Abstract
High glucose-induced protein synthesis in the glomerular epithelial cell (GEC) is partly dependent on reduction in phosphorylation of AMP-activated protein kinase (AMPK). We evaluated the effect of resveratrol, a phytophenol known to stimulate AMPK, on protein synthesis. Resveratrol completely inhibited high glucose stimulation of protein synthesis and synthesis of fibronectin, an important matrix protein, at 3 days. Resveratrol dose-dependently increased AMPK phosphorylation and abolished high glucose-induced reduction in its phosphorylation. We examined the effect of resveratrol on critical steps in mRNA translation, a critical event in protein synthesis. Resveratrol inhibited high glucose-induced changes in association of eIF4E with eIF4G, phosphorylation of eIF4E, eEF2, eEF2 kinase and, p70S6 kinase, indicating it affects important events in both initiation and elongation phases of mRNA translation. Upstream regulators of AMPK in high glucose-treated GEC were explored. High glucose augmented acetylation of LKB1, the upstream kinase for AMPK, and inhibited its activity. Resveratrol prevented acetylation of LKB1 and restored its activity in high glucose-treated cells; this action did not appear to depend on SIRT1, a class III histone deacetylase. Our data show that resveratrol ameliorates protein synthesis by regulating the LKB1-AMPK axis.
Keywords: protein synthesis, LKB1, AMP-activated protein kinase, diabetic nephropathy
1. Introduction
Polyphenols such as resveratrol and quercetin, commonly present in grapes and green tea, have drawn attention for their health-promoting effects. Resveratrol increases longevity and ameliorates diet-induced metabolic syndrome and diabetes [1, 2]. The mechanisms underlying effects of polyphenols are not fully known. They exert regulatory effects on a number of cell processes including oxidative metabolism, cell cycle events, cyclooxygenase metabolism, and, deacetylation of proteins by activation of Silent information regulator 1 (SIRT1) [1, 3, 4]. Although reseveratrol regulation of carbohydrate metabolism is widely studied, its potential effect on protein metabolism is not well understood. Among its multifarious effects on cell metabolism, resveratrol has been reported to stimulate AMP activated protein kinase (AMPK) activity in the liver and brain [5, 6]. AMPK, an energy sensor, has extensive regulatory effects on lipid and carbohydrate metabolism [7, 8]. Recent reports show that in the kidney, AMPK functions as an inhibitor of protein synthesis [9]. It regulates important steps in mRNA translation, which is a rate-limiting step in gene expression culminating in protein synthesis [9, 10]. The two cardinal manifestations of renal injury in diabetes, hypertrophy and accumulation of extracellular matrix proteins that contribute to progressive loss of kidney function, are dependent on increase in protein synthesis [10]. Kidney hypertrophy in rodents with type 1 diabetes is associated with reduction in AMPK activity, suggesting that AMPK normally acts as a break on protein synthesis and that its inhibition facilitates renal growth seen in diabetes [9]. Activation of AMPK depends on phosphorylation of alpha subunit on Thr172 by LKB1, its upstream kinase [11]. Whether resveratrol affects LKB1-AMPK axis and protein synthesis in kidney cells is not known; it is also not known whether the deacetylation reaction promoted by resveratrol is involved in these processes in the kidney cells. We evaluated the effect of resveratrol on high glucose-induced protein synthesis in glomerular epithelial cells (GEC), an important target of injury in diabetes [12]. Since fibronectin contributes to glomerular matrix changes in diabetic kidney disease, we studied modulation of high glucose regulation of that matrix protein. We also initiated investigation of resveratrol effect on LKB1, as a potential mechanism by which the polyphenol regulates AMPK.
2. Materials and methods
2.1. Cell culture
Rodent glomerular epithelial cells (GEC; provided by Dr. Jeffrey Kreisberg, Department of Surgery, University of Texas Health Science Center at San Antonio) were maintained in DMEM containing 7 % fetal bovine serum, 5 mM glucose, 100 U/mL penicillin, 100 ug/mL streptomycin and 2 mM glutamine as previously described [9]. GEC were incubated in DMEM containing either a normal (5 mM) or high (30 mM) concentration of glucose for 3 days with or without resveratrol (Sigma, St. Louis, MO, USA).
