Abstract
Objective
There is no information regarding the relationship between middle cerebral artery flow velocity (Vmca) and cerebral perfusion pressure in pediatric traumatic brain injury (TBI). We determined the incidence of low, normal and high mean Vmca when CPP is > 40 mm Hg in children with severe TBI.
Design
Prospective observational study
Setting
Level 1 pediatric trauma center
Patients
42 children < 17 years of age with an admission diagnosis of severe TBI (admission Glasgow Coma Scale [GCS] score < 9), TBI on computed tomography (CT) scan, tracheal intubation/mechanical ventilation and intracranial pressure (ICP) monitoring.
Interventions
None.
Measurements and Main Results
Bilateral middle cerebral arteries were insonated using transcranial Doppler ultrasonography (TCD) to calculate mean Vmca after TBI. Low mean Vmca was defined as Vmca < 2SD and high was defined as mean Vmca > 2SD. Patients were grouped by age (0.8–2.9, 3–5.9, 6–9.9, and 10–16.9 years) and gender to examine the relationship between CPP and low, high or normal mean Vmca. Potential confounders of the relationship between CPP and mean Vmca (ICP, PaCO2, hematocrit [Hct], sedation, fever and impaired autoregulation were examined). Most (33; 79%) children had normal mean Vmca but 4 (9%) patients had low mean Vmca and 5 children (12%) had high mean Vmca despite CPP > 40 mm Hg. There was no difference in potential confounders of the relationship between CPP and mean Vmca except for Hct, which was lower (25 ± 4 [range 21–30]) in children with high mean Vmca. An inverse relationship between mean Vmca and Hct was also found in boys 10–16.9 years.
Conclusions
Both low and/or high mean Vmca occur with CPP > 40 mm Hg in severe pediatric TBI. Of the potential confounders considered, only lower Hct was associated with high mean Vmca.
Keywords: cerebral blood flow velocity, cerebral perfusion pressure, pediatric trauma, brain injury, blood pressure, middle cerebral artery flow
Introduction
Traumatic brain injury (TBI) is the leading injury in pediatric trauma [1] and a leading cause of morbidity and mortality in children over 1 year of age [2]. Following TBI, secondary insults such as hypotension [3], worsen outcome. Consequently, current guidelines suggest maintaining 1) systolic blood pressure > 5th percentile for age and 2) cerebral perfusion pressure (CPP) > 40 mm Hg after severe TBI [4]. Yet, it is unclear whether achieving these SBP and or CPP thresholds are associated with optimal and adequate cerebral blood flow (CBF).
One important mechanism by which hypotension or even “normotension” might result in cerebral hypoperfusion is via derangement in the normal cerebral autoregulatory process which normally maintains CBF relatively constant between a range of blood pressures [5]. Cerebral hypoperfusion (CBF < 25ml/100gm/min) is the dominant derangement in pediatric TBI and is associated with cerebral ischemia and poor outcome [6]. Early hypotension, especially occurring during the first 6 h after injury, is associated with poor outcome in pediatric TBI [7, 8]. According to Hackbarth et al and others, the ability to maintain a CPP of ≥ 50 mm Hg was the single most important predictor of survival following pediatric TBI [9,10,11].
In 2003, the Pediatric Guidelines recommended that CPP < 40 mm Hg be avoided after severe TBI to prevent cerebral hypoperfusion and poor outcomes [4]. Although the guidelines suggest a CPP between 40 and 65 mm Hg likely represents an age-related continuum, there are no data documenting the relationship between CPP and CBF or that CPP > 40mmHg is associated with age-appropriate CBF. The paucity of such CBF data may be due to the fact that direct measurements of CBF using computed tomography based imaging in children with TBI pose certain challenges such as radiation risks. For these reasons, the use of transcranial Doppler (TCD) ultrasonography is appealing; this technology measures cerebral blood flow velocity (CBFV), which estimates CBF and correlates well with CBF because velocity of flow is proportional to the volume of flow through the vessels examined [12,13,14].
In this study, we hypothesized that maintaining CPP > 40 mm Hg is variably associated with normal mean velocity of the middle cerebral artery (Vmca), and therefore, aimed to determine the incidence of low and high mean Vmca when CPP is > 40 mmHg in children with severe TBI.
Materials and Methods
After Institutional Review Board approval from the University of Washington (Seattle, Washington), we prospectively examined CPP and Vmca in children with severe TBI at Harborview Medical Center (Level 1 pediatric trauma center). Consent was obtained from parents or legal guardians.
Study Participants
Children age < 17 years admitted to the Harborview Medical Center pediatric intensive care unit (PICU) with an admission diagnosis of severe TBI (Glasgow Coma Scale [GCS] score < 9), TBI on computed tomography (CT) scan, tracheal intubation/mechanical ventilation and intracranial pressure (ICP) monitoring were considered eligible. Children with extracranial injuries were also included. Patients with hemodynamic instability (per treating intensivist if significant hypotension or hypertension was present immediately before testing) or no available parent/guardian were excluded. We reviewed medical records for eligibility, relevant medical history, and physiologic data.
Measuring Middle Cerebral Artery Blood Flow Velocity
Following admission to the PICU, a single measure of each (right and left) Vmca were obtained within 0 to 72 hours by TCD ultrasonography (Multidop X;DWL. Corp., Sipplingen, Germany) with a 2-MHz ultrasound probe [15], at patient beside. Previously described age-appropriate depths were referenced and used to insonate the middle cerebral artery [16]. A registered vascular technologist, with more than 10 years of experience using TCD ultrasonography, insonated the middle cerebral arteries. Bilateral middle cerebral artery flow velocities (Vmca) were averaged and the mean Vmca was used in the analysis.
