Abstract
AIMS
In a systematic screening of the World Health Organization Adverse Drug Reaction database, VigiBase, in July 2008, a measure of association used to detect interactions (Omega) highlighted azithromycin with the individual statins atorvastatin, lovastatin and simvastatin and rhabdomyolysis. The aim was to examine all reports including rhabdomyolysis-azithromycin and statins in VigiBase to assess if the data were suggestive of an interaction.
METHODS
The individual case reports in VigiBase and the original files were reviewed. In order to investigate the reporting over time for rhabdomyolysis with azithromycin and statins to VigiBase, Omega values were generated retrospectively.
RESULTS
The reporting over time showed that rhabdomyolysis under concomitant use of azithromycin and statins was reported more often than expected from 2000 and onwards in Vigibase. After exclusion of possible duplicates and follow-up reports, 53 cases from five countries remained. Rhabdomyolysis occurred shortly after initiation of azithromycin in 23% of cases. In 11 patients an interaction had been suggested by the reporter. With the exception of one patient, the statin doses reported were within the recommended daily doses.
CONCLUSIONS
Case reports in VigiBase are suggestive that interactions between azithromycin and statins resulting in rhabdomyolysis may occur. This analysis showed the potential of the newly developed disproportionality measure, Omega, which can help to identify drug interactions in VigiBase in the future. The results also showed that reviewing spontaneous reports can add information to drug interactions not established previously.
Keywords: adverse drug reaction reporting systems, azithromycin, disproportionality measure, drug interaction, statins, WHO-ADR database
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Rhabdomyolysis is a serious but rare adverse effect of statins.
The mechanism of rhabdomyolysis with statins is poorly defined, but the occurrence is known to increase with dose/ concentration.
Some macrolides are known to interact with statins; however, azithromycin has not been described to interact with statins with the exception of two literature case reports.
WHAT THIS STUDY ADDS
A case series in the World Health Organization Adverse Drug Reaction (WHO-ADR) database was suggestive of a possible drug interaction between statins and azithromycin with rhabdomyolysis.
A disproportionality measure, Omega, was shown to identify previously not recognized suspected drug interactions within the WHO-ADR dataset.
Introduction
In general, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins) are well tolerated by most users [1, 2]. However, the clinical syndrome rhabdomyolysis has been demonstrated to be a rare but potentially life-threatening adverse effect of statins [3]. The mechanism of rhabdomyolysis with statins is poorly defined, but the occurrence is known to increase with dose/concentration [4]. Consequently, the risk increases when drugs that exclusively can cause rhabdomyolysis are used concomitantly with other drugs that can increase their effects via interactions. The macrolides erythromycin, clarithromycin, troleandomycin and telithromycin have been mentioned as potential interacting agents with statins in standard drug references [5, 6] since they are cleared through the same metabolic pathway, CYP3A4. The newer macrolide, azithromycin, is not suggested to cause clinically significant interactions with agents cleared through the cytochrome P450 enzyme system [7, 8]. Only one small human study has suggested that statins and azithromycin do not interact [9]. In that study 500 mg azithromycin used once daily orally for 3 days had no effect on pharmacokinetic parameters, such as AUC and Cmax of atorvastatin in 12 healthy volunteers. There are two case reports suggesting an interaction between azithromycin and statins [10, 11]. In the first case development of rhabdomyolysis was observed in a 51-year-old man using lovastatin the day after a completed 5-day course of azithromycin [10]. In the second case a 56-year-old man using simvastatin in combination with several other drugs, including the CYP3A4 inhibitor nefazodone developed rhabdomyolysis 5 days after the initiation of azithromycin [11].
Data mining methods [12, 13] have been extensively used in routine signal detection [14] of single drug–adverse drug reaction (ADR) combinations in pharmacovigilance. Measures of disproportionality have also been proposed in order to detect drug interactions [15–17]. Recently a disproportionality measure (Omega) was developed to study the co-reporting of two drugs and one ADR in order to detect drug interactions [18] in the World Health Organization (WHO)-ADR database, called VigiBase [19]. Since the statistical foundation of the method [18] and its ability to highlight interactions [18, 20] have been demonstrated, we have started to use the method in order to screen systematically for emerging drug interactions. In July 2008 the first systematic screening of VigiBase was done. In this screening the statistical measure was positive for atorvastatin, lovastatin and simvastatin with azithromycin and rhabdomyolysis. As this possible interaction has not previously been well described, we analysed the reporting of this potential interaction between azithromycin and statins in VigiBase.
