Abstract
AIMS
Imatinib mesylate (Gleevec®/Glivec®), which has revolutionized the treatment of chronic myeloid leukemias (CML) and gastrointestinal stromal tumours (GIST), has been reported to cause gastric upset. Consequently, proton pump inhibitors (PPI) are frequently co-administered with imatinib. Because PPI can elevate gastric pH and delay gastric emptying or antagonize ATP-binding-cassette transporters, they could influence imatinib absorption and pharmacokinetics. We aimed to evaluate whether use of omeprazole has a significant effect on imatinib pharmacokinetics.
METHODS
Twelve healthy subjects were enrolled in a two-period, open-label, single-institution, randomized cross-over, fixed-schedule study. In one period, each subject received 400 mg imatinib orally. In the other period, 40 mg omeprazole (Prilosec®) was administered orally for 5 days, and on day 5 it was administered 15 min before 400 mg imatinib. Plasma concentrations of imatinib and its active N-desmethyl metabolite CGP74588 were assayed by LC-MS, and data were analyzed non-compartmentally.
RESULTS
PPI administration did not significantly affect the imatinib area under the plasma concentration vs time curve (AUC) (34.1 µg ml−1 h alone vs 33.1 µg ml−1 h with omeprazole, P= 0.64; 80% power), maximum plasma concentration (Cmax) (2.04 µg ml−1 alone vs 2.02 µg ml−1 with omeprazole, P= 0.97), or half-life (13.4 h alone vs 14.1 h with omeprazole, P= 0.13).
CONCLUSIONS
Our results indicate that the use of omeprazole does not significantly affect the pharmacokinetics of imatinib, as opposed to, for example, dasatinib where PPI decreased AUC and Cmax two-fold.
Keywords: CML, GIST, imatinib, interaction, proton pump inhibitors
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Gastric upset is a side-effect of imatinib therapy and the concomitant use of proton pump inhibitors is common. With the increase in oral chemotherapy in cancer treatment, oral drug–drug interactions are becoming more relevant, as is exemplified by dasatinib which has already been shown to be absorbed to a much lesser extent when co-administered with antacids or proton-pump inhibitors. Because exposure to subtherapeutic concentrations of anticancer drugs such as imatinib and dasatinib may result in selection of resistant clones, and ultimately relapse, we studied the effect of the proton pump inhibitor omeprazole on the pharmacokinetics of imatinib.
WHAT THIS STUDY ADDS
Omeprazole may be co-administered with imatinib to treat the gastric side-effects of the latter without affecting the pharmacokinetics of imatinib and risking tumour relapse.
Introduction
Imatinib mesylate (Gleevec®, Glivec®), a potent inhibitor of Bcr-Abl, the tyrosine kinase created by the Philadelphia chromosome, is widely used to treat chronic myeloid leukaemias (CML) and gastrointestinal stromal tumours (GIST) [1–3]. Common side-effects associated with imatinib therapy include dyspepsia (18%) and nausea (43%) [3], so that proton pump inhibitors (PPI) are frequently co-administered with imatinib. PPI can increase the pH of gastric contents and delay gastric emptying [4, 5]. PPIs have also been reported to antagonize ATP-binding-cassette transporters, for which imatinib is a known substrate [6–8]. These effects could influence imatinib pharmacokinetics, possibly decrease its absorption and consequently cause imatinib concentrations to fall below therapeutic concentrations [9, 10]. Indeed, the PPI omeprazole is known to decrease the AUC and Cmax of the tyrosine kinase inhibitor dasatinib by 61% and 63%, respectively, [11]. Considering the potentially large and relevant effects of PPI, this study was performed with the aim of determining whether, and to what extent, the PPI omeprazole could influence the pharmacokinetics of imatinib.