Cerebral Hemodynamic Data
Recorded blood pressures were non-invasive (cuff) measurements and intraarterial catheter measurements in PICU. When both non-invasive and invasive blood pressure readings were available, we used the invasive measurements. Each available CPP, PaCO2, and ICP at the time of measuring Vmca, were abstracted and entered into Excel and SPSS datasheets. Mean Vmca values were compared to previously published Vmca data, which are normed for age and gender [16,17,18]. Information regarding hematocrit (Hct), temperature, and the use of sedation were recorded. When cerebral autoregulation testing was performed, these data were recorded and are included in the analysis. Autoregulatory status was determined using the commonly used and published Autoregulatory Index (ARI) which examines estimated cerebrovascular resistance (eCVR) and MAP [15]; ARI = % Δ eCVR/% Δ MAP; autoregulation was considered intact if the ARI was ≥ 0.4.
Statistical Analysis
Descriptive statistics were used to present clinical characteristics. We first examined the difference between the means of right and left Vmca using paired T-tests. Since no difference was found in Vmca between the two sides, we used mean Vmca in the analysis to examine the relationship between CPP and mean Vmca. Each mean Vmca was defined as low (< 2SD), normal or high (> 2SD) by age and gender according to previously published age and gender Vmca norms [16,17,18].
Patients were first divided into 4 age groups (0.8–2.9, 3–5.9, 6–9.9, and 10–16.9 years) based on previously published data [16,17,18] and then each age group was divided by gender, for a total of 8 categories of description for CPP and mean Vmca. The relationship between CPP and mean Vmca was examined separately by age and gender. With the exception of the group of 10–16.9 year old boys which are formally analyzed, the raw data are described without formal analysis (small sample group size) using scatter plots. These results are displayed via scatter plots. For the largest sample size (boys 10–16.9 years of age) relationships between mean Vmca and CPP, PaCO2, ICP, Hct and Temp were examined using Spearman’s rank correlation. For the 0.8–2.9 and 6–9.9 age groups, there are no gender norms, hence we examined data for these subjects by age only.
Student’s T tests of unequal variance were used to analyze differences in the variables that potentially confound the relationship between CPP and mean Vmca such as ICP, PaCO2 and hematocrit (Hct); these results are presented separately for patients with low, high, and normal mean Vmca. The relationship between the number of patients who had low PaCO2 (< 35 mm Hg), high ICP (> 20 mm Hg), fever (temperature > 38.5º C), sedation and impaired autoregulation (when data available) in low and high mean Vmca groups was examined using Fisher’s exact or χ2 test, as appropriate.
SPSS version 11.5 (SPSS Inc., Chicago, IL) was used for data entry and analysis. Data are presented as mean ± SD, or n (%). p < 0.05 was considered significant.
Results
Demographic and Baseline Clinical Characteristics (Table 1)
Table 1.
Clinical data of 42 children with severe traumatic brain injury. Data are presented as mean ± SD (range) or n (%).
| Age (years) | 8.9. ± 5.2 (0.8–16.0) |
| Male | 30 (71) |
| Admission Glasgow coma scale | 4 ± 1.2 (3–7) |
| Mechanism of injury | |
| Motor vehicle crash | 11 (26) |
| Fall | 11 (26) |
| Auto-pedestrian | 4 (10) |
| Inflicted trauma | 3 (7) |
| Bike | 6 (14) |
| Gun shot wound | 3 (7) |
| Other | 4 (10) |
| Associated injuries* | |
| Orthopedic | 13 (31) |
| Abdominal/pulmonary | 16 (38) |
| None | 16 (38) |
| Traumatic brain injury on computed tomography in emergency department* | |
| Diffuse axonal injury | 4 (9) |
| Subdural hematoma | 20 (48) |
| Epidural hematoma | 7 (15) |
| Subarachnoid hemorrhage | 18 (43) |
| Intracerebral hemorrhage | 8 (18) |
| Cerebral edema | 9 (19) |
| Skull fracture | 22 (52) |
| Cerebral infarction | 1 (2) |
| In-hospital mortality | 2 (4) |
Percentages exceed 100% because some patients have multiple injuries.
Forty-four children were enrolled and underwent TCD ultrasonography. Two children were excluded because PaCO2 data were out of acceptable range in the PICU, thereby leaving data from 42 children for analysis. Children were 8.9 ± 5.2 (0.8–16.0) years (Table 1). Most (71%) were male. Motor vehicle crash (26%) and falls (26%) accounted for the majority of injuries. Most patients had extracranial injuries, including 7 who had pulmonary injury (contusion or pneumothorax). Three children had inflicted trauma (7%). All patients received a head computed tomography scan in the emergency department. The exact time of TCD ultrasonography testing was recorded in 32 (76%) patients; in these patients, the median time of testing was 36 ± SEM 3.0 hours). During study testing, sedation with either propofol or midazolam/fentanyl was used in 27 (81%) patients; barbiturates were not used. Cerebral autoregulation measurements were available in only 27 (64%) patients. The average GCS was 4 ± 1.2 (3–7) on PICU admission. Two (4%) children died in hospital prior to discharge.
Incidence of low, normal and high mean Vmca
All 42 children had CPP > 40 mm Hg (42–87 mm Hg) at time of TCD ultrasonography. Table 2A presents the incidence of low, high and normal mean Vmca; most (33; 79%) children had normal mean Vmca but 4 (9%) patients had low mean Vmca and 5 children (12%) had high mean Vmca despite CPP > 40 mm Hg.
Table 2.
