Abstract
Background
Elevated coronary artery calcium (CAC) is a marker for increase risk of coronary heart disease (CHD). While the majority of CHD events occur among individuals with advanced CAC, CHD can also occur in individuals with little or no calcified plaque. In this study, we sought to evaluate the characteristics associated with incident CHD events in the setting of minimal (score ≤10) or absent CAC (score of zero).
Methods
Asymptomatic participants in the Multi-Ethnic Study of Atherosclerosis (MESA) (N=6,809), were followed for occurrence of all CHD events (including myocardial infarction(MI), angina, resuscitated cardiac arrest, or CHD death) and hard CHD events (MI or CHD death). Time to incident CHD was modeled using age-and gender-adjusted Cox regression.
Results
The final study population consisted of 3,923 MESA asymptomatic participants (mean age: 58±9years,39% males) had with CAC scores of 0-10. Overall no detectable CAC was seen in 3415 individuals, whereas 508 had CAC scores of 1-10. During follow up (median 4.1 years) there were 16 incident hard events, and 28 all CHD events in individuals with absent or minimal CAC. In age, gender, race and CHD risk factors adjusted analysis, minimal CAC (1-10) was associated with an estimated 3-fold greater risk of a hard CHD event (HR: 3.23, 95% CI: 1.17-8.95), or of all CHD event (HR: 3.66, 95% CI 1.71-7.85) compared to those with CAC=0. Former smoking (HR=3.57; 1.08-11.77), current smoking (HR=4.93; 1.20-20.30), and diabetes (HR=3.09; 1.07-8.93) were significant risk factors for events in those with CAC=0.
Conclusion
Asymptomatic persons with absent or minimal CAC are at very low risk of future cardiovascular events. Individuals with minimal CAC (1-10) were significantly increased to three fold increased risk for incident CHD events relative to those with CAC scores of zero.
Keywords: Computed Tomography, Prognosis, Coronary Artery Calcification, Atherosclerosis, Coronary Calcium Score, Cardiac Events
Introduction
Efforts to predict which individuals are at low or high-risk for coronary artery disease (CAD) events have occupied a prominent role in cardiology and in primary medical care over the last 50 years. Major reasons underlying this clinical focus include the high rates of morbidity and mortality in all industrialized nations due to CAD,1 as well as the observation that about half of individuals who present with clinical CAD have an initial presentation of either unexpected myocardial infarction (MI) or sudden death.2 Thus, identifying which asymptomatic patients might benefit from intensive preventive interventions to avert these sudden and unexpected outcomes of CAD is a recommended practice in various clinical guidelines.3
Coronary artery calcium (CAC) measurement has emerged as a noninvasive test that can risk stratify asymptomatic individuals into low-, intermediate-, and high-risk groups.4 In a recent meta-analysis absence of CAC was found to be associated with a very low risk of future cardiovascular events in asymptomatic as well symptomatic individuals.5 Nonetheless, previous studies have shown that occasionally individuals with low levels of CAC, or even some individuals with CAC=0, have gone on to develop clinical coronary events.4-6 Thus, despite an excellent prognosis in most individuals with either no detectable or low levels of CAC, clinical characteristics in the setting of these unexpected clinical events are not well defined. In addition, a recent study suggested that minimal CAC (scores 1-10) was significantly associated with all-cause mortality among a large cohort of self referred asymptomatic individuals mainly consisting of Caucasian ethnicity6. However whether the presence of minimal calcification (scores 1-10) is associated with higher CHD events in a prospective multi-ethnic population is not known.
To address these unanswered questions, in this prospective multi-ethnic cohort study of asymptomatic individuals initially free of cardiovascular disease at baseline, we sought to elucidate the risk factors for incident CHD events, and to assess whether individuals with minimal CAC (1-10) have similar or higher CHD event rates compared to those with CAC scores of zero.