Methods
Subjects
This pharmacokinetic study was conducted in 12 healthy subjects (six men, six women; ≥18 years of age; body mass index (BMI) < 31 kg m−2) after they signed an informed consent that had been approved by the University of Pittsburgh Institutional Review Board. Exclusion criteria were: abnormal bone marrow function (defined as leucocyte, neutrophil, or platelet counts outside the limits of the institution's normal ranges); renal dysfunction (proteinuria, estimated creatinine clearance < 60 ml min−1 1.73 m−2); impaired hepatic function (liver enzymes or bilirubin > the normal upper limit); pregnancy or breast-feeding; use of any medications (including over-the-counter products, herbal products or mineral supplements) within 2 weeks of start of the study, or an investigational new drug within 28 days of start of the study. Daily multivitamin preparations or oral contraceptives (for women) were allowed.
Study design
The study had a two-period, open-label, randomized cross-over, fixed-sequence design (Figure 1), and could detect a 30% difference in imatinib AUC with 80% power and a 5% type I error, assuming a within-subject variability of 30% [12]. In one phase, each subject received 400 mg imatinib (Gleevec®; Novartis Pharmaceuticals Corp, East Hanover, NJ) orally with 200 ml water, while in the other phase, 40 mg omeprazole (Prilosec®) was administered orally once a day for 5 days prior to the imatinib dose. On day 5, 40 mg omeprazole was administered orally 15 min before the 400 mg imatinib dose. The 5-day lead-in of omeprazole was chosen because PPI are reported to reach their maximum effect on gastric pH after 5 days [4]. The two imatinib doses were separated by a wash-out period of at least 14 days. In the case of administration of the combination on day 15, this meant that omeprazole was started on day 10.
Figure 1.
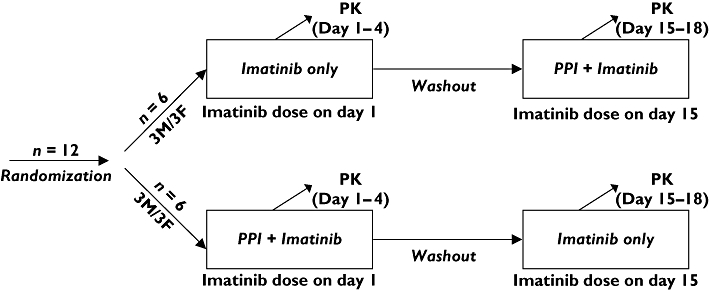
Study design
Pharmacokinetic sampling and bioanalysis
Subjects were asked to fast for approximately 8 h overnight before dosing and were given a normal diet on the day of pharmacokinetic sampling. Venous blood samples (n= 13 per subject, 6 ml each) were drawn from an indwelling catheter into heparinized Vacutainer™ tubes before and at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, and 72 h after imatinib administration. Blood samples were centrifuged at 4°C, 3000 ×g for 10 min, and the resulting plasma was aspirated and stored at −20°C or colder until analyzed.
Plasma samples were analyzed for imatinib and its active N-desmethyl metabolite (CGP74588) using an LC-MS method that was previously developed and validated in our laboratory [13, 14]. The assay was linear over the range of 10–1000 ng ml−1, and exhibited acceptable performance (imatinib: accuracy 13.1–9.8% and precision within 6.8% CV; CGP74588: accuracy 5.3–6.7% and precision within 15.0% CV).
Pharmacokinetic data analysis
The pharmacokinetic parameters of imatinib and CGP74588 were determined by standard non-compartmental methods, with PK Solutions 2.0 (Summit Research Services, Montrose, CO; http://www.summitPK.com). The maximum concentration (Cmax) and time to reach the maximum concentration (tmax) were determined by visual inspection of the plasma concentration vs time curves. The imatinib elimination rate constant (ke) was obtained using non-linear least-square regression of the terminal concentration vs time data. The imatinib area under the concentration vs time curve (AUC) was calculated by the trapezoidal rule with extrapolation to infinity (AUC(0,∞)). The percentage of AUC(0,∞) extrapolated beyond the last sample time (Clast), indicating the fraction of the AUC(0,∞) that was not based on plasma determinations, was calculated. Ideally, the percentage extrapolated is < 20%.
Statistical analysis
Whether or not omeprazole had a significant effect on the pharmacokinetics of imatinib was determined with SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). Pharmacokinetic parameters were log-transformed and compared non-parametrically with the two-tailed exact Wilcoxon signed rank test (paired data). Data were considered significantly different when P < 0.05.