2A and 2B. Relationship between cerebral perfusion pressure (CPP) and middle cerebral artery mean flow velocity (Vmca) in 42 children with CPP > 40 mm Hg. Cerebral autoregulation available in only 27 patients. Low mean Vmca = Vmca < 2SD and high mean Vmca = Vmca > 2SD for age and gender. ICP = intracranial pressure. Hct = hematocrit. (A) Data are presented as mean ± SD (range). (B) Data are presented n (%).
| A. | Low mean Vmca | Normal mean Vmca* |
High mean Vmca | |||
|---|---|---|---|---|---|---|
| (n=4; 9%) | p | (n=33; 79%) | (n=5; 12%) | p | ||
| PaCO2 (mm Hg) | 33 ± 5 (29–40) | 0.51≠ | 35 ± 3 (30–42) | 36 ± 4 (30–39) | 0.63 ≠ | |
| ICP (mm Hg) | 16 ± 16 (5–40) | 0.97≠ | 15 ± 7 (4–28) | 17 ± 5 (12–25) | 0.46≠ | |
| CPP (mm Hg) | 59 ± 4 (54–63) | .09≠ | 64 ± 14 (43–87) | 57 ± 11 (42–70) | 0.25≠ | |
| Hct (%) | 35 ± 5 (29–42) | 0.21≠ | 30 ± 4 (23–37) | 25 ± 4 (21–30) | 0.02≠ | |
| B. | Low mean Vmca | Normal mean Vmca* |
High mean Vmca | |||
|---|---|---|---|---|---|---|
| (n=4; 9%) | p | (n=33; 79%) | (n=5; 12%) | p | ||
| Number of patients with PaCO2 < 35 mm Hg |
3(75) | 0.41 ≠ | 18(55) | 2(40) | 0.45 ≠ | |
| Number of patients with ICP > 20 mm Hg |
1(25) | 0.66≠ | 10(30) | 1(20) | 0.55≠ | |
| Number of patients with temperature > 38.5ºC |
0(0) | -- | 5(15) | 2(40) | 0.05 ≠ | |
| Number of patients who received sedation |
3(75) | 0.59 ≠ | 27 (82) | 4(80) | 0.66 ≠ | |
| Number of patients with impaired autoregulation (N=10/27) |
3(75%) | 0.08≠ | 4(22%) | 3(60%) | 0.14≠ | |
| Median CPP (mm Hg) in patients with impaired autoregulation |
58 | -- | 78 | 64 | -- | |
Relationship between CPP and mean Vmca
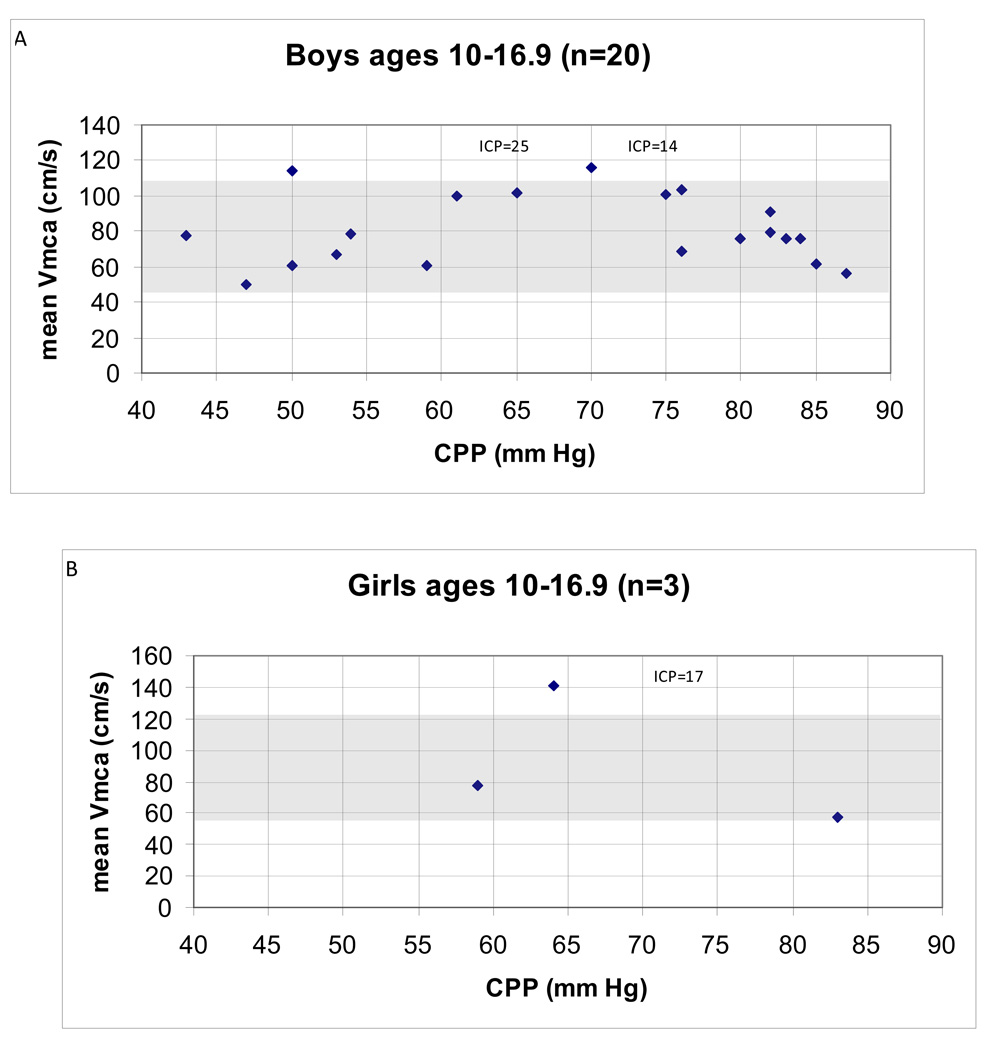
There was no obvious relationship between CPP and mean Vmca within the age and gender groups examined, including the group with the largest sample size (N=20) of boys 10–16.9 years old (Spearman’s ρ = −0.05; p = 0.84; Figure 1A).