Method
The WHO Collaborating Centre for International Drug Monitoring, the Uppsala Monitoring Centre (UMC), based in Uppsala, Sweden, collects spontaneously reported cases of suspected ADRs. These reports are forwarded from national centres in 86 countries [21]. The case reports are recorded in a common format, and processed and stored in a database, referred to as VigiBase [21]. Over 4 million case records are currently maintained by the centre, which provides a unique source of international ADR information. Each case report includes at least one drug suspected of causing the ADR, one ADR, reporting country and an identification number. Even though the data are not homogeneous with respect to origin or the probability that the pharmaceutical product caused the adverse reaction, spontaneous reporting of suspected ADR reports remains the cornerstone in the early detection of signals of previously unknown ADRs for one drug. Today quantitative screening is often an integral part of an overall knowledge discovery process for the detection of previously unsuspected ADRs in large collections of spontaneous reports [22].
There are several measures of pairwise association used to screen collections of spontaneous reports for excessive reporting of single drug–single adverse reaction combinations. In routine signal detection, the UMC uses the Information Component (IC) measure of association [12]. The IC is a logarithmic measure based on the observed-to-expected ratio for the number of reports on a drug and an ADR together, where the expected number is based on the total number of reports on the drug and the overall relative reporting rate of the ADR in the entire database. It is a Bayesian implementation of the observed-to-expected ratio, with a prior assumption of independence between drug and adverse reaction. The algorithm includes shrinkage of the observed-to-expected ratio towards the independence assumption when there is limited amount of data and observed and expected counts are small; effectively, this reduces the number of false-positive associations with little data support.
The IC is used to filter out combinations of particular drugs and ADR combinations that are present more frequently than expected according to the reporting of all reports for the particular drug and ADR and the total number of reports in VigiBase. The IC measure in conjunction with triage filters [23, 24] generates a list of drug–ADR combinations that are believed to be of greatest importance. This process is run quarterly on VigiBase. The drug–ADR combination characteristics of the quarterly search include positive IC credibility intervals, drugs recently marketed (introduced in the WHO Drug Dictionary within the last 5 years), serious reactions (critical terms within WHO-Adverse Reaction Terminology [19]), reported from more than one country and rapidly increasing IC value.
The analysis presented in this study is based on a measure of interaction, related to the IC, which is referred to as Omega, and contrasts the observed relative reporting rate in the database with its expected value estimated from the relative reporting rates of the ADR given and with the individual reporting of each drug. The observed number of cases is the number of reports reported to VigiBase for the two drugs together, with the ADR of interest. The expected number of cases is the number of reports, which we expect to be reported for the drug–drug–ADR (DDA).
No measure has yet been shown effective in highlighting emerging drug–drug interactions. This is at least in part due to limitations in the choice of measures used. However, a measure where the expected count is based on an additive model [25] will be more likely to highlight signals of drug–drug interactions. Such additive baseline models have proven more effective as a basis for drug–drug interaction surveillance than the multiplicative models on which standard methods such as log-linear models and logistic regression are based [18, 25]. If the Omega value is greater than zero the DDA is reported more often than expected. The measure is described in detail in by Norén et al. [18]. The lower limit of the 95% credibility interval provides a measure of the robustness and is called Omega_025, and Omega_025>0 is used as a threshold to screen for reporting patterns suggestive of drug–drug interaction. The credibility intervals become narrower when more information is added to the database in the form of case reports on the DDA. Narrow credibility intervals indicate the robustness in the reported value.
In the systematic screening of VigiBase in July 2008 some of the triage criteria used in routine signal detection were used. Atorvastatin, lovastatin and simvastatin were all reported more frequently than expected with azithromycin and rhabdomyolysis. The case reports within the database originated from more than one country. A more extensive search on rhabdomyolysis, azithromycin and all statins was then performed in order to investigate further the possible interaction. The case reports in VigiBase and original files, which generally provide more detailed information in the form of narrative text for the individual case report, were reviewed clinically. To investigate the evolution in time of the reporting pattern in VigiBase, Omega values and corresponding 95% credibility interval limits were computed for retrospectively recreated versions of VigiBase for rhabdomyolysis with azithromycin co-reported with all statins combined. Duplicate reports and follow-up reports are present in VigiBase and are identified by an automatic [26] or manual screening process. These reports were included in the calculation of Omega since both the observed and expected counts should be inflated by the same magnitude assuming absence of differential rates of duplication. However, these reports were excluded in the detailed case analysis.