We also performed an analysis of bioequivalence by calculating the 90% confidence intervals of the imatinib AUC ratio and the Cmax ratio, based on log-transformed data (individual ratios calculated first). Equivalence limits were defined as 80–125% [15].
Results
Thirteen subjects were enrolled to obtain 12 complete data sets. One female subject developed chills, hypertension, fever, nausea and headache after taking the combination of imatinib and omeprazole during her second study visit. Laboratory evaluations showed elevated transaminases and white cells, suggesting passage of a gall stone. Subsequent follow-up revealed complete resolution of clinical and laboratory abnormalities. Concentration vs time data from this subject was not used in the data analysis. All other subjects tolerated the administrations of imatinib and omeprazole well.
There was no sequence effect in the imatinib AUC, i.e. the pharmacokinetics were not different between the first and second administration of imatinib (P= 0.147). The pharmacokinetic parameter estimates for imatinib are shown in Table 1. The percentage of the AUC extrapolated beyond Clast was < 6% for imatinib, allowing us to interpret our data with confidence. Concentration vs time curves of imatinib and CGP74588 in the presence and absence of omeprazole, respectively, are shown in Figure 2.
Table 1.
Pharmacokinetic parameter estimates for imatinib and N-desmethyl-imatinib (CGP74588) after oral administration of imatinib alone and with co-administration of a PPI (omeprazole) (n= 12)
| AUC(0,∞) | Cmax | tmax | t1/2 | V/F | CL/F | ||
|---|---|---|---|---|---|---|---|
| Analyte | Arm | (µg ml−1 h) | (µg ml−1) | (h) | (h) | (l) | (l h−1) |
| Imatinib | Alone | 34.1 (11.1) | 2.04 (0.56) | 3.0 (0.9) | 13.4 (1.5) | 244 (130) | 14.1 (9.5) |
| + PPI | 33.1 (9.3) | 2.02 (0.65) | 3.1 (1.2) | 14.1 (1.9) | 273 (168) | 15.2 (12.6) | |
| P value | 0.64 | 0.97 | 0.78 | 0.13 | 0.14 | 0.64 | |
| CGP74588 | Alone | 6.95 (2.46) | 0.27 (0.06) | 2.6 (1.2) | 31.6 (10.3) | 2494 (839) | 62.8 (17.3) |
| + PPI | 6.77 (1.81) | 0.28 (0.07) | 3.7 (0.9) | 32.1 (12.6) | 2545 (801) | 63.2 (17.7) | |
| P value | 0.79 | 0.91 | 0.82 | 0.97 | 0.87 | 0.78 |
The data are expressed as mean (SD). The percentage of the AUC(0,∞) extrapolated beyond Clast was < 6% for imatinib and < 45% for CGP74588. P values were obtained after log-transformation of the parameters by use of the non-parametrically two-tailed exact Wilcoxon signed rank test (paired data).
Figure 2.
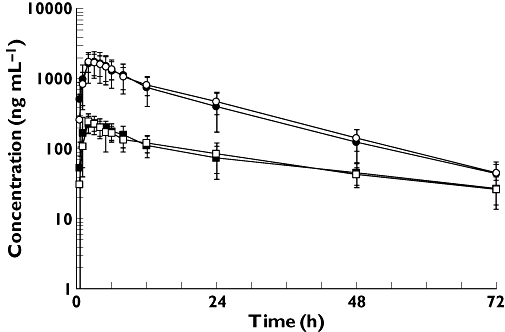
Mean (± SD) concentration vs time profile of imatinib (circles) and CGP74588 (squares) after oral administration of 400 mg imatinib without omeprazole (solid) and with omeprazole (open) to 12 healthy volunteers
There was no significant difference in imatinib plasma AUC after dosing of imatinib alone, when compared with the AUC after co-administration of imatinib and omeprazole (P= 0.64). The 90% confidence intervals of the imatinib AUC ratio (mean 1.07, 90% confidence interval 0.87, 1.07) and the Cmax ratio (mean 0.97, 90% confidence interval 0.87, 1.09), both fell well within the limits set for bioequivalence [15]. We also examined the effect of omeprazole on other pharmacokinetic parameters for imatinib and CGP74588 (Table 1 and Figure 3). We did not detect any statistically significant effect in any of the parameters tested.
