Abstract
Bone metastasis occurs frequently in advanced prostate cancer (PCa) patients; however, it is not known why this happens. The epidermal growth factor receptor (EGFR) ligand EGF is available to early stage PCa; whereas, TGF-α is available when PCa metastasizes. Since the microenvironment of metastases has been shown to play a role in the survival of the tumor, we examined whether the ligands had effects on cell survival and proliferation in early and late PCa. We used LNCaP cells as a model of early stage, non-metastatic PCa and the isogenic C4-2B cells as a model of late stage, metastatic PCa. We found that the proliferation factor MAPK and the survival factor AKT were differentially activated in the presence of different ligands. TGF-α induced growth of C4-2B cells and not of the parental LNCaP cells; however, LNCaP cells expressing a constitutively active AKT did proliferate with TGF-α. Therefore, AKT appeared to be the TGF-α-responsive factor for survival of the late stage PCa cells. LNCaP cells exposed to EGF produced more osteoprotegerin (OPG), an inhibitor of bone remodeling, than C4-2B cells with TGF-α, which had increased expression of RANKL, an activator of bone remodeling. In concordance, TGF-α-treated C4-2B conditioned medium was able to differentiate an osteoclast precursor line to a greater extent than EGF-treated C4-2B or TGF-α-treated LNCaP conditioned media. Taken together, these results suggest that the switch in EGFR ligand availability as PCa progresses affects cell survival and contributes to bone remodeling.
Keywords: prostate cancer, epidermal growth factor receptor, bone metastasis, cell signaling, TGF-α, EGF
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer of American men. The majority of men with advanced PCa will develop bone metastases to a greater extent than soft tissue metastases. Metastases to the skeleton significantly decrease a patient's quality of life by weakening the bone and causing fractures and nerve damage. The mechanisms that account for the predisposition of PCa to metastasize to bone are not well defined.
Epidermal growth factor receptor (EGFR) is an erbB-family receptor tyrosine kinase found on cells of epithelial origin. EGFR binds many different ligands including EGF, transforming growth factor alpha (TGF-α), beta cellulin, heparin-binding EGF-like factor (HB-EGF), epiregulin, and amphiregulin 1. Several studies looking at protein or RNA expression in PCa progression 2–7 have shown that EGF is the predominant EGFR ligand found in early, localized PCa. As the disease progresses to more advanced stages, TGF-α is more abundant than EGF due to the increase in availability of TGF-α and decrease in EGF. A reason for the shift in the availability of the ligands has not been elucidated.
Since TGF-α is readily available at later stages of PCa, when PCa metastasizes to bone, we hypothesized that TGF-α binding to EGFR contributes to PCa bone metastases. To test the effects of the ligand shift on the ability of PCa cells to initiate tumors in bone, we used a physiologically relevant human PCa system consisting of the isogenic cell lines LNCaP and C4-2B (reviewed in 8). We chose to use these two cell lines in our studies since they are isogenic, LNCaP cells do not grow in bone where C4-2B cells do, and the cells are not grown as xenografts, which, in our hands, are difficult to grow in bone possibly due to cell damage caused by the dissociation process. LNCaP cells are considered early stage human PCa cells with a minimal ability to metastasize in mice. C4-2B cells are a sub-line of the LNCaP cells isolated from a spontaneous metastasis to bone in mice, have a propensity to establish bone metastases in mice, and are considered advanced PCa. We used these two PCa cell lines to test the hypothesis that intracellular signals and bone remodeling proteins that are differentially activated by EGF compared to TGF-α affect PCa bone metastasis.
Even though EGFR is over expressed and constitutively active in a variety of tumors 9 and about 30% of PCa patients have EGFR over expression, many have speculated based on one clinical trial that EGFR does not play a physiologic role in PCa. Clinical trials with gefitinib, an EGFR-specific small molecule inhibitor, have shown no efficacy towards PCa. These data have left some to speculate that EGFR has no role in clinical PCa. However, Shah, et al. 10 have shown that EGFR over expression is strongly associated with advanced PCa but is not associated with any one metastatic site. Along with the data from Shah, et al. 10, preclinical and retrospective clinical data 11–21 indicate that EGFR over expression plays a role in prostate cancer aggressiveness which suggests that targeting EGFR should provide a clinical benefit to PCa patients. The patients in the gefitinib trial were not screened for EGFR over expression; therefore, a response in the patients that did express EGFR may have been easily over looked. Concordant with our hypothesis, erlotinib, an EGFR and HER-2 small molecule inhibitor, has been shown to have efficacy in lung cancer where gefitinib has failed 22. However, the data from a pilot trial for erlotinib in PCa had unclear results 23. Because of the inherent inadequacies in the gefitinib trial and differences between known EGFR inhibitor efficacies in other cancers, EGFR inhibitors require further study for the treatment of PCa.