Results
In a systematic screening of VigiBase in July 2008 the associations of rhabdomyolysis with azithromycin–atorvastatin, lovastatin and simvastatin were quantitatively highlighted. These findings prompted a more extensive analysis of azithromycin and all statins with rhabdomyolysis. Table 1 provides a summary of the statistical information provided for the 58 cases of rhabdomyolysis reported with azithromycin and one of the following statins: atorvastatin, cerivastatin, lovastatin, pravastatin, rosuvastatin or simvastatin. Fluvastatin was the only individual statin never co-reported to VigiBase with azithromycin and rhabdomyolysis. Other macrolides co-reported with any statin and rhabdomyolysis to VigiBase were clarithromycin (n= 118), erythromycin (n= 36), roxithromycin (n= 7) and telithromycin (n= 8). At the time of the study VigiBase included almost 4 million case reports, of those concerned approximately 160 000 statins. Rhabdomyolysis was reported to the database on 12 308 reports, >9000 of which concerned statins, with the majority including cerivastatin. In July 2008 VigiBase contained approximately 13 000 reports of azithromycin.
Table 1.
The reporting for rhabdomyolysis with azithromycin for each individual statin in VigiBase up to July 2008
| Statin | Observed DDA | Expected DDA | Omega | Omega_025 | IC for statin-rhabdo 2008q2 | IC_025 for statin-rhabdo 2008-2 |
|---|---|---|---|---|---|---|
| Atorvastatin | 24 | 7.10 | 1.69 | 1.05 | 2.98 | 2.90 |
| Cerivastatin | 17 | 11.62 | 0.53 | −0.24 | 6.86 | 6.82 |
| Lovastatin | 5 | 0.58 | 2.35 | 0.82 | 2.07 | 1.88 |
| Pravastatin | 2 | 1.22 | 0.54 | −2.05 | 2.13 | 1.95 |
| Rosuvastatin | 3 | 0.81 | 1.41 | −0.64 | 3.69 | 3.52 |
| Simvastatin | 20 | 8.73 | 1.15 | 0.45 | 3.62 | 3.55 |
| All statins | 58 | 35.39 | 0.70 | 0.31 | 4.21 | 4.18 |
DDA, drug–drug–ADR (represents azithromycin with the individual statin and rhabdomyolysis); Observed, the observed number of cases represents the number of reports actually reported to VigiBase for the two drugs together with the ADR of interest; Expected, the expected number of cases is number of reports which we expect to be reported for the drug–drug–ADR (DDA). The expected count is dependent on the total number of reports in VigiBase, per individual drug, for the combination of the two drugs and the ADR of interest; Omega, measure of disproportionality used to screen Vigibase for possible emerging drug–drug interactions; IC, measure of disproportionality used to screen Vigibase for possible emerging ADR side-effects.
Subsequent to the initiation of the clinical review, four additional case reports were entered to the database of azithromycin and statins rhabdomyolysis. When duplicates and follow-up reports were excluded, 53 cases were reviewed. In six cases, two or more statins were co-reported. The cases were reported from five countries: Canada (n= 3), France (n= 1), Germany (n= 1), Switzerland (n= 2) and the USA (n= 46). Table 2 includes a summary of all 53 patients. The age range was 41–85 years (median 60 years). Fourteen (26%) patients had been using the statin for ≥3 months before the onset of rhabdomyolysis. In 12 cases (23%) rhabdomyolysis occurred within 10 days after starting azithromycin in treatment. The reported statin doses were within the recommended daily doses [5, 6] with the exception of one patient, who had switched drug therapy from simvastatin to cerivastatin and by mistake taken the defined daily dose (0.8 mg) of cerivastatin, although twice daily. In one report it was explicitly noted that atorvastatin levels were increased, but plasma levels were not stated. In 12 cases azithromycin and the statin were the sole reported drugs. Of the remaining 41 reports, an established interacting agent [5, 6] known to increase the risk for rhabdomyolysis was found in 16 cases. Table 3 lists patient details of all 11 cases where an interaction has been suggested by the reporter, either by explicitly reporting the drug as interacting (n= 3) or providing a comment in the narrative text (n= 8). In total, azithromycin and a statin were both reported as suspected or interacting to cause rhabdomyolysis in 45% of the reports.