METHODS
Recruitment and Baseline Examination
The MESA cohort6 is a longitudinal, population-based study of 6,814 men and women, free of clinical cardiovascular disease, aged 45-84 at baseline recruited from six Field Centers: Baltimore, MD ; Chicago, IL; Forsyth County, NC ; Los Angeles, CA ; New York, NY ; and St. Paul, MN. Specific racial/ethnic groups enrolled included white, black, Hispanic and Chinese. Approximately 50% of the participants enrolled were female. Details of the MESA recruitment strategy are contained elsewhere.6 The baseline visit took place between July 2000 and September 2002. The study was approved by Institutional Review Boards at each site and all participants gave written informed consent. The purpose of the study is to examine the risk factors and progression of subclinical cardiovascular disease. The design of the study has been described in detail previously,7 but we describe the collection of pertinent variables here. Medical history, anthropometric measurements, and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000 to August 2002). Information about age, sex, ethnicity, and medical history were obtained by questionnaires. Current smoking was defined as having smoked a cigarette in the last 30 days, whereas former smoker defined as individual who is not currently smoker but had smoked ≥ 100 cigarettes in his/her lifetime. Alcohol use was defined as never, former, or current. Diabetes was defined as a fasting glucose ≥126 mg/dL or on hypoglycemic medication. Use of antihypertensive and other medications was based on clinic staff entry of prescribed medications. Resting blood pressure was measured 3 times in the seated position using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, FL), and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of medication together with a self-reported diagnosis of high blood pressure. Total and HDL cholesterol and triglyceride levels were measured from blood samples obtained after a 12–hour fast. LDL cholesterol was calculated with the Friedewald equation.8 C-reactive protein (CRP) was measured using the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc, Deerfield, IL) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Analytical intra–assay CVs ranged from 2.3% to 4.4% and interassay CVs ranged from 2.1% to 5.7%.
Computed Tomographic Scanning
Scanning centers assessed CAC by chest computed tomography using either a cardiac-gated electron-beam computed tomography scanner (Chicago, Los Angeles, and New York Field Centers) or a multi-detector computed tomography system (Baltimore, Forsyth County and St. Paul Field Centers). Certified technologists scanned all participants twice over phantoms of known physical calcium concentration. A radiologist or cardiologist read all CT scans at a central reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA in Torrance, CA). We used the average Agatston score9 for the two scans in all analyses. Carr et al have reported the details of the MESA computed tomography scanning and interpretation methods.10
Carotid intimal media thickness (IMT) Assessment
Trained technicians in each Field Center performed B-mode ultrasonography of the right and left near and far walls of the internal carotid and common carotid arteries 11.12 They used the Logiq 700 ultrasound device (General Electric Medical Systems, Waukesha, WI) to record images. An ultrasound reading center (Department of Radiology, New England Medical Center) measured maximal IMT of the internal and common carotid sites as the mean of the maximum IMT of the near and far walls of the right and left sides.
Ankle-brachial index (ABI)
After a 5-minute rest in a supine position, SBPs were measured in both arms and in the posterior tibial (PT) and dorsalis pedis (DP) arteries of both ankles, using appropriate-sized cuffs and a continuous wave Doppler probe. The ABI was computed separately for each leg, with the numerator the highest of the PT or DP systolic pressures and the denominator the highest of the right vs left brachial systolic pressures. The index ABI for the participant was the lower of the right vs left ABI.
Events Surveillance
At time of analysis, the cohort had been followed for incident cardiovascular events for a median of 49 months.13 At intervals of 9-12 months, a telephone interviewer contacted each participant to inquire about interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. In order to verify self-reported diagnoses, we requested copies of all death certificates and medical records for hospitalizations and outpatient cardiovascular diagnoses and conducted next-of-kin interviews for out of hospital cardiovascular deaths. We obtained records on 98% of reported hospitalized cardiovascular events. Some information was available on 95% of reported outpatient diagnostic encounters.
Trained personnel abstracted medical records suggesting possible cardiovascular events. Two physicians independently classified and assigned incidence dates. If, after review and adjudication, disagreements persisted, a full mortality and morbidity review committee made the final classification. For purposes of this study, we used all incident CHD events as the endpoint, including definite or probable MI, resuscitated cardiac arrest, fatal CHD, definite angina, and probable angina if accompanied by revascularization. Hard CHD events were classified as nonfatal MI or CHD-related death.
Definitions for each of these events are as follows. Reviewers classified MI as definite, probable, or absent, based primarily on combinations of symptoms, ECG, and cardiac biomarker levels. In most cases, definite or probable MI required either abnormal cardiac biomarkers (2 times upper limits of normal) regardless of pain or ECG findings; evolving Q waves regardless of pain or biomarker findings; or a combination of chest pain, and ST-T evolution or new LBBB, and biomarker levels 1-2 times upper limits of normal.