Results
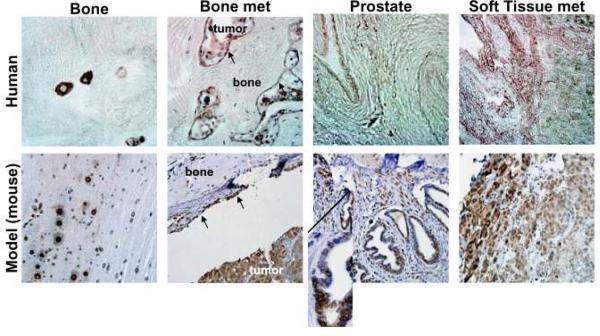
To validate that TGF-α is expressed in human osseous lesions of PCa and in bone in our mouse model system, both clinical tissues from the University of Michigan Rapid Autopsy Program 24 and tissues obtained from mice harboring PCa tumors from either orthotopically or intratibially injected C4-2B cells were stained for TGF-α (Figure 1); antibodies to EGF useful for immunohistochemical studies were not available. Both the human and mouse normal bone had small, distinct regions of TGF-α staining, and TGF-α was found on the interface that delineated the bone surface from PCa tumors. In contrast, soft tissue and bone metastases showed a diffuse staining in both human and mouse. Both the human and mouse normal prostate had little staining except in areas of increased epithelial cell density (Figure 1, inset). The staining was virtually identical between the human and mouse sections demonstrating that our model system recapitulates the human condition.
Figure 1. The C4-2B cell line is a good model for study of advanced prostate cancer.
Tissue from the human rapid autopsy program (top row), tibiae-injected (bone and bone met), and orthotopically injected (prostate and soft tissue met) C4-2B cells (bottom row) was stained for TGF-α. TGF-α was found in normal bone in distinct places, delineating the bone from the bone metastasis (arrows indicate interface), in the bone metastatic tumor, in the epithelial cells of the prostate, and in soft tissue metastases in both human and mouse model samples. Inset in model prostate section shows increased TGF-α in abnormal area of glandular epithelial cells.
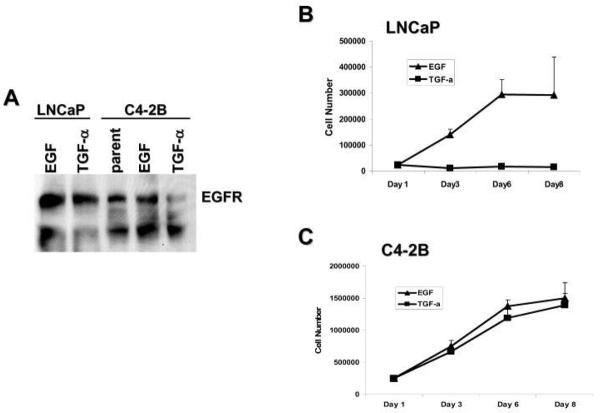
To investigate a functional role for TGF-α in PCa, we first determined that EGFR was present on our model cells, LNCaP and C4-2B. Both cell types contained physiological levels of EGFR (Figure 2A) even after 24 hr treatment with EGF or TGF-α. PCa cells were treated with fresh serum-free medium containing ligand every 48 hr for 3, 5, or 8 days and used for attachment-dependent growth assays. The LNCaP cells could only utilize EGF and ceased to grow in serum-free medium containing TGF-α (Figure 2B). In contrast, C4-2B cells utilized both of the ligands for growth (Figure 2C). The attachment-dependent growth assay data suggest that the EGFR ligand switch affects cell proliferation.
Figure 2. C4-2B cells can utilize TGF-α for growth, LNCaP cells cannot.
(A) Western blot of LNCaP and C4-2B cells treated with EGF or TGF-α probed for EGFR. (B) Attachment-dependent growth assay of LNCaP cells treated with EGF, TGF-α, or amphiregulin. Cells were plated, left for 24 hr, then treated at regular intervals with EGF, TGF-α, or amphiregulin. Cell counts were taken on the indicated days. (C) Attachment-dependent growth assay of C4-2B cells treated with EGF or TGF-α. Cells were plated, left for 24 hr, then treated every 48 hr with fresh serum-free medium containing EGF or TGF-α. Cell counts were taken on the indicated days.
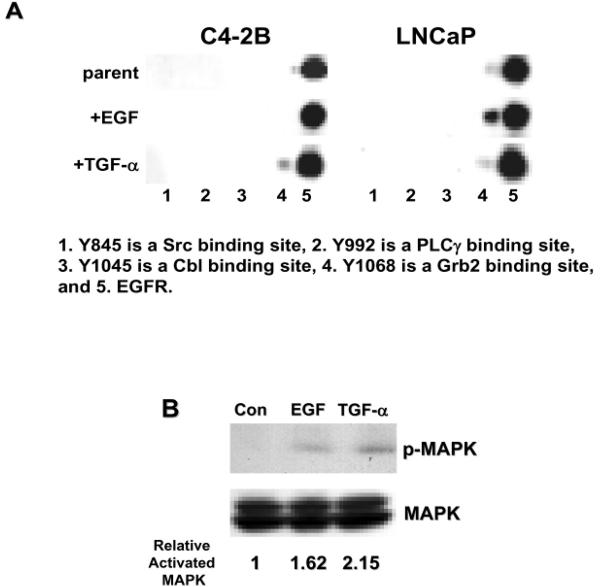
Cell proliferation and survival is controlled by cell signaling, which can originate from receptors on the cell surface. When EGFR binds ligand, the receptor differentially transautophosphorylates selected tyrosines in the cytoplasmic tail. The resulting tyrosine phosphorylation pattern determines which pathways are activated. To determine differential EGFR phosophorylation among the LNCaP and C4-2B cells in the presence of EGF and TGF-α, we used multiplex western blotting technology (Figure 3A). Y1068, the Grb2 binding site, was heavily phosphorylated in LNCaP cells in the presence of EGF but was minimally phosphorylated in the presence of TGF-α, consistent with the LNCaP cells being able to proliferate in EGF and not in TGF-α. Interestingly, EGFR in C4-2B cells in the presence of TGF-α had phosphorylated Y1068, but EGFR from C4-2B cells in EGF did not; even though, C4-2B cells could proliferate in EGF suggesting another proliferation mechanism not investigated here.