Table 2.
Descriptive data for the 53 patients reported with rhabdomyolysis with azithromycin and statins
| Statin | Tot reports | No of. countries reporting | Age range | Dose range | Solely two drugs | Overlapping therapy of statins and azithromycin |
|---|---|---|---|---|---|---|
| Atorvastatin | 18 | 2 | 41–85 | 10–80 mg (n = 10) | 6 | 4 |
| Cerivastatin | 12 | 3 | 43–74 | 0.2–0.8 mg (n = 10) | 2 | 4 |
| Lovastatin | 2 | 1 | 51* | – | 0 | 0 |
| Pravastatin | 2 | 2 | 60–74 | 20 mg (n = 1) | 0 | 1 |
| Rosuvastatin | 4 | 2 | 59–82 | 10 mg (n = 2) | 0 | 2 |
| Simvastatin | 22 | 3 | 46–83 | 20–80 mg (n = 11) | 4 | 1 |
Age was provided in only one patient. n, the number of patients where dose information was provided.
Table 3.
A summary of the 11 case reports in VigiBase of azithromycin–statin and rhabdomyolysis for which a drug interaction was suggested by the reporter
| Case | Sex | Age | Drugs | Other information |
|---|---|---|---|---|
| 1 | M | – | Azithromycin | Uncertainty whether atorvastatin or gemfibrozil was concomitantly used with azithromycin |
| Atorvastatin | ||||
| 2 | M | – | Azithromycin 500 mg (1 day) 250 mg (4 days) | Both drugs were discontinued. Outcome unknown |
| Atorvastatin 20 mg (2 years) | ||||
| 3 | M | 41 | Azithromycin | Atorvastatin levels were increased, although no plasma levels were given. Pharmacokinetic interaction related to CYP activity is discussed in the narrative text |
| Atorvastatin 40 mg | ||||
| 4 | F | 66 | Azithromycin | The reporter stated: multifactor cause of rhabdomyolysis including renal dysfunction, use of azithromycin–atorvastatin and viral infection. Pharmacokinetic interaction due to CYP activity is discussed in the narrative text |
| Atorvastatin 80 mg (3 years) | ||||
| Nicotinic acid | ||||
| 5 | M | 56 | Azithromycin | Atorvastatin and azithromycin discontinued. Patient recovered. Uncertainty whether atorvastatin or gemfibrozil was concomitantly used with azithromycin |
| Atorvastatin | ||||
| Gemfibrozil | ||||
| 6 | F | 43 | Azithromycin (9 days) | The patient recovered |
| Cerivastatin 1 dosage form (4 years) | ||||
| 7 | M | 51 | Azithromycin | |
| Lovastatin | ||||
| 8 | M | 56 | Azithromycin (5 days) | Simvastatin was increased from 40 to 80 mg day–1 since the LDL was elevated. Three weeks later the cholesterol was well controlled. The patient was then started on fexofenadine and AZ. Simvastatin, temazepam, nefazodone, isometheptene and fexofenadine were discontinued. The condition started to improve. In the report nefazodone was primarily suspected to be the culprit. However, it was not possible to exclude the contributing factor of azithromycin, since the ADR occurred 5 days after introduction of azithromycin |
| Simvastatin 80 mg (4 weeks) | ||||
| Temazepam | ||||
| Nefazodone | ||||
| Isometheptene | ||||
| Fexofenadine (5 days) | ||||
| 9 | M | – | Azithromycin | |
| Simvastatin | ||||
| 10 | M | 48 | Azithromycin (7 days) | Patient with diabetes mellitus, coronary artery disease, cardiomyopathy and history of renal failure secondary to diabetes (transplantation 15 years ago). Azithromycin was introduced; a week later the patient was admitted to hospital for acute renal failure, rhabdomyolysis and hepatic insufficiency. It was determined that the acute renal failure was due to rhabdomyolysis, which was caused by the combination of simvastatin and azithromycin. Simvastatin was discontinued. Hepatic insufficiency and rhabdomyolysis revealed, but renal failure persisted |
| Simvastatin | ||||
| 11 | F | 74 | Azithromycin (13 days) | Three days after a 10-day course of azithromycin the patient developed muscle pain. Pravastatin was discontinued and the symptoms resolved. Pharmacokinetic interaction due to CYP activity is discussed in the narrative. The reporter requested if a change in product information was adequate |
| Pravastatin 20 mg (2 years) |
Time intervals listed within parentheses state the duration of drug therapy prior to onset of rhabdomyolysis occurrence.