Reviewers classified resuscitated cardiac arrest when a patient successfully recovered from a full cardiac arrest through cardiopulmonary resuscitation (including cardioversion). Angina was classified, except in the setting of MI, as definite, probable, or absent. Definite or probable angina required symptoms of typical chest pain or atypical symptoms, as asymptomatic coronary artery disease is not a MESA clinical endpoint. Probable angina required, in addition to symptoms, a physician diagnosis of angina and medical treatment for it. Definite angina required one or more additional criteria, including CABG surgery or other revascularization procedure; 70% or greater obstruction on coronary angiography; or evidence of ischemia by stress tests or by resting ECG. We did not consider coronary revascularization or a physician diagnosis of angina or CHD, in the absence of symptoms, to be angina.
Fatal CHD required a documented MI within the previous 28 days, chest pain within the 72 hours before death, or a history of CHD, and required the absence of a known nonatherosclerotic or non-cardiac cause of death.
Statistical Methods
Event rates were estimated by dividing the number of events by the number of person-years at risk. Only the first CHD event for each participant was included. Within each subset defined by CAC score (CAC=0 or CAC<=10) time to incident CHD was modeled using Cox proportional hazards models. Each model was adjusted for age and gender. Larger multivariable models were not possible due to the limited number of events observed in these low risk subsets. All statistical analyses were performed using Stata version 10.0.
This study was funded by support from NHLBI. The authors are solely responsible for the design and conduct of this study; all study analyses, the drafting and editing of the paper and its final contents.
Results
Study Cohort
In the MESA population of 6814 at baseline, five participants were discovered to have had a cardiovascular event before enrollment and as a result were excluded from the analysis. In addition, 2890 individuals with CAC>10 were also excluded from the study population. The final study population consisted of 3,923 asymptomatic individuals free of known cardiovascular disease at baseline (mean age: 58±589years, 39% males). Overall 3415 individuals had no detectable CAC, whereas 508 had CAC scores of 1-10, respectively. As shown in table 1, those with minimal CAC were more likely to be males, older, of Caucasian ethnicity, have an adverse CHD risk profile as well as have greater internal carotid artery IMT, and a higher prevalence of ABI <0.9 (all p≤ 0.0001).