Figure 3.
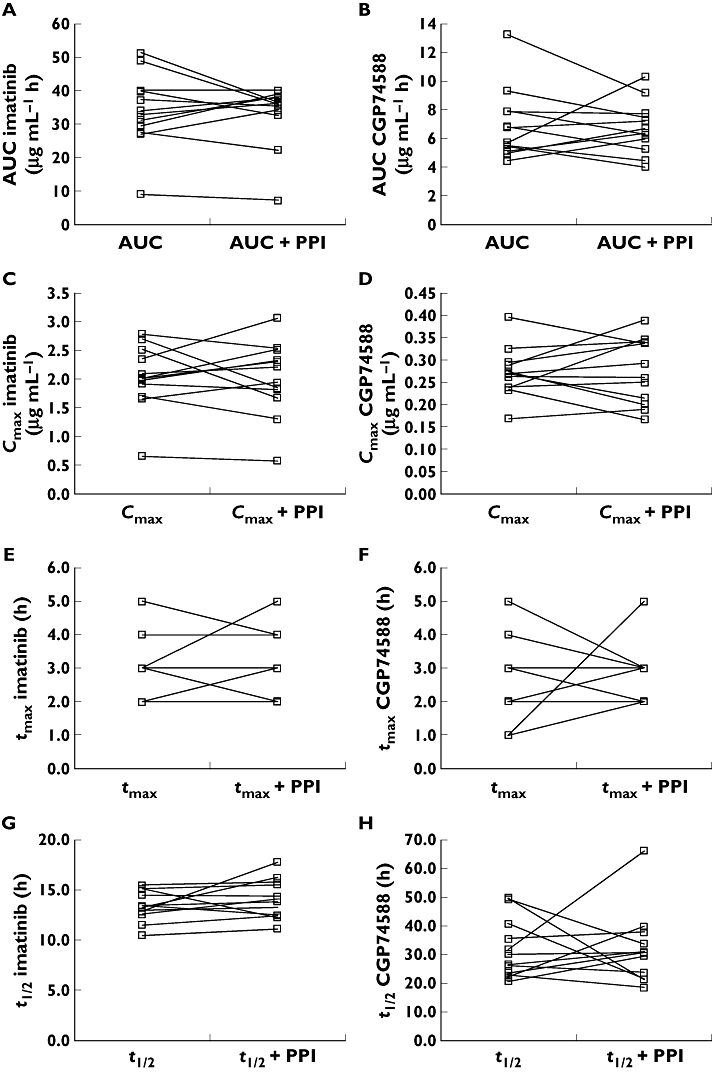
Intra-individual changes of imatinib (A, C, E, and G) and CGP74588 (B, D, F, and H) AUC (A and B), Cmax (C and D), tmax (E and F), and half-life (G and H) when oral imatinib was co-administered with oral omeprazole in 12 healthy volunteers
Discussion
This healthy volunteer study demonstrates that the use of omeprazole is not associated with a change in the pharmacokinetics of imatinib. The results are similar to results obtained when imatinib was co-administered with a Mg2+-Al3+-based antacid [13], but are in contrast to data demonstrating that PPI use is associated with a nearly two-fold decrease in dasatinib AUC and Cmax[11]. The reason for the interaction between PPI and dasatinib is not clear, but may be due to limited dissolution of the dasatinib base in an environment with elevated pH. Apparently, the elevated gastric pH associated with the use of a PPI is not as important for the absorption of imatinib. However, our results cannot be extrapolated to other PPIs, such as pantoprazole, which was shown to affect clearance mechanisms of imatinib in recent preclinical studies [16]. In conclusion, our results show that concomitant administration of the PPI omeprazole is not associated with alterations in imatinib pharmacokinetics.