2.2. Protein synthesis
Protein synthesis experiments were performed as previously described [9]. Serum-starved cells were labeled with 10 Ci/mL of [35S]-methionine (Met) for the final 2 hours of incubation. Cells were washed in PBS and lysed in radioimmunoprecipitation assay (RIPA) buffer, followed by centrifugation at 14,000 rpm for 20 minutes at 4°C. Protein contents in cell lysates were measured and 20ug of lysate was spotted onto the 3 MM filter paper (Whatman, Maidstone, Kent, UK). Filters were washed three times by boiling for 1 minute in 10% trichloroacetic acid (TCA) containing 0.1g/L methionine before determining radioactivity.
2.3. Immunoblotting
Equal amounts of cell lysate protein (15-30 ug) were separated by SDS-PAGE and transferred to a nitrocellulose membrane [9]. Membrane was probed with primary antibody for overnight at 4°C. All primary antibodies were from Cell Signaling (Danvers, MA, USA) except fibronectin, actin (Sigma) and acetyled-lysine (Upstate, Lake Placid, NY, USA). After extensive washing, the membrane was incubated with secondary antibodies linked to horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and proteins were visualized by chemiluminescence using ECL reagent. For assessment of association of eIF4E and eIF4G, cell lysate proteins (500 ug) were immunoprecipitated with an antibody against eIF4G (Cell Signaling) and immunoblotted with eIF4E antibody (Cell signaling).
2.4. Immunokinase assay
AMPK activity was measured after immunoprecipitating 500 ug protein with AMPK alpha antibody (Cell Signaling) with the SAMS peptide (Upstate), as described previously [9]. For LKB1 activity assay, equal amounts of cell lysate protein (400 ug) were immunoprecipitated with an antibody against LKB1 (Santa Cruz, Santa Cruz, CA, USA) and incubated at 30°C with 240 uM LKBtide (Upstate) in reaction buffer (50 mM Tris-Cl, pH 7.5, 0.1 mM EGTA, 0.1% 2-mercaptoethanol, 0.6 mM ATP, 60 mM MgCl2) with [32P]-ATP for 20 minutes. 20 ul of reaction mixture were spotted onto the phosphocellulose P81 paper (Upstate) and washed with phosphoric acid. Radioactivity on the filter paper was measured [13].
2.5. Silent information regulator 1 (SIRT1) activity
SIRT1 activity was measured with a Fluor de Lys-SIRT1 Assay Kit (Biomol, Plymouth Meeting, PA, USA). A 50 ul reaction mixture contained 25uM /fluor de Lys-SIRT1 substrate. After 30 minutes of incubation at room temperature, reactions were stopped by adding a developer, which generated a fluorophore. The fluorophore was excited at 360 nm and detected on a fluorometric plate reader. Resveratrol and suramin were employed as positive and negative controls as recommended by the manufacturer.
2.6. Statistical analysis
Data were obtained from at least 3 independent experiments and expressed as mean ± SE. Statistical comparisons between multiple groups were performed by ANOVA and Student-Neuman-Keuls method was applied for post-hoc analysis; p values <0.05 were considered statistically significant.
3. Results
3.1. Resveratrol inhibits high glucose-induced protein and fibronectin synthesis
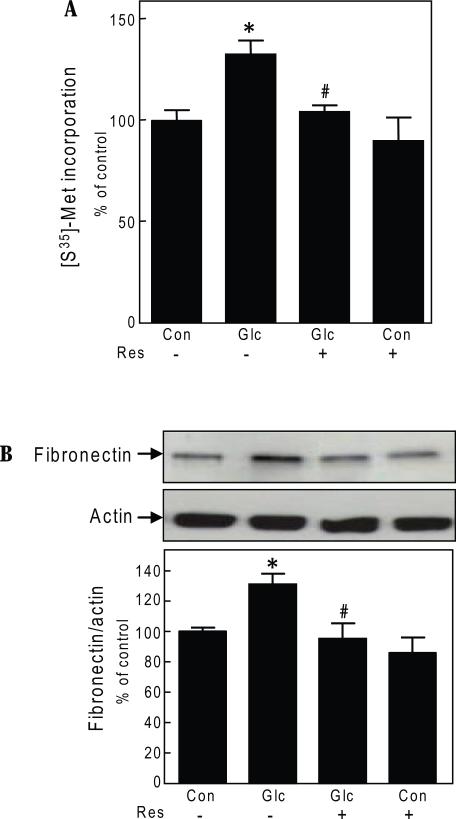
We examined if resveratrol modulated high glucose induced de novo protein synthesis. High glucose stimulated protein synthesis by 30% at 3 days in GEC (p<0.05) (Fig. 1A); equimolar mannitol does not affect protein synthesis in the GEC [9]. Simultaneous incubation with resveratrol (30 uM) abrogated high glucose-induced protein synthesis; resveratrol did not affect protein synthesis in control cells incubated with 5 mM glucose (Fig. 1A). Similar effect was found at reseveratrol concentration of 50 uM (data not shown). In addition to its effect on general protein synthesis, we studied whether resveratrol could inhibit high glucose-induced changes in amount of fibronectin, an important renal extracellular matrix protein which contributes to glomerular structural changes in diabetic nephropathy. High glucose incubation for 3 days resulted in 1.3-fold increment in fibronectin content in GEC lysates (p<0.05); resveratrol completely inhibited the increment induced by high glucose without affecting basal content (Fig. 1B). Resveratrol effect on protein synthesis was not due to cell toxicity as assessed by LDH release assay (data not shown).