Table 1.
Baseline Characteristics According to CAC Scores
| CAC=0 N=3415 (50%) | CAC 1-10 N=508 (8%) | P value | ||
|---|---|---|---|---|
| Study Characteristics | ||||
| Age (mean) | 58±9 | 62±10 | <0.0001 | |
| Gender (% male) | 37% | 49% | <0.0001 | |
| Ethnicity (%) | ||||
| Caucasian | 33% | 41% | ||
| Chinese | 12% | 12% | <0.0001 | |
| African-American | 31% | 26% | ||
| Hispanic | 24% | 21% | ||
| LDL-C (mg/dl) | 116±31 | 121±33 | 0.001 | |
| HDL-C (mg/dl) | 52±15 | 50±15 | <0.0001 | |
| Smoking Status (%) | ||||
| Never Smoker | 56% | 51% | ||
| Former Smoker | 31% | 34% | <0.0001 | |
| Current Smoker | 13% | 15% | ||
| Hypertension (%) | 35% | 44% | <0.0001 | |
| Diabetes Mellitus (%) | 11% | 13% | <0.0001 | |
| Internal Carotid IMT (mm) | 0.89±0.42 | 1.03±0.54 | <0.0001 | |
| PAD (ABI<0.9) | 1.3% | 2.2% | <0.0001 |
Coronary Events among participants with CAC=0 and CAC 1-10
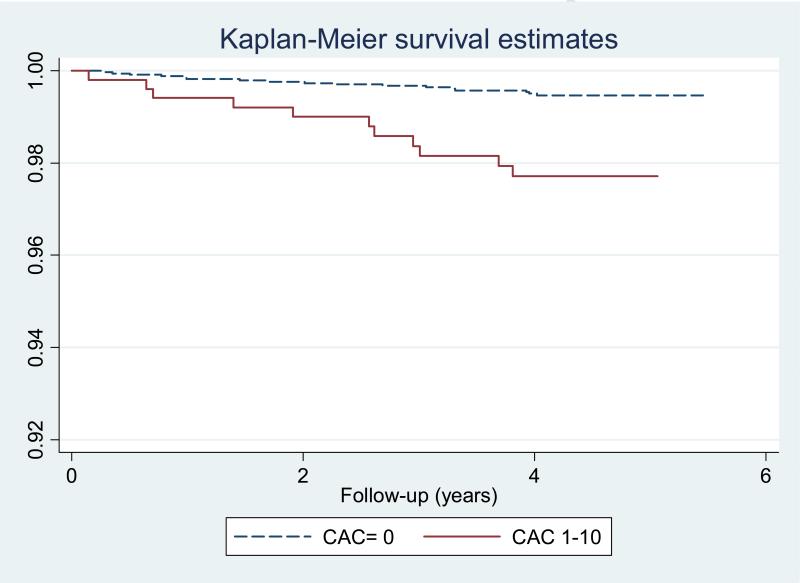
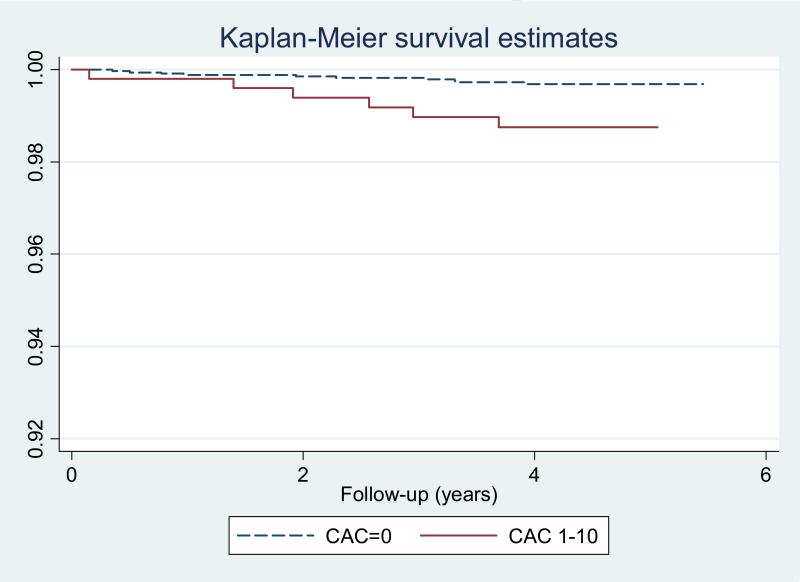
Overall, 108 incident hard CHD (1.59%) and 189 total CHD (2.78%) events were observed in the total study population over a median of 4.1 years follow-up. There were 17 all CHD events in those with no CAC (0.5%), and 11 in those with CAC 1 to 10 (2%). On the other hand 10 (0.3%) and 6 (1.2%) hard CHD events were noted among those with CAC=0 and CAC 1-10, respectively. The event rate for hard CHD among CAC=0 was 0.74 per 1,000 participants (95% confidence intervals [CI] 0.40-1.37), increasing to 3.01 (95% CI 1.35-6.70) among those with CAC scores of 1-10. For all CHD, those with CAC=0 had 1.25 (95% CI: 0.78-2.02.) events per 1,000 person years at risk, compared to those with CAC=1-10, who had 5.54 (3.07-10-01) events per 1,000 person years. Figures 1 and 2 demonstrate time to event curves for all and hard CHD events among those with CAC=0 and CAC 1-10, respectively.
Figure 1.
Kaplan Meyer Survival Curve for All CHD Events Among those with Zero CAC and Minimal CAC (1-10)
Figure 2.
Kaplan Meyer Survival Curve for Hard CHD Events Among those with Zero CAC and Minimal CAC (1-10)
In age and gender adjusted analysis minimal CAC (1-10) was associated with an estimated 3-fold higher risk of a hard event (HR: 3.23, 95% CI: 1.17-8.95), or of any coronary event (HR: 3.66, 95% CI 1.71-7.85). After further adjustments for race, hypertension, LDL, HDL, diabetes, smoking, cholesterol lowering medications, internal Carotid IMT, the relationship remained robust with CAC 1-10 associated with a hazard ratio of 3.11 (95% CI: 1.10-8.80) for hard CHD and 3.01 (95% CI: 1.36-6.67) for any CHD events, respectively.