Figure 1.
(A–B). Relationship between cerebral perfusion pressure (CPP) and middle cerebral artery mean flow velocity (Vmca) in children post-TBI between ages 10–16.9 years. ICP (mmHg) data are presented for the two patients whose Vmca are high for age and gender. (A) N = 20 boys. Normal Vmca (cm/s) 75 ± 16 cm/s (43–107)** for age and gender denoted by shaded area. Mean Vmca (cm/s) 77 ± 22 (32–123). Mean CPP (mm Hg) 66 ± 16 (34–87). Spearman’s ρ = −0.08; p = 0.7. (B) N = 3 girls. Normal Vmca (cm/s) 89 ± 16 (57–121)** for age and gender denoted by shaded area. Mean Vmca (cm/s) 79 ± 37 (52–134). Mean CPP (mm Hg) 59 ± 22 (31–83).
**Source: Vavilala et al., 2005 [17] and Tontisirin et al., 2007 [18]
Factors associated with low and high mean Vmca (N=42)
At the time of TCD ultrasonography, PaCO2 for the entire cohort was 35 ± 3 (30–42) mm Hg, ICP was 15 ± 7 (4–28) mm Hg and Hct was 30 ± 4% (23–37). There were also no differences in PaCO2, ICP or Hct between patients with low mean Vmca vs. normal mean