Fig. 1.
Resveratrol abolishes high glucose effects on protein synthesis and fibronectin expression in glomerular epithelial cells.
A. Following 3-day exposure to 5 mM glucose (Con) or 30 mM glucose (Glc) with or without resveratrol (Res, 30 uM), de novo protein synthesis was measured by incorporation of 35S-methionine (Met) into TCA-precipitable protein. Composite data from 3 experiments are shown in a graph (*p<0.05 high glucose Vs control; #p<0.05 high glucose Vs high glucose+ resveratrol, by ANOVA). B. Following 3-day incubation with 5 mM or 30 mM glucose (Glc) with or without resveratrol (Res, 30 uM), equal amounts of cell lysate protein were separated by SDS PAGE and immunoblotted with fibronectin and actin antibodies. A representative blot from 5 experiments is shown. Lower panel shows composite densitometric data from 5 experiments in a graph (*p<0.05 high glucose Vs control; #p<0.05 high glucose Vs high glucose+ resveratrol, by ANOVA).
3.2. Resveratrol stimulates AMPK phosphorylation
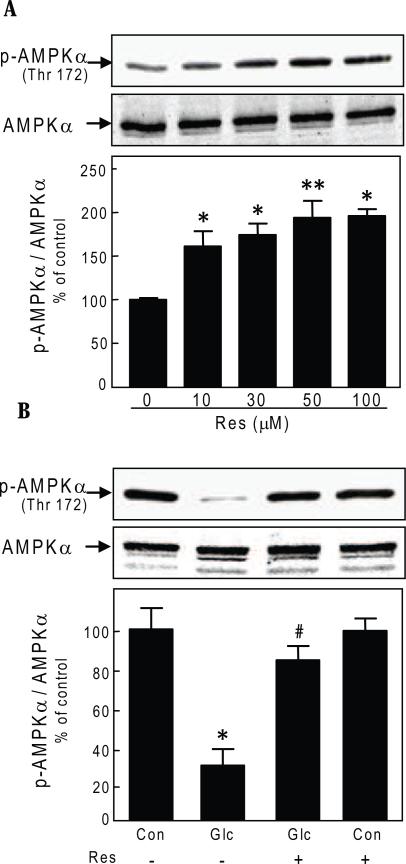
We have reported that high glucose-induced protein synthesis in the GEC is abrogated by AMPK activation[9]. Therefore, we next examined if resveratrol effect involved changes in AMPK phosphorylation. Resveratrol dose-dependently increased AMPK phosphorylation nearly 2-fold, peaking at 30-50 uM at 24 hours (Fig. 2A). Stimulation of protein synthesis by high glucose (Fig. 1A) was associated with reduction in Thr172 phosphorylation of AMPK by 70% (p<0.001) (Fig. 2B). Resveratrol prevented AMPK dephosphorylation induced by high glucose (Fig. 2B) without affecting basal level of phosphorylation in control cells.
Fig. 2.