Table 2 provides the hazard ratios for all CHD events (age-gender adjusted) for CHD risk factors and carotid IMT among those with an absence of CAC (CAC=0) and CAC 1-10. After age and gender adjustment former (HR=3.57; 1.08-11.77) or current smoking (HR=4.93; 1.20-20.3), and diabetes (HR=3.09; 1.07-8.93) were significant predictors of events in this cohort. For hard CHD (MI or CHD death), cigarette smoking and internal carotid IMT appeared to be associated with a trend towards increased event (tables 3).
Table 2.
Risk Factor Profile of Individuals with CHD Events and CAC=0 and CAC 1-10
| CACS | Hard CHD | Age | Gender | Race | Cig Smok | DM | HTN | LDL | HDL | LLM | FH HD | CIMT | ABI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Yes | 53 | Male | AA | Former | No | No | 84 | 38 | No | No | 0.81 | 1.27 |
| 0 | Yes | 54 | Female | White | Former | No | No | 145 | 62 | No | No | 0.80 | 1.06 |
| 0 | Yes | 55 | Male | AA | Former | No | Yes | 75 | 38 | No | No | 1.05 | 1.22 |
| 0 | Yes | 56 | Female | White | Never | No | No | 152 | 62 | No | Yes | 0.59 | 1.18 |
| 0 | Yes | 58 | Male | Hispanic | Former | No | No | 171 | 39 | No | Yes | 1.93 | 1.12 |
| 0 | Yes | 63 | Female | White | Former | No | Yes | 106 | 81 | No | No | 0.99 | 0.94 |
| 0 | Yes | 66 | Male | Hispanic | Current | Yes | Yes | 97 | 27 | No | No | 1.52 | 1.02 |
| 0 | Yes | 67 | Male | White | Current | No | No | 140 | 44 | No | Yes | 1.48 | 1.06 |
| 0 | Yes | 68 | Female | Hispanic | Never | Yes | Yes | 87 | 49 | Yes | Yes | 1.56 | 1.12 |
| 0 | Yes | 72 | Female | White | Former | No | Yes | 106 | 59 | Yes | Yes | - | 1.07 |
| 0 | No | 51 | Male | Hispanic | Current | Yes | Yes | 69 | 33 | No | Yes | 0.49 | 1.37 |
| 0 | No | 55 | Male | White | Former | No | No | 158 | 42 | No | Yes | 0.75 | 1.09 |
| 0 | No | 55 | Female | White | Current | No | No | 75 | 44 | Yes | No | 0.61 | 1.04 |
| 0 | No | 59 | Female | AA | Former | Yes | No | 124 | 50 | Yes | No | 1.58 | 1.06 |
| 0 | No | 68 | Male | Hispanic | Never | No | No | 148 | 29 | No | Yes | 1.14 | 1.15 |
| 0 | No | 70 | Female | AA | Former | No | No | 105 | 48 | No | No | 0.99 | 1.11 |
| 0 | No | 82 | Male | Hispanic | Never | Yes | Yes | 71 | 35 | No | No | 0.89 | 1.18 |
| 2 | Yes | 70 | Male | White | Former | No | Yes | 136 | 38 | No | Yes | 2.10 | 1.26 |
| 2 | Yes | 75 | Female | Hispanic | Former | No | Yes | 82 | 95 | Yes | No | 2.77 | 1.10 |
| 4 | Yes | 57 | Male | AA | Never | No | No | 153 | 50 | Yes | Yes | 0.92 | 1.20 |
| 4 | Yes | 60 | Male | White | Current | No | Yes | 149 | 44 | Yes | Yes | 1.74 | 1.10 |
| 5 | Yes | 60 | Male | AA | Current | No | No | 148 | 35 | No | No | 0.70 | 1.03 |
| 7 | Yes | 48 | Male | Hispanic | Current | No | No | 103 | 34 | No | Yes | 0.90 | 1.09 |
| 5 | No | 65 | Male | White | Never | No | No | 114 | 44 | No | No | 0.71 | 1.34 |
| 6 | No | 53 | Female | AA | Never | Yes | Yes | 194 | 103 | Yes | Yes | 1.63 | 1.00 |
| 7 | No | 72 | Female | White | Former | No | No | 91 | 60 | Yes | Yes | 0.81 | 1.05 |
| 8 | No | 57 | Female | White | Never | No | Yes | 63 | 45 | No | Yes | 0.65 | 1.14 |
| 8 | No | 59 | Male | Hispanic | Former | Yes | No | 110 | 30 | No | No | 0.61 | 1.12 |
AA: African Americans; Cig: Cigarette smoking; DM: Diabetes Mellitus; HTN: hypertension; FH: family history; LLM: lipid lowering medication; CIMT: Internal carotid IMT; ABI: ankle brachial index
Table 3.
