Indication
Eculizumab is effective in paroxysmal nocturnal haemoglobinuria (PNH). This rare disease is characterized by intermittent complement-dependent haemolysis. The cause of PNH is a genetic defect in one of the natural complement inhibitors, CD59. CD59 protects erythrocytes from the membrane attack complex (MAC) that is the final effector pathway of complement activation, and this protective mechanism is deficient in PNH.
Mechanism
Complement C5 is split by C5 convertase into C5a and C5b. C5a increases the permeability of blood vessels and attracts inflammatory cells by chemotaxis. C5b binds to other complement components (C6, C7 and C8). The C5b-8 complex is expanded with C9 to form the MAC. MAC binds and permeabilizes bacterial walls (e.g. Neisseria), thereby killing the microorganism.
Eculizumab is a long-acting humanized monoclonal antibody targeted against complement C5. It inhibits the cleavage of C5 into C5a and C5b and hence inhibits deployment of the terminal complement system including the formation of MAC. In PNH patients, eculizumab profoundly inhibits haemolysis.
Adverse effects
PNH patients should be vaccinated against Neisseria meningitides before treatment with eculizumab, since this predictably increases their susceptibility to meningococcal infection. Increased susceptibility to other infections (e.g. urinary, respiratory and gastrointestinal) also occurs.
Available at http://www.emea.europa.eu/humandocs/Humans/EPAR/soliris/soliris.htm
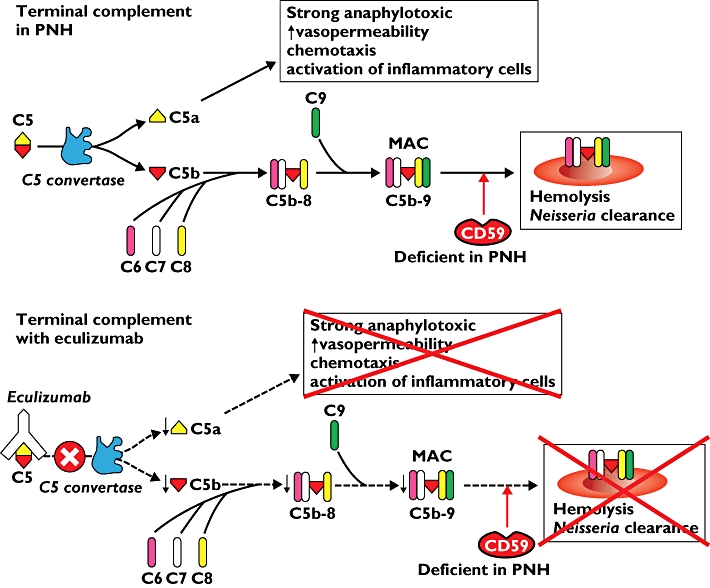
The membrane attack complex (MAC), the end-product of the terminal complement system, normally does not attack the body's own red blood cells, because CD59 prevents this. However, patients with paroxysmal nocturnal haemoglobinuria (PNH) lack CD59, which allows MAC to attack the red blood cells and cause lysis Eculizumab binds complement C5, thereby inhibiting the entire terminal complement system including the generation of the MAC. In PNH patients this results in a strong decrease in haemolysis, but also reduced clearance of Neisseria
Literature
- Parker CJ, Kar S, Kirkpatrick P. FRESH FROM THE PIPELINE: Eculizumab. Nat Rev Drug Discov. 2007;6:515–16. doi: 10.1038/nrd2369. [DOI] [PubMed] [Google Scholar]