Figure 3. Different EGFR ligands cause differential cell signaling.
(A) Multiplex western blot of EGFR immunoprecipitations from C4-2B or LNCaP cells treated with different ligands were probed for phosphorylation on cytoplasmic tyrosines. 1.Y845 (Src binding), 2.Y992 (PLCγ binding), 3.Y1045 (Cbl binding), 4.Y1068 (Grb 2 binding), 5. EGFR. (B) Western blot of C4-2B cells treated wih EGF or TGF-α and probed for phosphor-p42/44MAPK and p42/44MAPK.
Phosphorylated Y1068 on EGFR binds to the adapter molecule Grb2. Grb 2 can then activate the Ras-Raf-MAPK pathway. MAPK is generally thought to be a proliferation factor 25,26. To verify that MAPK was indeed activated as suggested by the multiplex EGFR phosphorylation data, C4-2B cells treated with EGF or TGF-α were probed for MAPK and activated MAPK. The amount of phosphorylated MAPK was undetectable in our low passage (p17) LNCaP cells, even when the cells were stimulated with EGF (data not shown). Concordant with our EGFR phosphorylation data, we observed an increase in activated MAPK in C4-2B cells treated with TGF-α compared to untreated cells or to C4-2B cells treated with EGF (Figure 3B). Even though C4-2B cells did not have phosphorylation of Y1068 on EGFR in the presence of EGF, MAPK is still activated in these cells, indicating another mechanism for MAPK activation.
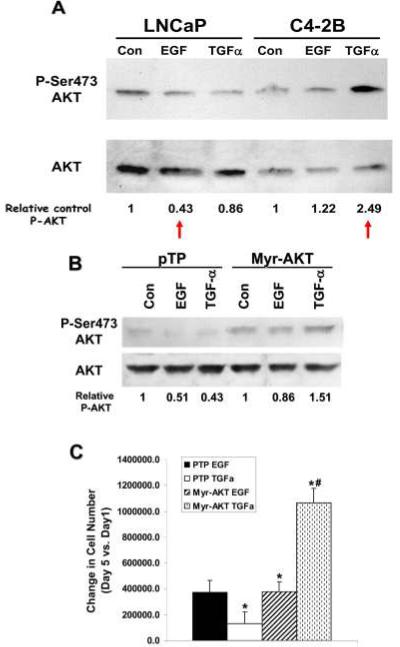
To identify other pathways that could play a role in PCa cell survival in the bone, we conducted a multiplex western screen for activation of 20 potential signaling molecules (data not shown). We identified 5 proteins which were differentially expressed or activated between LNCaP cells in EGF versus C4-2B cells which had been incubated with TGF-α (Table 1). We did find proteins that were differentially activated in the same cells that were treated with different ligands, but since we were interested in the differences present in early stage PCa versus late stage PCa, here we report only the differences between LNCaP cells in EGF versus C4-2B cells in TGF-α. Among the proteins found to have differential activation was AKT. AKT is a serine/threonine kinase that plays a role in cell survival 27. To verify the multiplex results, we ran conventional western analyses for AKT and phospho-S473-AKT (Figure 4A). LNCaP cells in the presence of EGF had 57% less AKT activity (by serine 473 phosphorylation) than LNCaP cells without EGFR ligand supplementation (control). C4-2B cells in the presence of TGF-α had 249% more AKT activity compared to control. Thus, the survival factor AKT is activated in metastatic cells with late stage ligand. These data suggest that survival of PCa metastases is facilitated by TGF-α-mediated EGFR activation of AKT.
Table 1.
Proteins differentially regulate by signaling pathways initiated by EGFR ligands as determined by multiplex western analysis. The plus symbol indicates presence on multiplex western blot.
| Cells plus ligand | Cell Signaling Proteins | Bone remodeling Proteins | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AKT | p-PDK11 | p-PKC pan2 | p-PKC α/β3 | p-PKC δ4 | RANKL | OPG | SMAD1 | SMAD5 | SMAD8 | |
| LNCaP/EGF | − | − | − | + | + | − | + | + | + | − |
| C4-2B/TGF-α | + | + | + | − | − | + | − | + | − | + |
Serine241
Serine 660
Threonine 410/403
Serine 643
Figure 4. AKT activation promotes late stage PCa cell proliferation.
(A) Protein blots of whole cell lysate proteins from LNCaP and C4-2B cells treated with EGF or TGF-α probed for phosphorylated serine 473 on AKT (top) and total AKT (bottom). Amounts of phospho-AKT relative to control are given below the blots. “Control (Con)” is the cells in serum-free medium without supplemented EGFR ligands. (B) Protein blot of proteins from LNCaP cells containing constitutively active AKT (Myr-AKT) or vector (pNG) probed for AKT. Relative amounts of phospho-AKT are given below the blots (C) Attachment-dependent growth assay of LNCaP cells containing constitutively active AKT (Myr-AKT) or vector (pNG) in the presence of EGF or TGF-α. Cells were plated, left for 24 hr, then treated at regular intervals with EGF or TGF-α. Cell counts were taken on the indicated days one (plating efficiency) and five. Since the plating efficiencies for the vector-only (pTP) cells were vastly different compared to the Myr-AKT cells, the differences in cell number between day 5 and day 1 are graphed to show the differences in the proliferation rates. Experiment was repeated twice. The differences for Myr-AKTcells in EGF versus Myr-AKT cells in TGF-α were not significant. The p-value was <0.007 for *PTP cells in EGF vs. PTP cells in TGF-α and Myr-AKT cells in EGF or TGF-α. The p-value was <0.02 for #PTP cells vs Myr-AKT cells in TGF-α.