Competing interests
M.J.E. has served as a consultant to Novartis Pharmaceuticals and is receiving grant support from Novartis Pharmaceuticals. L.R.A. has served as a principal investigator for two clinical trials that were supported and/or sponsored by Novartis Pharmaceuticals.
We thank the nursing staff of the University of Pittsburgh Clinical Translational Research Center for their invaluable assistance, and the University of Pittsburgh Cancer Institute Hematology/Oncology Writing Group for constructive suggestions regarding the manuscript. This work was supported by Novartis Pharmaceuticals Corporation (East Hanover, NJ), and NIH/NCRR/CTSA Grant UL1 RR024153.
REFERENCES
- 1.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CDM, Joensuu H. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344:1038–42. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 4.Howden CW. Clinical pharmacology of omeprazole. Clin Pharmacokinet. 1991;20:38–49. doi: 10.2165/00003088-199120010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Tougas G, Earnest DL, Chen Y, Vanderkoy C, Rojavin M. Omeprazole delays gastric emptying in healthy volunteers: an effect prevented by tegaserod. Aliment Pharmacol Ther. 2005;22:59–65. doi: 10.1111/j.1365-2036.2005.02528.x. [DOI] [PubMed] [Google Scholar]
- 6.Burger H, van Tol H, Boersma AW, Brok M, Wiemer EA, Stoter G, Nooter K. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 7.Breedveld P, Zelcer N, Pluim D, Sonmezer O, Tibben MM, Beijnen JH, Schinkel AH, van Tellingen O, Borst P, Schellens JH. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004;64:5804–11. doi: 10.1158/0008-5472.CAN-03-4062. [DOI] [PubMed] [Google Scholar]
- 8.Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–82. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- 9.Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, Lassalle R, Marit G, Reiffers J, Begaud B, Moore N, Molimard M, Mahon FX. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–9. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, Wang Y, Wehrle E, Blanke C, Joensuu H, von Mehren M. Correlation of imatinib plasma levels with clinical benefit in patients (Pts) with unresectable/metastatic gastrointestinal stromal tumors (GIST) ASCO Gastrointestinal Cancers Symposium.
- 11.Bristol-Myers Squibb Company. Prescribing Information Sprycel(R) (dasatinib)
- 12.Cohen MH, Williams G, Johnson JR, Duan J, Gobburu J, Rahman A, Benson K, Leighton J, Kim SK, Wood R, Rothmann M, Chen G, U KM, Staten AM, Pazdur R. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8:935–42. [PubMed] [Google Scholar]
- 13.Sparano BA, Egorin MJ, Parise RA, Walters J, Komazec KA, Redner RL, Beumer JH. Effect of antacid on imatinib absorption. Cancer Chemother Pharmacol. 2009;63:525–8. doi: 10.1007/s00280-008-0778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parise RA, Ramanathan RK, Hayes MJ, Egorin MJ. Liquid chromatographic-mass spectrometric assay for quantitation of imatinib and its main metabolite (CGP 74588) in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;791:39–44. doi: 10.1016/s1570-0232(03)00206-x. [DOI] [PubMed] [Google Scholar]
- 15.de Campos DR, Vieira NR, Bernasconi G, Barros FA, Meurer EC, Marchioretto MA, Coelho EC, Calafatti SA, Sommer C, Couto JM, Buranello S, Silva AR, Amarante AR, Abib E, Junior JP. Bioequivalence of two enteric coated formulations of pantoprazole in healthy volunteers under fasting and fed conditions. Arzneimittelforschung. 2007;57:309–14. doi: 10.1055/s-0031-1296624. [DOI] [PubMed] [Google Scholar]
- 16.Oostendorp RL, Buckle T, Beijnen JH, van Tellingen O, Schellens JH. The effect of P-gp (Mdr1a/1b), BCRP (Bcrp1) and P-gp/BCRP inhibitors on the in vivo absorption, distribution, metabolism and excretion of imatinib. Invest New Drugs. 2009;27:31–40. doi: 10.1007/s10637-008-9138-z. [DOI] [PubMed] [Google Scholar]