Figure 1 shows the reporting of azithromycin and statins with rhabdomyolysis from 1994 up to July 2008. From 2000 onwards Omega and Omega _025 were both positive, indicating that the DDA was reported more often than expected in comparison with general reporting to VigiBase. The credibility intervals become narrower over time, which indicates a robustness of the values provided. There is a general reporting increase of rhabdomyolysis in particular with statins to VigiBase from 2000 and onwards. Neither a proportional increase nor an absolute such increase was seen for azithromycin alone with rhabdomyolysis.
Figure 1.
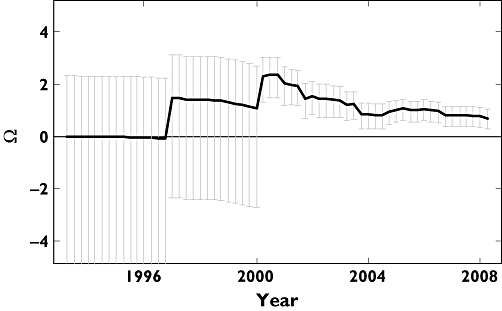
A trend of disproportionality measures with the 95% credibility intervals over time in VigiBase for atorvastatin, cerivastatin, lovastatin, pravastatin, rosuvastatin, simvastatin with azithromycin and rhabdomyolysis
Discussion
A statistical method used for detecting interactions in VigiBase, Omega, triggered a manual review of the case reports concerning all statins, azithromycin and rhabdomyolysis. Azithromycin has previously been described not to interact with statins [4, 9], with the exception of two case reports [10, 11]. The 53 case reports in VigiBase are suggestive that interactions between azithromycin and atorvastatin, cerivastatin, lovastatin, pravastatin, rosuvastatin or simvastatin resulting in rhabdomyolysis may occur. As the reports were received from a variety of countries, this reduces the possibility that the volume of reports was purely due to selective reporting due to country- or region-specific publication bias. The raw number of reports per combination cannot be interpreted as an estimate of incidence, while the raw number is higher than expected based on general reporting of the drug and adverse reactions. This leads to positive disproportionality scores that have proved predictive of emerging but currently recognized ADR issues [27].
It is noteworthy that the reports were not restricted exclusively to the elderly (e.g. >65 years [28]); this, in combination with the fact that no other drugs than azithromycin and the statin was reported in 12 cases, increases the plausibility of an association. The long-term use of statins with a rapid onset within 10 days of rhabdomyolysis from initiation of azithromycin is also indicative of a drug interaction [29, 30]. Nevertheless, there are several alternative explanations to why rhabdomyolysis might occur and be reported after combined azithromycin and statin therapy. It is possible that the rhabdomyolysis onset during statin therapy after initiation of azithromycin could be a coincidental temporal association. Alternatively, the statin may have interacted with other drugs co-prescribed concomitantly, whether listed on the reports or otherwise. Well-established interacting agents such as gemfibrozil, clarithromycin and erythromycin were co-reported in 16 cases [2, 4–7, 9]. Various infectious agents may also cause rhabdomyolysis [31], consequently the underlying infection for which azithromycin was given could have led to rhabdomyolysis without any drug effect. Moreover, other risk factors such as decreased renal function and patient obesity were listed on some of the reports and therefore may have contributed to the disease onset.