To determine if AKT was the factor that enabled the C4-2B cells to survive in TGF-α, we transfected LNCaP cells with a constitutively active form of AKT, Myr-AKT 28. We confirmed the increased activation of AKT in the Myr-AKT expressing cells by western blot analysis probed for phospho-S473 AKT (Figure 4B). We then subjected the Myr –AKT expressing cells and the vector-only control cells to attachment-dependent growth assays. The cells were plated, treated every 48 hr with EGF or TGF-α in fresh serum-free medium, and counted on days 3 and 5 after plating (Figure 4C). Since the cells with the vector and the cells with Myr-AKT had vastly different plating efficiencies (data not shown), we compared the differences in cell growth on the 5th day to the plating efficiency (Figure 4D). LNCaP cells expressing constitutively active AKT were able to grow in the presence of TGF-α (Figure 4C), unlike the parental cells (Figure 2B). Control-transfected LNCaP cells were unable to grow in TGF-α, similar to the parental cells (Figure 2A). Student T-tests showed that the Myr-AKT cells in TGF-α had increased proliferation capacity compared to cells containing vector and treated with either EGF or TGF-α (p=0.002 and 0.013, respectively). A student T-test also showed that the Myr-AKT cells grow just as well in EGF as in TGF-α (p=0.55); therefore, constitutively active AKT conferred the ability to grow in response to TGF-α upon the LNCaP cells. We attempted to do the reciprocal experiment: knocking out AKT in C4-2B cells using shRNA to AKT and subjecting them to attachment-dependent growth assay analysis. However, C4-2B cells with a blockage of AKT died at the first passage, so the experiment was not done (data not shown).
Since TGF-α is present during late stage PCa and late stage PCa metastasizes primarily to the bone, we determined if TGF-α influenced the ability of C4-2B to remodel bone. We conducted multiplex western analysis to screen for differences in bone remodeling proteins between LNCaP in EGF versus C4-2B in TGF-α (data not shown). We identified 5 proteins that were differentially expressed (Table 1). Two of the differentially expressed proteins were the receptor activator of NFκB ligand (RANKL) and osteoprotegerin (OPG). In bone remodeling, osteoblast cells make new bone and release RANKL which binds to its receptor, RANK, on osteoclast precursors causing the cells to mature and absorb bone 29. OPG is a soluble, decoy RANKL receptor which controls the effects of RANKL 30.
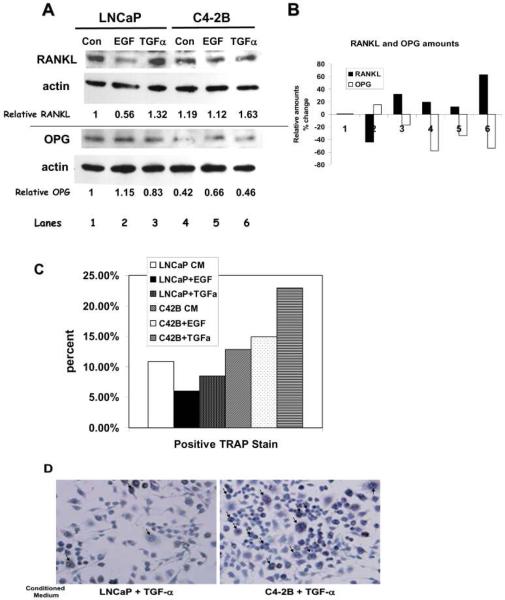
To verify the multiplex results, we conducted conventional western analysis for OPG and RANKL (Figure 5A and B). There was a 44% decrease of RANKL in LNCaP cells plus EGF and a 163% increase with C4-2B plus TGF-α. Concordant with the RANKL data, there also was a 115% increase of OPG with LNCaP cells plus EGF and a 54% decrease of OPG in C4-2B cells plus TGF-α. Therefore, the most RANKL and the least OPG were expressed by C4-2B cells in the presence of TGF-α and the least RANKL and the most OPG were expressed by LNCaP in the presence of EGF. These data show that the early stage cells (LNCaP) with the early stage ligand (EGF) produced more inhibitor of bone remodeling (OPG) than bone metastatic cells (C4-2B) with late stage ligand (TGF-α), which produced more activator of bone remodeling (RANKL).
Figure 5. LNCaP cells with EGF have more OPG and less RANKL; C4-2B cells with TGF-α have more RANKL and less OPG.