Vmca (Table 2A). However, Hct was lower (25 ± 4 [range 21–30]) in children with high versus normal mean Vmca groups (p = 0.02; Table 2A). There was no relationship between number of patients with low PaCO2, high ICP, or number of patients with fever, sedation or impaired autoregulation in the high and low mean Vmca groups when compared to the normal mean Vmca group (Table 2B).
Mean Vmca and confounder data in boys 10–16.9 years old (N=20)
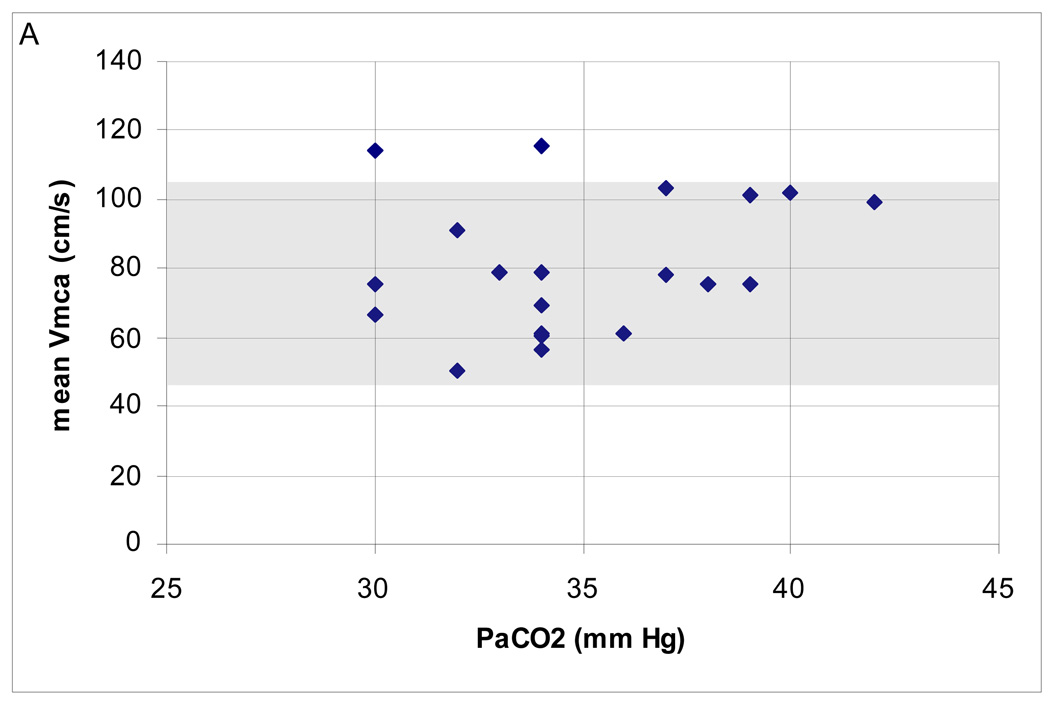
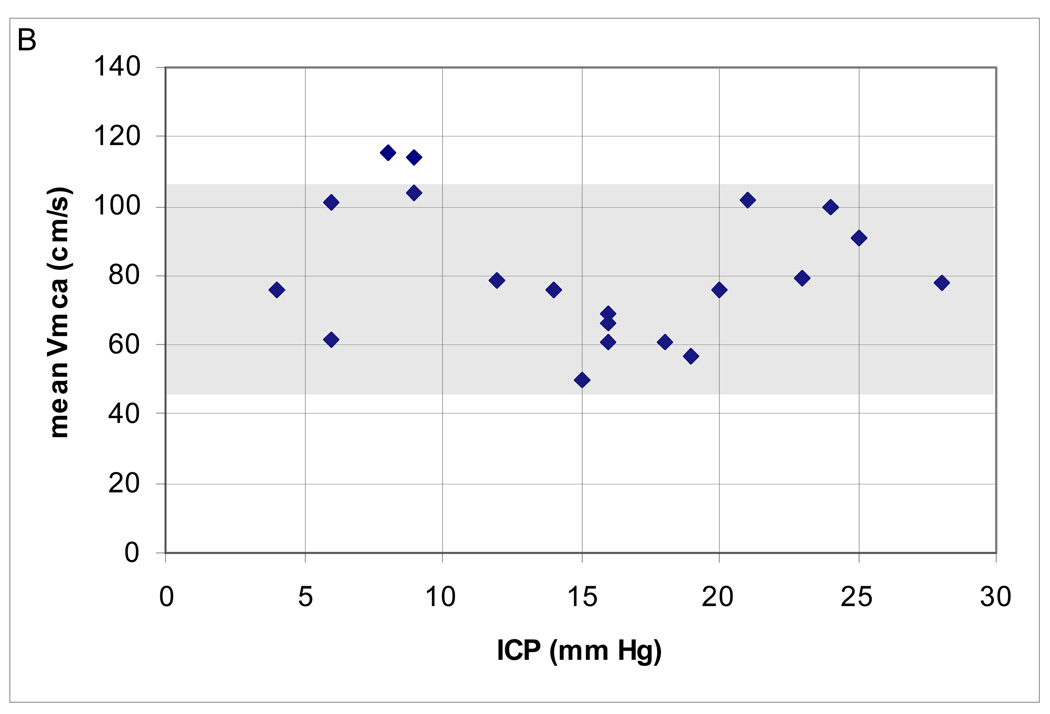
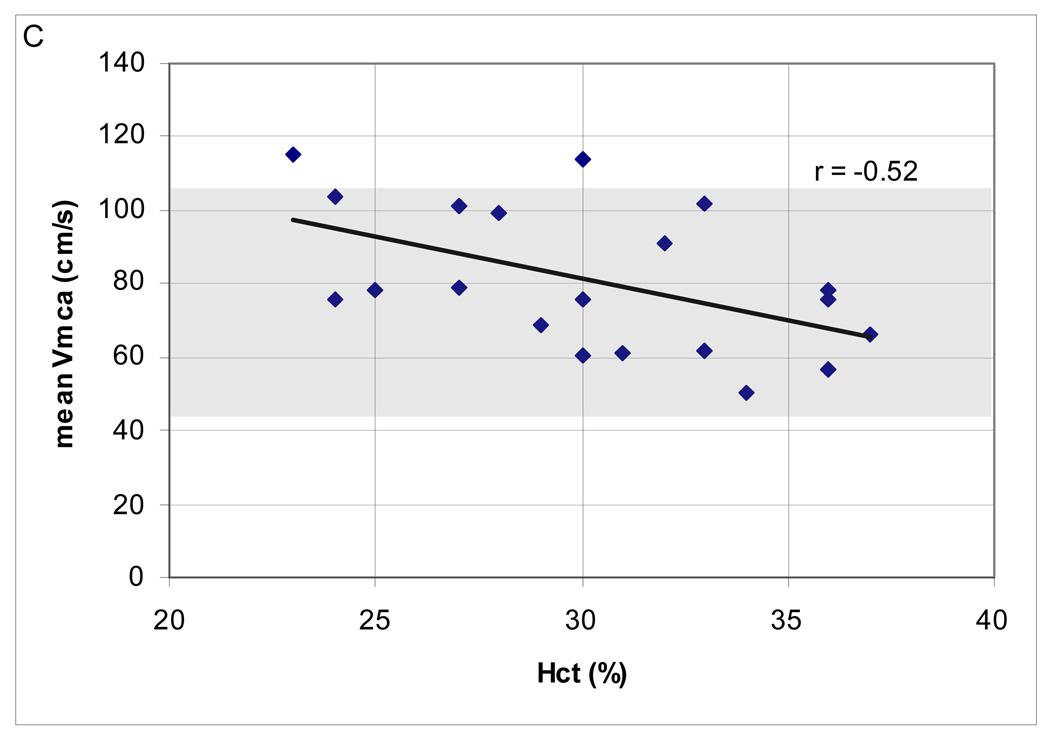
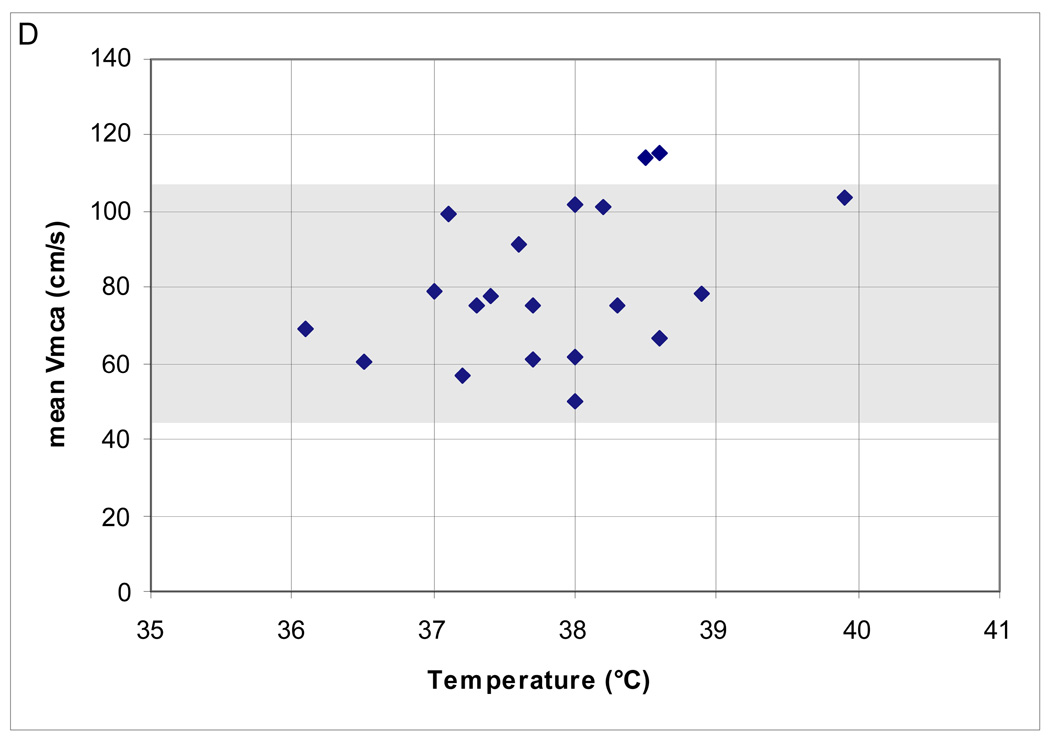
In the range of CPP examined, the majority (18; 90%) had normal mean Vmca. Two (10%) patients had high Vmca. There was a negative correlation between Hct and mean Vmca (Spearman’s ρ = −0.52; p = 0.02) but no relationship between mean Vmca and PaCO2, ICP and temperature (Figure 2A–D). Cerebral autoregulation was examined in all of these patients; it was intact in 15 (75%) and impaired in 5 (25%).