The observed number cases reported for rhabdomyolysis with azithromycin and the individual statins is two to three times greater than the expected number of cases, which leads to positive Omega values, as well as positive lower 95% credibility intervals (Table 1), although for three individual DDAs, cerivastatin, pravastatin and rosuvastatin, the lack of data (low number of reports) is shown by negative statistics for the lower limit of the 95% credibility interval, Omega_025. However, selective underreporting of the DDA combination by reporting of one drug or the other with rhabdomyolysis would lead to an inflated expected count relative to the observed count – this bias is one sided and could deflate the Omega value. In our dataset azithromycin was co-reported more often with statins than the well-established interacting agent erythromycin. Selective underreporting of already established associations cannot be excluded. However, the quantitative analysis of spontaneous reports is particularly difficult when considering well-established adverse effects of drugs, since increased publicity of specific issues leads to much increased reporting, irrespective of whether there is an underlying causal relationship [32]. This has been referred to as notoriety bias [33]. This is clearly the case with statins and rhabdomyolysis [33, 34], due to the worldwide withdrawal of cerivastatin in 2001. Consequently, we examined how the reporting of this potential interaction has changed over time. As seen in Figure 1, rhabdomyolysis with azithromycin and the group of statins was already highlighted in the first quarter of 2000, which suggests that the interaction could have been detected at that time if the Omega measure had been available. The positive Omega value preceding the recent statin publicity strongly shows that this association cannot be explained by the peculiarities of statin reporting observed in recent years. The relative reporting of rhabdomyolysis with azithromycin and statins as a group decreases eventually, although the observed reporting remains higher than expected and the influence was more robust over time up to July 2008. The potential role for routine screening for emerging positive Omega to aid in drug–drug interaction signal detection is clear. The reporting of individual statins and rhabdomyolysis to the database has increased considerably from 2000 and onwards. However, this increase has not been seen for azithromycin alone with rhabdomyolysis. Nevertheless, a drug known to cause a specific adverse reaction is more likely to be reported as suspected than other agents with no or limited established evidence [35]. Consequently, other possible interacting agents may not be reported or identified as suspected or interacting. Therefore there may be patients who appear from the reports to have received solely a statin and had rhabdomyolysis who in fact were co-prescribed azithromycin and the reporter assumed the macrolide was an innocent bystander.
Since muscular and liver function disorders may have a similar mechanism involved as rhabdomyolysis, these associations were also analysed (results not shown). Even though some hepatic reactions were more frequently reported with certain statins and azithromycin, no consistent pattern was observed, probably reflecting the fact that muscular and liver function disorders are general reactions, in contrast to the more specific reaction rhabdomyolysis and may have a number of different causes.
Although spontaneous reporting systems remain the basis in the early detection of rare and previously unknown ADRs, such data cannot be used to establish a definite causal relationship between drugs and reactions [32]. The inherent limitations also include reporting bias, underreporting, lack of information regarding drug therapy and clinical details. Furthermore, there is no available information on the number of persons at risk. Additionally, more data on the reported cases are often needed to be able to evaluate potential drug interaction signals, this when comparing to routine signal detection. For example, detailed information regarding time relationships between the reactions and the medications and laboratory value are required in order to identify overlapping therapy or increased plasma concentrations. Nevertheless, our analysis of VigiBase is indicative of a possible interaction between azithromycin and statins resulting in rhabdomyolysis.
The exact mechanism of statin-induced rhabdomyolysis remains unclear. Pharmacokinetic as well as pharmacodynamic interactions may be involved [36]. When treatment is initiated with a drug that inhibits the metabolism of a statin, by inhibiting cytochrome P450 enzyme activity (mainly CYP3A4), the risk for rhabdomyolysis will increase. Although this mechanism is reported for most of the drug interactions caused by macrolides [37], drugs affecting transporter P-glycoprotein, organic anion transporting polypeptide 1B1 (OATP1B1), or other hepatic uptake transporters, membrane transporters can also affect the disposition of statins [38]. Since azithromycin only is a weak inhibitor of CYP3A4 [37], it is possible that other metabolic or transporter mechanism can explain the drug interaction between statins and azithromycin. Azithromycin has previously been reported to increase the bioavailability of ivermectin, a substrate for the transporter P-glycoprotein and CYP3A4 [39]. The effect of azithromycin on these cell membrane transporters and the possible influence on the disposition of statins need to be further explored.
In conclusion, our study has demonstrated the usefulness of the newly developed disproportionality measure, Omega, in order to identify drug interactions in spontaneous reporting datasets. Case reports are suggestive of a drug interaction between statins and azithromycin with rhabdomyolysis. The limited evidence base, in combination with our results, motivates further studies of this potential interaction. This is particularly important given the widespread use of statins, the desire on occasion to co-prescribe macrolides, and the seriousness of rhabdomyolysis as an adverse reaction.
Competing interests
None declared.