(A) Protein blots of proteins from PCa cells treated with either EGF or TGF-α probed for RANKL/actin (top) or OPG/actin (bottom). RANKL and OPG amounts shown are relative to actin then made relative to LNCaP control. (B) Graph of relative amounts of RANKL and OPG taken from the densitometry calculations in panel A. (C) TRAP stain on RAW cells treated with conditioned media from LNCaP or C4-2B cells that were treated with EGF or TGF-α. Untreated LNCaP and C4-2B conditioned media were used as controls. Straight serum-free medium with either EGF or TGF-α was also used and showed no response. Experiment was repeated four times; a representative experiment is shown. (D) Representative pictures from a TRAP stain experiment. Cells were serum starved for 24hr then incubated with ligand for 24 hours. Conditioned medium was placed on serum starved RAW cells for 24 hr before staining. Pictured are RAW cells in the presence of LNCaP/TGF-α medium (on left) and C4-2B/TGF-α medium (on right). Arrows point to some examples of TRAP positive staining.
Our data showed that EGFR ligands affected the expression of proteins that have effects on osteoclast differentiation, suggesting that early stage ligands trigger a blocking response to osteolytic bone remodeling and late stage ligands enhance osteoclast differentiation. Thus, we determined if conditioned medium from the cell lines that were grown with the different ligands could drive osteoclast differentiation. C4-2B cell medium from the TGF-α treatment mediated the highest level of osteoclast differentiation as seen by TRAP stain (p=0.02); whereas, the cells treated with conditioned medium from EGF-treated LNCaP cells showed the least amount of differentiation (p=0.08) (Figure 5C and D). The TRAP experiment was repeated four times with the same trends, the most TRAP staining was seen with TGF-α treated C4-2B cells, but with different overall numbers, making the errors seem larger than they were. Taken together, our data suggest that late stage PCa cells with late stage ligand are able to drive bone remodeling.
Discussion
About 30% of PCa patients have EGFR over expression, which correlates with PCa progression 10. Preclinical and retrospective clinical data 10–15, 17–21 indicate that EGFR plays a role in PCa progression. Thus, we were interested in the effects of EGFR ligands on PCa progression, specifically in bone metastases, which occurs in almost 80% of advanced stage prostate cancer patients 31. TGF-α is available to late stage PCa when most late stage PCa metastasizes to the bone; therefore, we determined if TGF-α played a role in PCa survival and in bone remodeling. Here we have shown that TGF-α binding to EGFR activates survival pathways such as AKT and MAPK and that bone metastatic C4-2B PCa cells can utilize both TGF-α and EGF for proliferation; whereas, nonmetastatic LNCaP cells could proliferate in EGF. The LNCaP proliferation data suggest that EGF and TGF-α mediated-activation of EGFR activate different intra-cellular signals. Since the C4-2B cells in the presence of TGF-α can grow and have activated AKT, we expressed a constitutively active AKT in LNCaP cells. The LNCaP cells that contained the constitutively active AKT could proliferate in the presence of TGF-α. Taken together, the data presented here suggest that AKT activation mediated by TGF-α binding to EGFR allows advanced PCa cells to proliferate. Since TGF-α is found in the bone, it is conceivable that the bone TGF-α mediates PCa cell survival and proliferation through activation of AKT.
We have also shown that TGF-α mediates the release of the osteoclast differentiation factor RANKL; whereas, EGF-mediated signaling releases OPG to block osteoclast differentiation. Therefore, late stage cells with late stage ligand express factors which promote a lytic reaction in bone and early stage cells with early stage ligand express factors which block lytic bone remodeling. In concordance with our bone remodeling protein expression data, TGF-α-treated C4-2B conditioned medium was able to promote maturation of an osteoclast precursor cell line better than EGF-treated C4-2B or TGF-α-treated LNCaP conditioned media.
Taken together, our data show that TGF-α treated C4-2B cells can mature cells that break down bone. However, C4-2B tumors in bone tend to be mostly osteoblastic with a small lytic component. Hall, et al. 32–34 has put forth a model of bone metastasis in which a lytic reaction may be needed to establish the tumor before the osteoblastic reaction can occur. Consistent with Hall's model, we have shown that soluble RANK (sRANK), an analog of OPG, can block C4-2B-mediated bone remodeling in tibiae of mice by blocking osteoclast-mediated bone destruction 35, indicating that the lytic action of osteoclasts is necessary for C4-2B bone metastasis formation. Therefore, our data, which show that C4-2B cells in TGF-α can force osteoclast differentiation, suggests that C4-2B cells themselves produce the components necessary to mediate the lytic reaction.
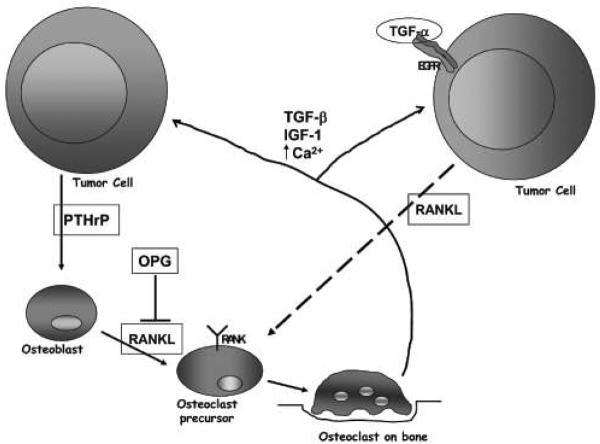
The vicious cycle model of tumor growth in bone 36–38 states that the tumor cell releases parathyroid hormone related protein (PTHrP) (Figure 6). PTHrP then causes the osteoblast precursors to mature and release RANKL. RANKL binds to RANK on the surface of osteoclasts, causing them to mature and reabsorb bone. In areas of reabsorbed bone, TGF-α and other growth factors are released. These growth factors are used by the tumor cell to proliferate, and the cycle starts again.
