum 16α-OHE1 and SBP after adjusting for multiple covariates. 16-ketoE2, an EM similar in structure to 16α-OHE1, also demonstrated an inverse relationship with SBP in this population. However, contrary to our hypothesis, we did not find an inverse relationship between 17β-E2 and SBP in either women or men.
Previous research has shown that endothelial production of the vasodilator prostacyclin can be induced by 16α-OHE1.7 16α-OHE1 also has potent antioxidant effects.8 These properties may contribute to the association between 16α-OHE1 and lower blood pressure among postmenopausal women. In addition, at least one study suggests that 16α-OHE1 can form covalent bonds with estrogen receptors, possibly leading to longer-lasting estrogenic effects.13 Within the arterial vasculature, these effects may include increased vasodilator production, enhanced endothelial cell production, inhibition of reactive oxygen species, and inhibition of VSMC proliferation.5 A prolonged effect of 16α-OHE1 may explain its greater association with SBP than 17β-E2, which may exert shorter-term effects.
Previous research suggests lower serum concentrations of 16α-OHE1 and 16-ketoE2 among postmenopausal compared to premenopausal women. In a recent article describing an assay for EMs, the mean serum concentrations of 16α-OHE1 and 16-ketoE2 among premenopausal women (N = 4) were 21.4 pg/ml (s.d. = 16.1) and 25.4 pg/ml (s.d. = 16.6), respectively.10 Among postmenopausal women (N = 2), the mean serum concentrations of 16α-OHE1 and 16-ketoE2 were 8.8 pg/ml (s.d. = 0.6) and 11.0 pg/ml (s.d. = 0.9), respectively.10 Due to the small sample sizes in that article, neither of the metabolite concentration differences associated with menopausal status was significant. Our study population included only postmenopausal women and the mean serum concentrations for 16α-OHE1 and 16-ketoE2 were comparable to the mean values among postmenopausal women in the previous report.10 The trend toward decreased serum 16α-OHE1 and 16-ketoE2 among postmenopausal women is consistent with our hypothesis that a decline in one of these metabolites contributes to increased blood pressure among postmenopausal women.
Among men in our study population, SBP was not associated with 17β-E2, 16α-OHE1, or 16-ketoE2. This is not surprising given results of animal and human studies which show sex differences in vascular function. For example, endothelium-dependent vascular relaxation is greater among female than male spontaneously hypertensive rats,14 total endothelial NO release is greater in premenopausal women than in men,15 superoxide production is greater in the blood vessels of male compared to female rats,5 and less endothelin-1 is released from female spontaneously hypertensive rat endothelial cells than from those of male spontaneously hypertensive rats.16 Therefore, our findings do not contradict research demonstrating important sex differences in vascular physiology.
A limitation of this study is its relatively small sample size. Because we did not find correlations between endogenous estrogens and SBP among men, our analysis focused on the 32–33 postmenopausal women for whom all EM and covariate data were available. Therefore, our results should be viewed as preliminary until replicated in a larger cohort of postmenopausal women. Another potential limitation of this study was our use of total (conjugated plus unconjugated) serum EMs. As 16α-OHE1 circulates primarily unbound to sex hormone binding globulin,17 the total concentration of this metabolite may approximate its unbound (and therefore metabolically active) concentration. In contrast, most other EMs are significantly bound to sex hormone binding globulin and the lack of association between total serum concentration of those metabolites and SBP may be due more to their unavailability to estrogen receptors than to their inability to induce vasodilatory changes. Future studies should evaluate the relationship between SBP and both conjugated and unconjugated EMs.10 Finally, our study was cross-sectional in design. Therefore, we cannot infer that higher serum concentrations of 16α-OHE1 resulted in lower SBP among postmenopausal women. The reverse may be true or a third factor may account for this inverse relationship. Causal inferences will require prospective analysis of the relationship between serum EMs and SBP.
The mechanism by which 16α-OHE1 and 16-ketoE2 are associated with lower blood pressure in postmenopausal women is unknown but we hypothesize that 16α-OHE1 is the active metabolite whereas 16-ketoE2 simply serves as a marker for 16α-OHE1. This hypothesis is based upon studies which show that 16α-OHE1 can induce endothelial prostacyclin production,7 has antioxidant effects,8 and can bind covalently to estrogen receptors.13 If our findings are replicated, future studies should investigate dietary and genetic predictors of 16α-OHE1 production. Such information may lead to screening tests which identify women at risk for hypertension. It may also lead to therapies which mimic the potentially vasculoprotective effects of 16α-OHE1.
Acknowledgments
This work was supported by a National Institute on Aging Career Development Award 5K08AG027200-02 (principal investigator: C.M. Masi) and a National Institute on Aging R01 AG034052-01 (principal investigator: J.T. Cacioppo). This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Ostchega Y, Dillon CF, Hughes JP, Carroll M, Yoon S. Trends in hypertension prevalence, awareness, treatment, and control in older U.S. adults: data from the National Health and Nutrition Examination Survey 1988 to 2004. J Am Geriatr Soc. 2007;55:1056–1065. doi: 10.1111/j.1532-5415.2007.01215.x. [DOI] [PubMed] [Google Scholar]
- 4.Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. doi: 10.1152/ajpregu.00338.2003. [DOI] [PubMed] [Google Scholar]
- 5.Qiao X, McConnell KR, Khalil RA. Sex steroids and vascular responses in hypertension and aging. Gend Med. 2008;5 A:S46–S64. doi: 10.1016/j.genm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Seeger H, Mueck AO, Lippert TH. Effect of estradiol metabolites on prostacyclin synthesis in human endothelial cell cultures. Life Sci. 1999;65:PL167–170. doi: 10.1016/s0024-3205(99)00383-5. [DOI] [PubMed] [Google Scholar]
- 8.Seeger H, Mueck AO, Lippert TH. Effect of estradiol metabolites on the susceptibility of low density lipoprotein to oxidation. Life Sci. 1997;61:865–868. doi: 10.1016/s0024-3205(97)00588-2. [DOI] [PubMed] [Google Scholar]
- 9.Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychol Aging. 2006;21:152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 12.Bulun SE, Adashi EY. The Physiology and pathology of the female reproductive axis. In: Larson PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. 10th. Saunders; Philadelphia: 2003. pp. 587–664. [Google Scholar]
- 13.Swaneck GE, Fishman J. Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci USA. 1988;85:7831–7835. doi: 10.1073/pnas.85.21.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 15.Forte P, Kneale BJ, Milne E, Chowienczyk PJ, Johnston A, Benjamin N, Ritter JM. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension. 1998;32:730–734. doi: 10.1161/01.hyp.32.4.730. [DOI] [PubMed] [Google Scholar]
- 16.Fetalvero KM, Martin KA, Hwa J. Cardioprotective prostacyclin signaling in vascular smooth muscle. Prostaglandins Other Lipid Mediat. 2007;82:109–118. doi: 10.1016/j.prostaglandins.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Fishman J, Martucci C. Biological properties of 16 alpha-hydroxyestrone: implications in estrogen physiology and pathophysiology. J Clin Endocrinol Metab. 1980;51:611–615. doi: 10.1210/jcem-51-3-611. [DOI] [PubMed] [Google Scholar]