Resveratrol reverses high glucose effects on AMPK phosphorylation in glomerular epithelial cells. A. Cells were incubated with different concentrations of resveratrol (Res) for 24 hours. Equal amounts of cell lysate protein were separated by SDS PAGE and immunoblotted with antibody against phosphorylated Thr172 on alpha subunit of AMPK or AMPK antibody. A representative blot from 3 experiments is shown. Lower panel shows composite densitometric data from 3 experiments in a graph (*p<0.01, **p<0.001 for resveratrol Vs control, by ANOVA). B. Cells were treated as described in Fig. 1, panel B. Equal amounts of cell lysate protein were separated by SDS PAGE and immunoblotted with antibody against phosphorylated Thr172 on alpha subunit of AMPK or AMPK antibody. A representative blot from 3 experiments is shown. Lower panel shows composite densitometric data from 3 experiments in a graph (*p<0.001 high glucose Vs control; #p<0.01 high glucose Vs high glucose+ resveratrol, by ANOVA).
3.3. Resveratrol effect on mRNA translation
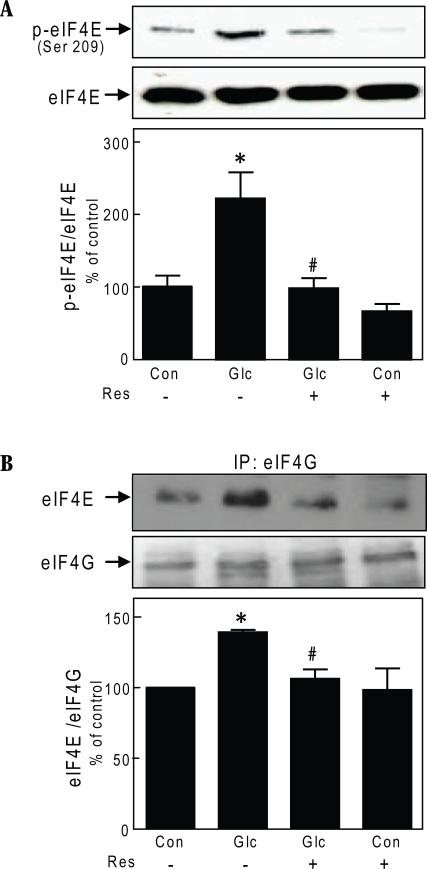
Stimulation of protein synthesis and induction of GEC hypertrophy by high glucose involves regulation of initiation and elongation phases of mRNA translation [9]. The initiation phase of translation is controlled by several eukaryotic factors (eIFs) including the mRNA cap binding protein eIF4E. After stimulation, eIF4E is released from its complex with its binding protein, 4E-BP1. Free eIF4E binds eIF4G to form eIF4F complex and promotes mRNA translation [10]. High glucose increased phosphorylation of eIF4E at Ser209 (p<0.001) that was abolished by resveratrol (p<0.01) (Fig.3A). We next examined the effect of resveratrol on the association of eIF4E and eIF4G. High glucose promoted association between eIF4E and eIF4G by nearly 50% (p<0.01); resveratrol inhibited increased binding of eIF4E to eIF4G induced by high glucose (p<0.01) (Fig. 3B).
Fig. 3.
Resveratrol regulates the initiation phase of mRNA translation in glomerular epithelial cells. A. Cells were treated as described in Fig. 1, panel B, and immunoblotting was done with antibody against phosphorylated Ser209 on eIF4E or eIF4E antibody. Representative blots from 3 experiments are shown. Lower panel shows composite densitometric data from 3 experiments in a graph (*p<0.001 high glucose Vs control; #p<0.01 high glucose Vs high glucose+resveratrol by ANOVA). B. Glomerular epithelial cells were incubated with 30 mM glucose (Glc) and resveratol (Res) for 3 days and cell lysates (500 ug) were immunoprecipitated with eIF4G antibody and immunoblotted with eIF4E antibody. The membrane was stripped and re-probed with eIF4G antibody. A representative blots from 3 experiments are shown. Lower panel shows composite densitometric data from 3 experiments in a graph (*p<0.01 high glucose Vs control; #p<0.01 high glucose Vs high glucose+resveratrol by ANOVA).