Figure 6. Vicious cycle of tumor metastasis bone remodeling.
Tumor cell on the left is initiating the classic bone remodeling vicious cycle as described in the text. Tumor cell on the right has EGFR activation by TGF-α initiating the vicious cycle at the point of the osteoclast precursor as our preliminary data suggests.
Our data suggest that the lytic reaction in C4-2B tumor formation in bone is initiated by the tumor cells themselves and not by osteoblast precursors. Thus, we are putting forth a model where the apex of the vicious cycle is the activation of EGFR by TGF-α which results in the release of RANKL (Figure 6), suggesting that osteoclast differentiation is directly mediated by the tumor cell in response to TGF-α. However, the source of TGF-α is still in question. We have observed TGF-α staining in normal bone, at the tumor bone interface, and in the bone metastasis. We hypothesize that the TGF-α utilized by the bone metastasis is present in the microenvironment since TGF-α message is found in LNCaP cells (data not shown); however, the question of whether the TGF-α utilized by PCa cells is endogenously or exogenously produced is the subject of further studies.
Although 30% of PCa have EGFR over expression, our studies were obtained with cells that have normal expression of EGFR. Therefore, our data indicate that normal EGFR activation by TGF-α contributes to bone metastasis and that over expression of EGFR may facilitate bone metastasis. Shah, et al. 10 showed that EGFR expression was strongly correlated with hormone-refractory status. Since 80% of hormone-refractory PCa produce bone metastases, it is possible that EGFR over expression can drive bone metastases.
The data presented in this manuscript suggest that PCa cells exposed to TGF-α are able to survive, proliferate, and mediate remodeling in bone. The involvement of EGFR in the initiation and survival of PCa bone metastasis suggests that EGFR-specific inhibitors should abrogate PCa bone metastasis. Based on our data, we are now studying the use of shRNA to EGFR and of EGFR inhibitors to inhibit PCa bone metastases. If EGFR-specific treatments are successful, use of EGFR inhibition in the clinic for bone metastatic PCa will be an improvement over the current situation, where bone metastases are incurable.
Materials and Methods
Cell culture
LNCaP cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum and 1x penicillin/streptomycin. C4-2B cells, isolated from bone-metastasizing LNCaP cells 39,40, were maintained in T-medium (80% DMEM-20% Ham's F12 (GIBCO), 5μg/ml Insulin, 13.6 pg/ml triiodothyronine, 5μg/ml transferrin, 0.25 μg/ml biotin, 25 μg/ml adenine (Sigma, St. Louis, MO), 1x penicillin/streptomycin and 5% FBS). For experiments using growth factors, cells were serum starved for 24 hr in Ham's F-12 medium containing 0.1% bovine serum albumin, 0.5 μg/ml fungizone, 5 μg/ml gentamycin, 5 mM ethanolamine, 10 mM HEPES, 5 μg/ml transferrin, 10 μM T3, 50 μM selenium, and 1 μg/ml hydrocortisone. 1nM TGF-α or 10 ng/ml EGF were added for 24 hr after the serum starvation and before the measurement.
Myr-AKT expressing HME cells 28 were grown in Ham's F12 media supplemented with 0.1% bovine serum albumin, 0.5 μg/ml fungizone, 5 μg/ml gentamycin, 5 mM ethanolamine, 10 mM HEPES, 5 μg/ml transferrin, 10 μM T3, 50 μM selenium, and 1 μg/ml hydrocortisone and 5 μg/ml insulin. pTP cell 41 medium was the same as Myr-AKT medium but was further supplemented with 10 ng/ml EGF.
IHC
Tissues were isolated as per protocols approved by the University of Michigan Animal Care and Use Committee and the University of Michigan Internal Review Board. If necessary, tissues were decalcified in Cal-Ex II (Fisher Scientific, Hampton, NH). All tissues were paraffin embedded and histological sections were stained for TGF-α (Catalog #905-579, Assay Designs, Ann Arbor, MI).
Attachment-dependent growth assay s
Cells were plated at 1.0 × 104 in triplicate in six-well plates. Plating efficiencies were obtained one day after plating, after which ligands, if appropriate, were added. Cells were then counted 3 times over the time course for the individual experiments. All cell counts were performed using a Coulter model Z1 (Coulter Corporation, Miami, FL). All growth curves were done in triplicate and repeated at least twice.
Western analysis
Cells were lysed in a buffer containing 20 mM Tris·HCl, pH 8.0, 137 mM NaCl, 1% NP-40, 10% glycerol, 1 mM Na3VO4, 1 mM PMSF, 1% aprotinin, and 20 μg/ml leupeptin. Protein concentrations were equalized using the Løwry method. For whole cell lysates, Laemmeli sample buffer was added and the samples were boiled. Equal amounts of protein were separated by SDS-PAGE. The proteins were blotted to PVDF membranes and probed for AKT (Catalog #9272, Cell Signaling Technologies, Danvers, MA) and phospho-AKT (Catalog #9271, Cell Signaling), p42/44 MAPK (Catalog #9102, Cell Signaling Technologies) and phospho-p42/44 MAPK (Catalog #4370, Cell Signaling Technologies), RANKL (Catalog #5557-100, BioVision, Mountain View, CA ) and OPG (Catalog # 5312-100, BioVision) or EGFR (Catalog #28-8763, Zymed/Invotrogen, Carlsbad, CA). All blots were repeated at least twice.