We thank Johan Hopstadius for his help in producing figures and statistics presented here. The authors are indebted also to the National Centres that contributed data. The opinions and conclusions in this study are not necessarily those of the various centres or of the WHO.
REFERENCES
- 1.Lane R, Phillips M. Rhabdomyolysis. BMJ. 2003;327:115–6. doi: 10.1136/bmj.327.7407.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans M, Rees A. Effects of HMG-coa reductase inhibitors on skeletal muscle: are all statins the same? Drug Saf. 2002;25:649–63. doi: 10.2165/00002018-200225090-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bolego C, Baetta R, Bellosta S, Corsini A, Paoletti R. Safety considerations for statins. Curr Opin Lipidol. 2002;13:637–44. doi: 10.1097/00041433-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Molden E, Andersson KS, Jacobsen D. Interactions between statins and macrolide antibiotics. Tidsskr Nor Laegeforen. 2007;127:1660–1. [PubMed] [Google Scholar]
- 5.Thompson Micromedex Database. DrugDex
- 6.Datapharm Communications Ltd. The Medicines Compendium. Electronic Medicine Compendium. Electronic version.
- 7.Pai MP, Graci DM, Amsden GW. Macrolide drug interactions: an update. Ann Pharmacother. 2000;34:495–513. doi: 10.1345/aph.19138. [DOI] [PubMed] [Google Scholar]
- 8.Amsden GW. Macrolides versus azalides: a drug interaction update. Ann Pharmacother. 1995;29:906–17. doi: 10.1177/106002809502900913. [DOI] [PubMed] [Google Scholar]
- 9.Amsden GW, Kuye O, Wei GC. A study of the interaction potential of azithromycin and clarithromycin with atorvastatin in healthy volunteers. J Clin Pharmacol. 2002;42:444–9. [PubMed] [Google Scholar]
- 10.Grunden JW, Fisher KA. Lovastatin-induced rhabdomyolysis possibly associated with clarithromycin and azithromycin. Ann Pharmacother. 1997;31:859–63. doi: 10.1177/106002809703100710. [DOI] [PubMed] [Google Scholar]
- 11.Skrabal MZ, Stading JA, Cannella CA, Monaghan MS. Two cases of rhabdomyolysis associated with high-dose simvastatin. Am J Health Syst Pharm. 2003;60:578–81. doi: 10.1093/ajhp/60.6.578. [DOI] [PubMed] [Google Scholar]
- 12.Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, De Freitas RMA. Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54:315–21. doi: 10.1007/s002280050466. [DOI] [PubMed] [Google Scholar]
- 13.Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJ, Yuen N. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82:157–66. doi: 10.1038/sj.clpt.6100258. [DOI] [PubMed] [Google Scholar]
- 14.Edwards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf. 1994;10:93–102. doi: 10.2165/00002018-199410020-00001. [DOI] [PubMed] [Google Scholar]
- 15.Egberts AC, Meyboom RH, van Puijenbroek EP. Use of measures of disproportionality in pharmacovigilance: three Dutch examples. Drug Saf. 2002;25:453–8. doi: 10.2165/00002018-200225060-00010. [DOI] [PubMed] [Google Scholar]
- 16.van Puijenbroek EP, Egberts AC, Heerdink ER, Leufkens HG. Detecting drug–drug interactions using a database for spontaneous adverse drug reactions: an example with diuretics and non-steroidal anti-inflammatory drugs. Eur J Clin Pharmacol. 2000;56:733–8. doi: 10.1007/s002280000215. [DOI] [PubMed] [Google Scholar]
- 17.Szarfman A, Machado SG, O'Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA's spontaneous reports database. Drug Saf. 2002;25:381–92. doi: 10.2165/00002018-200225060-00001. [DOI] [PubMed] [Google Scholar]
- 18.Noren GN, Sundberg R, Bate A, Edwards IR. A statistical methodology for drug–drug interaction surveillance. Stat Med. 2008;27:3057–70. doi: 10.1002/sim.3247. [DOI] [PubMed] [Google Scholar]
- 19.Lindquist M, Edwards IR. The WHO Programme for International Drug Monitoring, its database, and the technical support of the Uppsala Monitoring Center. J Rheumatol. 2001;28:1180–7. [PubMed] [Google Scholar]