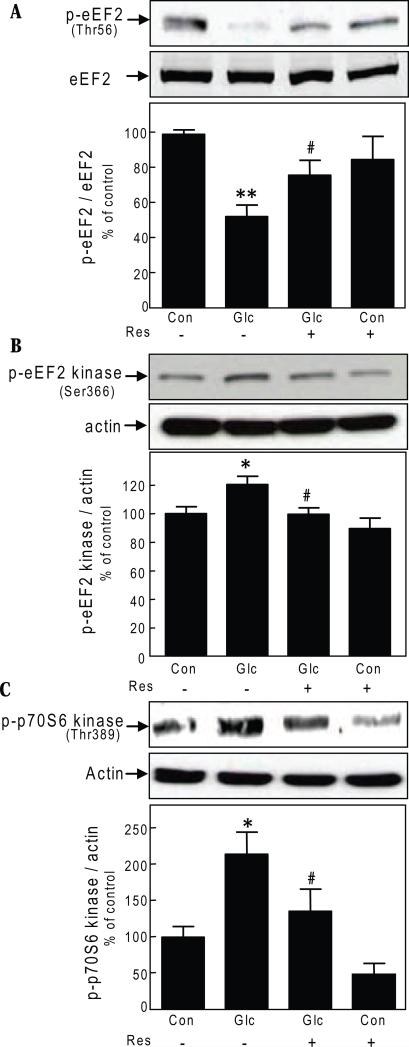
During elongation phase of mRNA translation, growth and propagation of polypeptide chain occurs. Movement of aminoacyl tRNA from the A (aminoacyl) site to the P (peptidyl) site on the ribosome during elongation is facilitated by eukaryotic elongation factor 2 (eEF2), which is active when dephosphorylated on Thr56 [14]. We examined if resveratrol inhibition of high glucose-induced protein synthesis involved elongation stage of mRNA translation. High glucose induced a reduction in phosphorylation of Thr56 on eEF2 in the GEC by 50% (p<0.01) that was reversed by incubation with resveratrol (p<0.05) (Fig. 4A). Phosphorylation of Thr56 is under the control of eEF2 kinase, a calcium calmodulin-dependent protein kinase III [15]. Activity of eEF2 kinase is reduced upon Ser366 phosphorylation, which contributes to reduction in content of Thr56-phosphorylated eEF2 [16]. High glucose-induced Thr56 dephosphorylation of eEF2 is associated with increase in Ser366 phosphorylation of eEF2 kinase in renal proximal tubular epithelial cells [17]. High glucose significantly augmented Ser366 phosphorylation of eEF2 kinase (p<0.05) in the GEC that was completely inhibited by resveratrol (p<0.05) (Fig. 4B). p70S6 kinase phosphorylates eEF2 kinase on Ser366 in non-renal cells [16]. We examined the effect of resveratrol on high glucose-induced changes in Thr389 phosphorylation of p70S6 kinase, which correlates with its activation. High glucose increased Thr389 phosphorylation of p70S6 kinase by more than 2-fold (p<0.05) that was restored to baseline by resveratrol (p<0.05) (Fig. 4C). These data show that ameliorative effect of resveratrol on high glucose-induced protein synthesis involves regulation both initiation and elongation phases of mRNA translation. Since Thr389 phosphorylation of p70S6 kinase is under the direct control of mTOR [18], these data show that high glucose induced mTOR activation is inhibited by resveratrol. Since AMPK is upstream of mTOR [9], taken together these data suggest that site of action of resveratrol lies upstream of mTOR in GECs incubated with high glucose.
Fig. 4.
Resveratrol regulates the elongation phase of mRNA translation in glomerular epithelial cells. A, B & C. Cells were treated as described in Fig. 1, panel B, and immunoblotting was done with indicated antibodies. Representative blots from 3-4 experiments are shown. Lower panel shows composite densitometric data from 3-4 experiments for each parameter in a graph (*p<0.05, **p<0.01, high glucose Vs control; #p<0.05, high glucose Vs high glucose+resveratrol by ANOVA).