Figure 2.
(A–D). Relationship between mean middle cerebral artery flow velocity (Vmca) and potential confounders in 20 boys ages 10–16.9 years, post-TBI. (A) PaC02 (B) intracranial pressure [ICP] (C) hematocrit [Hct]; Spearman’s ρ = −0.52; p = 0.02 and (D) Temperature.
Data from 9 children with low or high mean Vmca (N=42)
Of the 9 patients whose mean Vmca fell out of range for age and gender, 5 had high (>2 SD) and 4 had low (<2 SD) mean Vmca in 5 boys and 4 girls. There was a non-significant relationship between high Vmca and fever and low Vmca with impaired cerebral autoregulation (Table 2A–B)
Discussion
There are currently no data examining the relationship between CPP and CBF in severe pediatric TBI. The main finding of this study is that mean Vmca can be low, high or normal for age and gender despite CPP > 40 mm Hg. We also found that patients with high mean Vmca had lower Hct than patients with normal mean Vmca. Since this study is not powered to formally examine relationships between Vmca and the variables examined, we cannot definitively conclude that high mean Vmca is not associated with PaCO2, high ICP, fever or sedation; however, we did not observe such an association.
In healthy children, CBF is highest during early childhood [19], and gender differences in mean Vmca and Vbas (basilar) are observed prior to puberty [17,18]. Healthy girls between ages 4–16 have higher mean Vmca than do age-matched boys. Children with TBI have lower mean Vmca than children without TBI [20]. These differences in age and gender biology make it important in order to determine the association between blood pressure and mean Vmca by age and gender. Historically, CBF < 20 ml/100g/min signified hypoperfusion, and neuronal death occurs when CBF is 10–15 ml/100g/min. Although cerebral hypoperfusion is associated with cerebral ischemia and poor outcome after TBI [21], high CBF is also problematic and can lead to hyperemia, increased ICP and poor outcome [19,20]. In either case, we assume that CBF is involved in the casual pathway between CPP and outcome. Consistent with the 2003 Pediatric Guidelines, we chose CPP > 40 mm Hg as a threshold blood pressure because outcomes are better when CPP exceeds 40 mm Hg [4]. The relatively high incidence of both low and high mean Vmca during “normotension” are similar to previously published data in adult TBI [22,23]. We examined the relationship between CPP and mean Vmca for each group to explore the presence of a threshold CPP by age but there was no such pattern. The fact that there was no obvious relationship between Vmca and CPP and that most Vmca values were normal for age and gender is generally consistent with the 2003 Pediatric Guidelines. However, the variability in mean Vmca, despite categories of what clinicians would consider acceptable CPP, argues for both individual and advanced neuromonitoring in pediatric TBI.
In this study, we explored some potential causes of low and high mean Vmca even when CPP was > 40 mm Hg. In this small sample, low mean Vmca was not associated with hypocarbia, sedation or high ICP. None of the subjects were treated with hypothermia or barbiturates and we did not have data on cerebral metabolic rate of oxygen (CMRO2), thus cannot comment on whether low CMRO2 led to low mean Vmca. However, since flow–metabolism coupling is typically intact even in severe TBI, the observed low mean Vmca may be in response to changes in CMRO2. While our patients had CPP > 40 mm Hg, we cannot be certain that mean Vmca was low because the lower limit of autoregulation (LLA) exceeded the CPP 40 mm Hg threshold. In fact, the LLA in pediatric TBI is not known and may vary considerably given the heterogeneous nature of TBI. In this study, high mean Vmca was associated with low Hct. The relationship between Hct and mean Vmca is well described; the rheological properties of anemia result in vasodilation and increased CBF [24]. However, there is little information regarding optimal Hct in pediatric TBI [25]. Finally, impaired cerebral autoregulation could lead to either cerebral hypoperfusion or hyperemia.
Although we used mean Vmca to describe cerebral hemodynamics other measures have also been used in previous studies. Low diastolic velocities (Vd) and high pulsatility indices (PI) have been shown to be associated with poor outcome in children and adults with TBI [26, 27, 28]. Despite the correlations between PI and CPP [29, 30] and the fact that measuring PI is independent of insonation angle, we used mean Vmca as our measure primarily because of existing data on age and gender norms for Vmca. We wanted to consider these developmental differences in our analysis of the relationship between CPP and Vmca.