Multiplex western analysis
Whole cell lysates were prepared as above. Lysates were separated on 10%, one-well SDS-PAGE gels. Proteins were blotted to PVDF, the membranes were blocked, and the membranes were put into a Miniblotter 28 (Immunetics, Cambridge, MA) and probed with EGFR-phosphorylation specific antibodies or with 20 different cell signaling antibodies that were obtained as groups from Cell Signaling Technologies (Catalog #9922 (EGFR); Catalog #9912, #9910, #9931, #9916). Proteins were visualized by chemiluminescence after secondary HRP antibody incubation.
TRAP staining
RAW 264.7 cells were maintained in RPMI 1640 medium containing 10% FBS and antibiotics on non-tissue culture coated plates. Cells were seeded onto chamber slides (2 wells per slide) and fed with conditioned media for 24 hr. Cells were stained according to manufacturers instructions (Catalog # 387A-1KT, Sigma Chemical, St. Louis, MO). Briefly, cells were fixed then stained with 1:1 mix of Fast Garnet GBC Base solution and Sodium Nitrate Solution at 37°C for 1 hr. Slides were rinsed in dH2O and counterstained with for 2 min with Hematoxylin Solution, Gill No.3 then rinsed in tap H2O and viewed under a phase microscope.
Acknowledgements
These experiments were funded, in part, by National Institutes of Health grant CA098513.
References
- 1.Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- 2.Seth D, Shaw K, Jazayeri J, Leedman PJ. Complex post-transcriptional regulation of EGF-receptor expression by EGF and TGF-alpha in human prostate cancer cells. Br. J. Cancer. 1999;80:657–669. doi: 10.1038/sj.bjc.6690407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher HI, et al. Changing pattern of expression of the epidermal growth factor receptor and transforming growth factor alpha in the progression of prostatic neoplasms. Clin. Cancer Res. 1995;1:545–550. [PubMed] [Google Scholar]
- 4.Liu XH, Wiley HS, Meikle AW. Androgens regulate proliferation of human prostate cancer cells in culture by increasing transforming growth factor-alpha (TGF-alpha) and epidermal growth factor (EGF)/TGF-alpha receptor. J. Clin. Endocrinol. Metab. 1993;77:1472–1478. doi: 10.1210/jcem.77.6.8263129. [DOI] [PubMed] [Google Scholar]
- 5.Myers RB, Kudlow JE, Grizzle WE. Expression of transforming growth factor-alpha, epidermal growth factor, and epidermal growth factor receptor in adenocarcinoma of the prostate and benign prostatic hyperplasia. Mod. Pathol. 1993;6:733–737. [PubMed] [Google Scholar]
- 6.Ching KZ, et al. Expression of mRNA for epidermal growth factor, transforming growyh factor-alpha, and their receptor in human prostate tissue and cell lines. Mol. Cell. Biochem. 1993;126:151–158. doi: 10.1007/BF00925693. [DOI] [PubMed] [Google Scholar]
- 7.Torring N, Hansen FD, Sorensen BS, Orntoft TF, Nexo E. Increase in amphiregulin and epiregulin in prostate cancer xenograft after androgen deprivation-impaact of specific HER1 inhibition. The Prostate. 2005;64:1–8. doi: 10.1002/pros.20214. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis CJ, Lin S-H. Osteoblasts in prostate cancer metastasis to bone. Nature Reviews Cancer. 2005;5:21–28. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- 9.Gusterson B. Identification and interpretation of epidermal growth factor and c-erbB-2 overexpression. Eur J Cancer. 1992;28:263–267. doi: 10.1016/0959-8049(92)90429-6. [DOI] [PubMed] [Google Scholar]
- 10.Shah RB, Ghosh D, Elder JT. Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: correlation with androgen independence. Prostate. 2006;66:1437–1444. doi: 10.1002/pros.20460. [DOI] [PubMed] [Google Scholar]
- 11.Mimeault M, Pommery N, Henichart JP. New advances on prostate carcinogenesis and therapies: involvement of EGF-EGFR transduction system. Growth Factors. 2003;21:1–14. doi: 10.1080/0897719031000094921. [DOI] [PubMed] [Google Scholar]
- 12.Mimeault M, et al. Combined targeting of epidermal growth factor receptor and hedgehog signaling by gefitinib and cyclopamine cooperatively improves the cytotoxic effects of docetaxel on metastatic prostate cancer cells. Mol Cancer Ther. 2007;6:967–978. doi: 10.1158/1535-7163.MCT-06-0648. [DOI] [PubMed] [Google Scholar]
- 13.Mimeault M, et al. Novel combination therapy against metastatic and androgen-independent prostate cancer by using gefitinib, tamoxifen and etoposide. Int J Cancer. 2007;120:160–169. doi: 10.1002/ijc.22268. [DOI] [PubMed] [Google Scholar]
- 14.Normanno N, Maiello M, De Luca A. Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs): simple drugs with a complex mechanism of action? J Cell Physiol. 2003;194:13–19. doi: 10.1002/jcp.10194. [DOI] [PubMed] [Google Scholar]
- 15.Normanno N, Bianco C, De Luca A, Maiello M, Salomon D. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment. Endocr Relat Cancer. 2003;10:1–21. doi: 10.1677/erc.0.0100001. [DOI] [PubMed] [Google Scholar]
- 16.Festuccia C, et al. Epidermal growth factor modulates prostate cancer cell invasiveness regulating urokinase-type plasminogen activator activity. EGF-receptor inhibition may prevent tumor cell dissemination. Thromb Haemost. 2005;93:964–975. doi: 10.1160/TH04-09-0637. [DOI] [PubMed] [Google Scholar]