3.4. Resveratrol effect on LKB1, the upstream kinase of AMPK
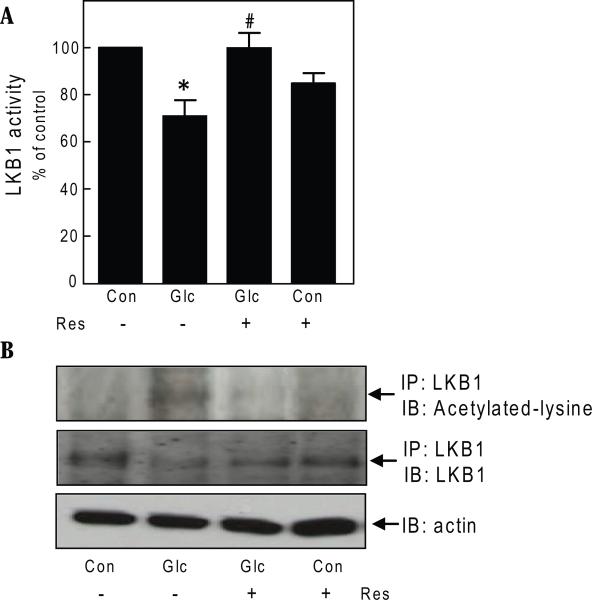
Next, we investigated events upstream of AMPK that may be regulated by resveratrol. Previous observations had shown that changes induced in AMP and ATP contents by high glucose did not sufficiently explain inhibition of AMPK activity in the GEC [9]. We tested the effect of resveratrol on activity of LKB1, the upstream kinase that catalyzes activating phosphorylation of AMPK on Thr172 [19], by performing an immunokinase assay using LKBtide as a substrate. High glucose decreased LKB1 activity by 24% (p<0.05) that was completely reversed by resveratrol (p<0.05) (Fig. 5A).
Fig. 5.
Resveratrol regulates LKB1 activation in glomerular epithelial cells. A. Cells were incubated with 30 mM glucose (Glc) and resveratol (Res) for 3 days and processed for LKB1 activity using LKBtide substrate. Composite data from 3 experiments are shown in a graph (*p<0.05 high glucose Vs control; #p<0.05 high glucose+resveratrol Vs high glucose by ANOVA). B. Cells were treated as described in Fig. 1, panel B. Cell lysates (500 ug) were immunoprecipitated with anti-LKB1 antibody and immunoblotted with anti-acetylated lysine antibody. The blot was stripped and probed with an antibody against LKB1. To assess loading, equal amounts of cell lysate protein were separated by SDS PAGE and immunoblotted with antibody against actin. Representative blots from 4 experiments are shown.
Resveratrol and other polyphenols are known to stimulate deacetylation of proteins [3]. Accordingly, we tested if reseveratrol affected acetylation of lysine residues in LKB1. Immunoprecipitation with LKB1 antibody and immunoblotting with anti-acetylated lysine antibody showed that high glucose increased acetylation of lysine on LKB1 (p<0.05); resveratrol promoted deacetylation of LKB1 (p<0.05) (Fig. 5B). These data suggested that high glucose-induced reduction in LKB1 activity may be due to acetylation of the latter.
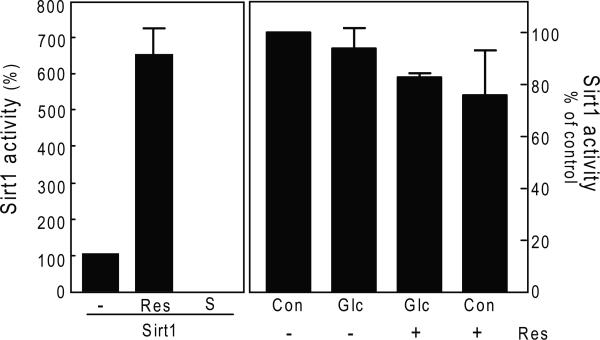
As resveratrol is known to augment the activity of SIRT1 deacetylase [3] and LKB1 deacetylation was induced by the polyphenol, we explored whether SIRT1 could be involved. We tested the activity of SIRT1 by a fluorometric assay. High glucose did not significantly affect the activity of SIRT1 and resveratrol did not augment Sirt1 activity in the GEC (Fig. 6). In the cell free assay system we used resveratrol as a positive control and suramin as a negative control; the data showed expected stimulation and inhibition, respectively, compared to untreated control showing validity of the assay. Since LKB1 was deacetylated by exposure to resveratrol it appears that a deacetylase other than SIRT1 is involved.
Fig. 6.
GEC were incubated with 30 mM glucose (Glc) and resveratol (Res) for 3 days and processed for SIRT1 activity using SIRT1 fluorometric activity kit. Recombinant human SIRT1 was incubated with resveratrol (100uM, Res) as positive control and suramin sodium (100uM, S), as a negative control for SIRT1 activity in a cell-free system. Composite data from 3 experiments are shown in a graph.