- 20.Strandell J, Bate A, Lindquist M, Edwards IR. Drug interaction signal detection in spontaneous reports. Pharmacoepidemiol Drug Saf. 2007;16(Suppl. 2):S256–540. [Google Scholar]
- 21.Edwards IR, Olsson S. WHO programme – global monitoring. In: Mann RD, Andrews EB, editors. Pharmacovigilance. Chichester: John Wiley & Sons, Ltd; 2002. pp. 169–82. [Google Scholar]
- 22.Bate A, Lindquist M, Edwards IR. The application of knowledge discovery in databases to post-marketing drug safety: example of the WHO database. Fundam Clin Pharmacol. 2008;22:127–40. doi: 10.1111/j.1472-8206.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 23.Ståhl M, Lindquist M, Edwards IR, Brown EG. Introducing triage logic as a new strategy for the detection of signals in the WHO Drug Monitoring Database. Pharmacoepidemiol Drug Saf. 2004;13:355–63. doi: 10.1002/pds.894. [DOI] [PubMed] [Google Scholar]
- 24.Lindquist M. Use of triage strategies in the WHO signal-detection process. Drug Saf. 2007;30:635–7. doi: 10.2165/00002018-200730070-00014. [DOI] [PubMed] [Google Scholar]
- 25.Thakrar BT, Grundschober SB, Doessegger L. Detecting signals of drug–drug interactions in a spontaneous reports database. Br J Clin Pharmacol. 2007;64:489–95. doi: 10.1111/j.1365-2125.2007.02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norén GN, Orre R, Bate A, Edwards IR. Duplicate detection in adverse drug reaction surveillance. Data Mining Knowl Discovery. 2007;14:305–28. [Google Scholar]
- 27.Lindquist M, Stahl M, Bate A, Edwards IR, Meyboom RH. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf. 2000;23:533–42. doi: 10.2165/00002018-200023060-00004. [DOI] [PubMed] [Google Scholar]
- 28.Bentzen N, Bridges-Webb C. An international glossary for general/family practice. Fam Pract. 1995;12:341–69. doi: 10.1093/fampra/12.3.267. [DOI] [PubMed] [Google Scholar]
- 29.Kogan ADOS. Lovastatin-induced acute rhabdomyolysis. Postgrad Med J. 1990;66:294–6. doi: 10.1136/pgmj.66.774.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med. 2005;165:2671–6. doi: 10.1001/archinte.165.22.2671. [DOI] [PubMed] [Google Scholar]
- 31.Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473–94. doi: 10.1128/CMR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyboom RH, Hekster YA, Egberts AC, Gribnau FW, Edwards IR. Causal or casual? The role of causality assessment in pharmacovigilance. Drug Saf. 1997;17:374–89. doi: 10.2165/00002018-199717060-00004. [DOI] [PubMed] [Google Scholar]
- 33.Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007;30:891–8. doi: 10.2165/00002018-200730100-00007. [DOI] [PubMed] [Google Scholar]
- 34.McAdams M, Staffa J, Dal Pan G. Estimating the extent of reporting to FDA: a case study of statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2008;17:229–39. doi: 10.1002/pds.1535. [DOI] [PubMed] [Google Scholar]
- 35.Strandell J, Bate A, Lindquist M, Edwards IR Swedish, Finnish, Interaction X-referencing Drug–Drug Interaction Database (SFINX Group) Drug–drug interactions – a preventable patient safety issue? Br J Clin Pharmacol. 2008;65:144–6. doi: 10.1111/j.1365-2125.2007.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther. 2006;80:565–81. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Westphal JF. Macrolide-induced clinically relevant drug interactions with cytochrome P-450A (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. Br J Clin Pharmacol. 2000;50:285–95. doi: 10.1046/j.1365-2125.2000.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuvonen PJ, Backman JT, Niemi M. Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin. Clin Pharmacokinet. 2008;47:463–74. doi: 10.2165/00003088-200847070-00003. [DOI] [PubMed] [Google Scholar]
- 39.El-Tahtawy A, Glue P, Andrews EN, Mardekian J, Amsden GW, Knirsch CA. The effect of azithromycin on ivermectin pharmacokinetics – a population pharmacokinetic model analysis. PLoS Negl Trop Dis. 2008;2:e236. doi: 10.1371/journal.pntd.0000236. [DOI] [PMC free article] [PubMed] [Google Scholar]