- 17.Festuccia C, et al. Additive antitumor effects of the epidermal growth factor receptor tyrosine kinase inhibitor, gefitinib (Iressa), and the nonsteroidal antiandrogen, bicalutamide (Casodex), in prostate cancer cells in vitro. Int. J. Cancer. 2005;115:630–640. doi: 10.1002/ijc.20917. [DOI] [PubMed] [Google Scholar]
- 18.Angelucci A, et al. Suppression of EGF-R signaling reduces the incidence of prostate cancer metastasis in nude mice. Endocr Relat Cancer. 2006;13:197–210. doi: 10.1677/erc.1.01100. [DOI] [PubMed] [Google Scholar]
- 19.Legrier M, et al. Potentiation of antitumour activity of docetaxel by combination with trastuzumab in a human prostate cancer xenograft model and underlying mechanisms. Br J Cancer. 2007;96:269–276. doi: 10.1038/sj.bjc.6603553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formento P, et al. Gefitinib-trastuzumab combination on hormone-refractory prostate cancer xenograft. Eur J Cancer. 2005;41:1467–1473. doi: 10.1016/j.ejca.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Kim S, et al. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor-associated endothelial cells for therapy of androgen-independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003;9:1200–1210. [PubMed] [Google Scholar]
- 22.Wong A, et al. Evidence for disease control with erlotinib after gefitinib failure in typical gefitinib-sensitive Asian patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:400–404. doi: 10.1097/JTO.0b013e318168c801. [DOI] [PubMed] [Google Scholar]
- 23.Wilding G, Soulie P, Trump D, Das-Gupta A, small E. Results from a pilot phase 1 trial of Gefitinib combined with docetaxel and Estramustine in patients with hormaone refractory prostate cancer. Cancer. 2006;106:1917–1924. doi: 10.1002/cncr.21831. [DOI] [PubMed] [Google Scholar]
- 24.Rubin MA, et al. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin. Cancer Res. 2000;6:1038–1045. [PubMed] [Google Scholar]
- 25.Oliver B, Sha'afi R, JJ H. Transforming growth factor-alpha and epidermal growth factor activate mitogen-activated protein kinase and its substrates in intestinal epithelial cells. Proc Soc Exp Biol Med. 1995;210:162–170. doi: 10.3181/00379727-210-43936. [DOI] [PubMed] [Google Scholar]
- 26.Marte B, et al. NDF/heregulin activates MAP kinase and p70/p85 S6 kinase during proliferation or differentiation of mammary epithelial cells. Oncogene. 1995;10:167–175. [PubMed] [Google Scholar]
- 27.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 28.Woods Ignatoski KM, Livant DL, Markwart S, Grewal NK, Ethier SP. The role of PI3'kinase and its downstream signals in erbB-2-mediated transformation. Mol Cancer Res. 2003;1:551–560. [PubMed] [Google Scholar]
- 29.Udagawa N, et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone. 1999;25:517–523. doi: 10.1016/s8756-3282(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 30.Itonaga I, Sabokbar A, Murray D, Athanasou N. Effect of osteoprotegerin and osteoprotegerin ligand on osteoclast formation by arthroplasty membrane derived macrophages. Ann Rheum Dis. 2000;59:26–31. doi: 10.1136/ard.59.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah RB, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 2004;64:9209–9216. doi: 10.1158/0008-5472.CAN-04-2442. [DOI] [PubMed] [Google Scholar]
- 32.Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Research. 2005;65:7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- 33.Hall C, Kang S, MacDougald O, Keller E. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97:661–672. doi: 10.1002/jcb.20735. [DOI] [PubMed] [Google Scholar]
- 34.Hall C, Keller E. The role of Wnts in bone metastases. Cancer Metastasis Rev. 2006 doi: 10.1007/s10555-006-9022-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Woods Ignatoski K, et al. RANKL inhibition is an effective adjuvant for docetaxel in a prostate cancer bone metastases model. The Prostate. 2008;68:820–829. doi: 10.1002/pros.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chirgwin JM, Mohammad KS, Guise T, Guise A. Tumor-bone cellular interactions in skeletal metastases. J Musculoskelet Neuronal Interact. 2004;4:308–318. [PubMed] [Google Scholar]
- 37.Mundy G. Preclinical models of bone metastases. Semin Oncol. 2001;28:2–8. doi: 10.1016/s0093-7754(01)90225-8. [DOI] [PubMed] [Google Scholar]
- 38.Mundy G. Mechanisms of bone metastasis. Cancer. 1997;80:1546–1556. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 39.Wu HC, et al. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 40.Thalmann GN, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Research. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 41.Woods Ignatoski KM, LaPointe AJ, Radany EH, Ethier SP. ErbB-2 overexpression in human mammary epithelial cells confers growth factor independence. Endocrinology. 1999;140:3615–3622. doi: 10.1210/endo.140.8.6939. [DOI] [PubMed] [Google Scholar]