This study has some limitations. First, given the small numbers of patients in each age/gender group, this study is largely descriptive. The number of subjects was too small to allow formal examination of CPP and mean Vmca by age and gender for each group and this study is not powered to state conclusions regarding relationships between Vmca and the variables examined in Tables 2a and 2B, including ICP and PaCO2. Our findings may not be generalized to children less than 4 years or between 8 and 10 years because of the small size in these groups. However, we thought it important to describe the data from younger children since young children have the worse outcome after TBI. While, we could not determine the correlation between CPP and mean Vmca within each group, data from this study (specifically the variability data) will allow for formal power calculations needed to better examine the relationship between CPP and mean Vmca for each age and gender group. Also due to the small sample size, we could not examine all potential confounders. We chose to examine the most potent determinants of Vmca such as ICP, Hct, and PaCO2 but were not powered to test for different levels (doses, duration, types) of sedation or temperature (intensity, duration) and therefore grouped patients into broader categories to provide preliminary examination of these potential confounders. Also, CBF and or CBFV may represent the underlying pathophysiology and as such may differ despite similar clinical neurological scores, such as the GCS. However, the number of patients with each distinguishing pathophysiology is quite small. Additionally, as autoregulation testing was not done in all patients and not all patients had time of testing recorded, we only presented groups that had available data. Similarly, we were not able to determine the incidence of cerebral ischemia, not all patients had autoregulation testing and we do not have CMRO2 data. This is important because low CBF may not be problematic following TBI if CMRO2 is low and there is a compensatory increase in oxygen extraction fraction [31]. We did not consider ventilator pressures or pulmonary compliance as potential confounders of Vmca. Finally, we do not have direct measures of CBF. The lack of relationship between fever and high Vmca and impaired autoreuglation with low Vmca is likely due to the small number of patients. Although a standard tool for use in complications of sickle cell disease [32] and cerebral vasospasm following subarachnoid hemorrhage [33], it is largely a research tool in TBI [34]. While we cannot advocate the use of TCD ultrasonography in pediatric TBI, the absence of other feasible determinants of CBF in these patients renders it a potentially useful [14], noninvasive tool for use at the bedside. Despite these limitations, however, these new data provide new insight into the variation in mean Vmca, and possibly CBF, with regard to current recommendations of adequate CPP. Furthermore, we speculate the use of advanced neuromonitoring, which may include TCD, may guide CPP management in the future.
In summary, this is the first study to document the variation in mean Vmca following pediatric TBI when CPP is greater than 40 mm Hg. Both low and high mean Vmca were observed with what is currently considered acceptable blood pressure in severe pediatric TBI. Advanced neuromonitoring is needed to better understand whether the variability in mean Vmca reflects the variability in CMRO2 and whether empiric CPP management is appropriate in severe pediatric TBI.
Acknowledgements
We would like to thank Jin Wang, Ph.D, Research Consultant, for review of statistical methods”.
Funding: NICHD/NIH/K23
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- 1.Ward JD, Buntain WL. Craniocerebral injuries. Philadelphia: W.B. Saunders; 1995. pp. 177–188. Management of pediatric trauma. [Google Scholar]
- 2.Langlois JGK. Traumatic Brain Injury in the United States: Assessing Outcomes in Children. Atlanta (GA): National Center for Injury Prevention and Control, Centers for Disease Control and Prevention (CDC); 2001. [Google Scholar]
- 3.Vavilala MS, Bowen A, Lam AM, Uffman JC, Powell J, Winn HR, et al. Blood pressure and outcome after severe pediatric traumatic brain injury. J Trauma Dec. 2003;55(6):1039–1044. doi: 10.1097/01.TA.0000101759.23607.57. [DOI] [PubMed] [Google Scholar]
- 4.Adelson PD, Bratton SL, Carney NA, et al. American Association for Surgery of Trauma; Child Neurology Society; International Trauma Anesthesia and Critical Care Society; Society of Critical Care Medicine; World Federation of Pediatric Intensive and Critical Care Societies. Guidelines for the acute medical management of severe traumatic brain injury in infants, children and adolescents. Pediatr Crit Care Med. 2003;4 Suppl 3:S1–S75. doi: 10.1097/01.CCM.0000067635.95882.24. [DOI] [PubMed] [Google Scholar]
- 5.Sahuquillo J, Munar F, Baguena M, Poca MA, et al. Evaluation of cerebrovascular CO2 reactivity and autoregulation in patients with post-traumatic diffuse brain swelling (diffuse injury III) Acta Neurochir Suppl. 1998;71:233–236. doi: 10.1007/978-3-7091-6475-4_67. [DOI] [PubMed] [Google Scholar]
- 6.Coles JP, Fyer TD, Smielewski P, et al. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J Cereb Blood Flow Metab. 2004;24:202–211. doi: 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- 7.Samant UB, Mack CD, Koepsell T, Rivara FP, Vavilala MS. Time of hypotension and discharge outcome in children with severe traumatic brain injury. J Neurotrauma. 2008 May;25(5):495–502. doi: 10.1089/neu.2007.0491. [DOI] [PubMed] [Google Scholar]