4. Discussion
Our data demonstrate that resveratrol inhibits high glucose-induced de novo protein synthesis and increment in matrix protein fibronectin in the GEC. High glucose augments acetylation of LKB1 and reduces its activity and phosphorylation of its downstream target, AMPK; resveratrol reverses this effect and restores LKB1 activity along with AMPK phosphorylation. SIRT1 does not appear to be the deacetylase involved in resveratrol regulation of LKB1. Resveratrol inhibits high glucose-induced mTOR and p70S6 kinase activities, restoring high glucose-induced changes in initiation and elongation stages in mRNA translation to normal.
The potential role of LKB1 in high glucose regulation of AMPK has not been studied in detail in the kidney. Our data suggest that the ameliorative effect of resveratrol on high glucose-induced protein and fibronectin synthesis involves activation of LKB1. Immunokinase assays with LKBtide, a specific substrate, showed that high glucose reduced the activity of LKB1, an effect that was associated with increase in its acetylation. Resveratrol-induced activation of LKB1 was associated with deacetylation of the latter, thus suggesting that high glucose-induced acetylation of LKB1 impairs its activity. Deacetylation of LKB1 by resveratrol has also been reported in 293T cells [20]. Although LKB1 deacetylation was due to SIRT1 in 293T cells, the identity of deacetylase involved in resveratrol regulation of LKB1 in the GEC is not clear from our study. Activity assays of SIRT1 showed no changes with either high glucose incubation or following addition of resveratrol to the GEC, suggesting involvement of other deacetylases. Recently, non-requirement of SIRT1 in resveratrol-induced LKB1 activation has also been reported in neurons [5]. Skeptical views have been expressed regarding the need for SIRT1 activation to explain resveratrol effects [4, 21]. In 293T cells, Lys98 appears to be the site of acetylation of LKB1 that is targeted for deacetylation by resveratrol [20]. The lysine residues involved in high glucose-induced acetylation in the GEC and the key residues that undergo deacetylation by resveratrol need to be identified. Previously, we had reported that changes in AMP and ATP levels did not provide an adequate explanation for high glucose inhibition of AMPK in the GEC [9]. Similarly, changes in AMP and ATP levels could not account for AMPK activation in neurons incubated with resveratrol [5]. AMP may not directly regulate LKB1-induced phosphorylation of Thr172 on AMPK alpha subunit [22], suggesting that other mechanisms may be involved in LKB1 catalytic regulation of AMPK.
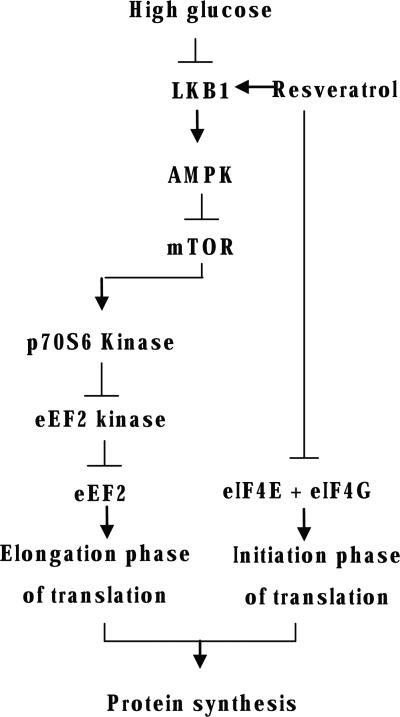
High glucose-stimulated protein synthesis and hypertrophy in the GEC are partly dependent on inhibition of AMPK activity in the GEC [9]. This was associated with stimulation of initiation and elongation phases of mRNA translation; stimulation of AMPK activity by metformin and AICAR restored high glucose-induced changes in initiation and elongation events to normal as well as inhibiting high glucose-induced protein synthesis [9]. The current study shows that resveratrol exerted similar effects in high glucose-treated GEC. Exploration of signaling pathways suggested that high glucose inhibition of AMPK phosphorylation was associated with activation of mTOR as shown by increase in Thr389 phosphorylation of p70S6 kinase. Stimulation of p70S6 kinase resulted in Ser366 phosphorylation of eEF2 kinase inhibiting its activity and leading to reduction in Thr56 phosphorylation of eEF2, culminating in stimulation of the elongation phase of translation (Fig. 7). Whether AMPK is involved in resveratrol regulation of eIF4E phosphorylation and formation of eIF4F complex needs to be determined.
Fig. 7.
Schematic of signaling pathways involved in high glucose stimulation of protein synthesis in the GEC and its inhibition by resveratrol.