- 8.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993;28:310–316. doi: 10.1016/0022-3468(93)90223-8. [DOI] [PubMed] [Google Scholar]
- 9.Hackbarth RM, Rzeszutko KM, Sturm G, Donders J, et al. Survival and functional outcome in pediatric traumatic brain injury: a retrospective review and analysis of predictive factors. Crit Care Med. 2002 Jul;30(7):1630–1635. doi: 10.1097/00003246-200207000-00038. [DOI] [PubMed] [Google Scholar]
- 10.Kokosa ER, Smith GS, Pittman T, Weber TR. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. J Pediatr Surg. 1998 Feb;33(2):333–338. doi: 10.1016/s0022-3468(98)90457-2. [DOI] [PubMed] [Google Scholar]
- 11.Downard C, Hulka F, Mullins RJ, Piatt J, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000 Oct;49(4):654–659. doi: 10.1097/00005373-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57(6):769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 13.Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25(4):793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- 14.Fisher AQ, Truemper EJ. Babikian VL, Wechsler LR. Applications in the neonate and child. In: Transcranial Doppler ultrasonography. 2nd ed. Boston: Butterworth-Heinemann; 1999. pp. 355–376. [Google Scholar]
- 15.Strebel S, Lam AM, Matta B, Mayberg TS, et al. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995 Jul;83(1):66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Bode H. Application of Pediatric Transcranial Doppler Ultrasonography. Vienna, Springer Verlag; 1988. [Google Scholar]
- 17.Vavilala MS, Kinkaid MS, Muangman SL, Suz P, et al. Gender differences in cerebral blood flow velocity and autoregulation between anterior and posterior circulations in healthy children. Pediatr Res. 2005;58:574–578. doi: 10.1203/01.PDR.0000179405.30737.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tontisirin N, Muangman SL, Suz P, et al. Early childhood gender differences in anterior and posterior cerebral blood flow velocity and autoregulation. Pediatrics. 2007;119:610–615. doi: 10.1542/peds.2006-2110. [DOI] [PubMed] [Google Scholar]
- 19.Wintermark M, Lepori D, Cotting J, et al. Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics. 2004;113:1642–1652. doi: 10.1542/peds.113.6.1642. [DOI] [PubMed] [Google Scholar]
- 20.Vavilala MS, Lee LA, Boddu K, et al. Cerebral autoregulation in pediatric traumatic brain injury. Pediatr Crit Care Med. 2004;5:257–263. doi: 10.1097/01.pcc.0000123545.69133.c3. [DOI] [PubMed] [Google Scholar]
- 21.Chan KH, Miller JD, Dearden NM. Mechanism and treatment principle for cerebral vessel spasm caused by concussion. Chin J Traumatol. 2002 Dec;5(6):380–384. [PubMed] [Google Scholar]
- 22.Obrist WD, Gennarelli TA, Segawa H, Dolinskas CA. Relation of cerebral blood flow to neurological status and outcome in head-injured patients. J Neurosurg. 1979;51:292–300. doi: 10.3171/jns.1979.51.3.0292. [DOI] [PubMed] [Google Scholar]
- 23.Miller JD, Gudeman SK. Wilkins RH. Cerebral vasospasm after head injury. Amsterdam, TheNetherlands. Baltimore: Williams Wilkins; 1980. pp. 476–479. Cerebral arterial spasm. Proc 2nd Int Workshop. [Google Scholar]
- 24.Hébert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin. 2004;20(2):187–212. doi: 10.1016/j.ccc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Lacroix J, Hébert PC, Hutchison JS, Hume HA, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 26.Trabold F, Meyer PG, Blanot S, Carli PA, Orliaguet GA. The prognostic value of transcranial Doppler studies in children with moderate and severe head injury. Intensive Care Med. 2004;30(1):108–112. doi: 10.1007/s00134-003-2057-8. [DOI] [PubMed] [Google Scholar]
- 27.Ract C, Le Moigno S, Bruder N, Vigué B. Transcranial Doppler ultrasound goal-directed therapy for the early management of severe traumatic brain injury. Intensive Care Med. 2007;33(4):645–651. doi: 10.1007/s00134-007-0558-6. [DOI] [PubMed] [Google Scholar]
- 28.Jaffres P, Brun J, Declety P, Bosson JL, Fauvage B, et al. Transcranial Doppler to detect on admission patients at risk for neurological deterioration following mild and moderate brain trauma. Intensive Care Med. 2005 Jun;31(6):785–790. doi: 10.1007/s00134-005-2630-4. [DOI] [PubMed] [Google Scholar]
- 29.Chan KH, Miller JD, Dearden NM, Andrews PJ, Midgley S. The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurg. 1992 Jul;77(1):55–61. doi: 10.3171/jns.1992.77.1.0055. [DOI] [PubMed] [Google Scholar]
- 30.Czosnyka M, Matta BF, Smielewski P, Kirkpatrick PJ, Pickard JD. Cerebral perfusion pressure in head-injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J Neurosurg. 1998;88(5):802–808. doi: 10.3171/jns.1998.88.5.0802. [DOI] [PubMed] [Google Scholar]
- 31.Diringer MN, Videen TO, Yundt K, Zazulia AR, Aiyagari V, et al. Regional cerebrovascular and metabolic effects of hyperventilation after severe traumatic brain injury. J Neurosurg. 2002;96:103–108. doi: 10.3171/jns.2002.96.1.0103. [DOI] [PubMed] [Google Scholar]
- 32.Mazumdar M, Heeney MM, Sox CM, Lieu TA. Preventing stroke among children with sickle cell anemia: an analysis of strategies that involve transcranial Doppler testing and chronic transfusion. Pediatrics. 2007 Oct;120(4):e1107–e1116. doi: 10.1542/peds.2006-2002. [DOI] [PubMed] [Google Scholar]
- 33.Sviri GE, Ghodke B, Britz GW, Douville CM, Haynor DR, et al. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery. 2006;59:360–366. doi: 10.1227/01.NEU.0000223502.93013.6E. [DOI] [PubMed] [Google Scholar]
- 34.White H, Venkatesh B. Applications of transcranial Doppler in the ICU: a review. Intensive Care Med. 2006 Jul;32(7):981–994. doi: 10.1007/s00134-006-0173-y. [DOI] [PubMed] [Google Scholar]