Recent reports have examined mechanism underlying resveratrol regulation of carbohydrate and lipid metabolism. Resveratrol ameliorated diet-induced weight gain and insulin resistance in rodents, and extended their life span [1, 2]. In mice fed a high fat diet, resveratrol improved insulin sensitivity and reduced the levels of IGF-1. It induced an increase in activity of hepatic AMPK leading to reduction in hepatic steatosis [1]. Resveratrol also increased mitochondrial number by deacetylation and activation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC 1α) [2]. Preliminary data from our lab show that resveratrol inhibits renal hypertrophy in mice with type 1 diabetes without affecting elevated blood glucose levels (data not shown). These observations are in accordance with inhibitory effect of resveratrol on high glucose-induced increment in protein synthesis in the GEC. Resveratrol effect on the kidney may not be limited to LKB1/AMPK axis as it is known to have regulatory effects on diverse pathways including protein kinase C, NF-κB, mitochondrial ATP synthase and complex III, fatty acid synthase and TNF alpha [4]. Data contained in this report suggest that a common ingredient of grapes and green tea deserves deeper investigation as an additional tool in the management of diabetic nephropathy.
Acknowledgements
This study was supported by the NIH (grants DK077295 to BSK, DK050190 to GGC), NIDDK O'Brien Kidney Center Grant (BSK, DF), Veterans Research Service (BSK, GGC), American Diabetes Association (grant 7-05-RA-60 to BSK), Juvenile Diabetes Research Foundation (grant 3-2007-245 to MMM/BSK, grant 1-2008-185 to GGC), and, American Heart Association (grant SDG 0630283 N to DF). GGC is a recipient of VA Research Career Scientist Award. We thank Dr. Nicolas Musi and Dr. Koh for helpful suggestions on LKB1 activity assay.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 4.Kaeberlein M, Rabinovitch PS. Nature. 2006;444(7117):280–281. doi: 10.1038/nature05308. [DOI] [PubMed] [Google Scholar]
- 5.Dasgupta B, Milbrandt J. Proc Natl Acad Sci U S A. 2007;104(17):7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Diabetes. 2006;55(8):2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 7.Carling D. Trends Biochem Sci. 2004;29(1):18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Hardie DG, Scott JW, Pan DA, Hudson ER. FEBS Lett. 2003;546(1):113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, Foretz M, Viollet B, Weinberg JM, Choudhury GG, Kasinath BS. Am J Physiol Renal Physiol. 2007;292(2):F617–627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 10.Kasinath BS, Mariappan MM, Sataranatarajan K, Lee MJ, Feliers D. J Am Soc Nephrol. 2006;17(12):3281–3292. doi: 10.1681/ASN.2006050488. [DOI] [PubMed] [Google Scholar]
- 11.Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. Curr Biol. 2003;13(22):2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Susztak K, Raff AC, Schiffer M, Bottinger EP. Diabetes. 2006;55(1):225–233. [PubMed] [Google Scholar]
- 13.Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ. Mol Cell Biol. 2006;26(22):8217–8227. doi: 10.1128/MCB.00979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redpath NT, Price NT, Severinov KV, Proud CG. Eur J Biochem. 1993;213(2):689–699. doi: 10.1111/j.1432-1033.1993.tb17809.x. [DOI] [PubMed] [Google Scholar]
- 15.Nairn AC, Palfrey HC. J Biol Chem. 1987;262(36):17299–17303. [PubMed] [Google Scholar]
- 16.Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Embo J. 2001;20(16):4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sataranatarajan K, Mariappan MM, Lee MJ, Feliers D, Choudhury GG, Barnes JL, Kasinath BS. Am J Pathol. 2007;171(6):1733–1742. doi: 10.2353/ajpath.2007.070412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holz MK, Ballif BA, Gygi SP, Blenis J. Cell. 2005;123(4):569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 19.Hong SP, Leiper FC, Woods A, Carling D, Carlson M. Proc Natl Acad Sci U S A. 2003;100(15):8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan F, Cacicedo JM, Ruderman N, Ido Y. J Biol Chem. 2008;283(41):27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaeberlein M, Kennedy BK. Aging Cell. 2007;6(4):415–416. doi: 10.1111/j.1474-9726.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanders MJ, Grondin PO, Hegarty BD, Snowden MA, Carling D. Biochem J. 2007;403(1):139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]