Risk Factors for All CHD Events in Participants with CAC=0 and CAC 1-10
| |
CAC=0 (n=3415) |
CAC 1-10 (n=508) |
||||
|---|---|---|---|---|---|---|
| No Event (n=3398) Mean or N (%) | Event (n=17) Mean or N (%) | HR*(95% CI) | No Event (n=497) Mean or N (%) | Event (n=11) Mean or N (%) | HR*(95% CI) | |
| Age | 58 | 62 | 1.04 (0.99- 1.10) | 62 | 61 | 1.00 (0.94-1.06) |
| Male | 1240 (37) | 9 (53) | 2.10 (0.80-5.45) | 242 (47) | 7 (64) | 1.84 (0.53-6.36) |
| Race | ||||||
| White | 1120 (33) | 7 (41) | ref | 205 (41) | 5 (45) | ref |
| Chinese | 399 (12) | 0 (0) | NA | 63 (13) | 0 (0) | NA |
| African American | 1068 (31) | 4 (24) | 0.56 (0.16-1.91) | 128 (26) | 3 (27) | 0.98 (0.23-4.12) |
| Hispanic | 811 (24) | 6 (35) | 1.19 (0.40- 3.57) | 101 (20) | 3 (27) | 1.24 (0.30-5.23) |
| Hypertension | 1192 (35) | 7 (41) | 1.06 (0.38-2.90) | 220 (44) | 5 (45) | 1.16 (0.34-3.97) |
| LDL cholesterol (per 1mg/dl) | 116 | 113 | 1.00 (0.98-1.01) | 121 | 121 | 1.00 (0.98-1.02) |
| HDL cholesterol (per 1mg/dl) | 53 | 46 | 0.96 (0.92-1.00) | 50 | 52 | 1.02 (0.98-1.06) |
| Lipid lowering meds | 359 (11) | 4 (24) | 2.23 (0.72-6.96) | 101 (20) | 5 (45) | 3.98 (1.14-31.93) |
| Cigarette Smoking | ||||||
| Never | 1900 (56) | 4 (24) | ref | 254 (51) | 4 (36) | ref |
| Former | 1036 (31) | 9 (52) | 3.57 (1.08-11.77) | 171 (35) | 4 (36) | 1.34 (0.32-4.88) |
| Current | 447 (13) | 4(24) | 4.93 (1.20-20.30) | 72 (14) | 3(27) | 4.93 (0.58-12.96) |
| Diabetes | 358 (11) | 5 (29) | 3.09 (1.07-8.93) | 62 (13) | 2 (18) | 1.67 (0.36-7.77) |
| Family history of heart attack | 1195 (37) | 15 (47) | 1.60 (0.61-4.17) | 204 (41) | 7 (64) | 3.42 (0.87-13.44) |
| Internal carotid thickness ( per 1 mm) | 0.89 | 1.07 | 1.75 (0.75-4.09) | 1.03 | 1.23 | 1.76 (0.77-4.01) |
| Peripheral arterial disease (ABI<0.9) | 17 (0.50) | 0 (0) | NA | 11 (2) | 0 (0) | NA |
age and gender adjusted
Discussion
This large, population based multi-ethnic study demonstrated a remarkably low rate of cardiovascular events among those individuals with calcium scores of zero. This is concordant with prior study results.5 Conventional cardiovascular risk factors, particularly smoking and diabetes mellitus, are associated with a higher relative risk of CHD with no CAC, although absolute event rates remain low. Individuals with low CAC scores (1-10) had a 3-fold increased risk of CHD compared to those with no CAC, suggesting that individuals with low but non-zero represent a distinct risk group.
A recent meta-analysis compromising of 13 studies assessed the relationship of CAC with adverse cardiovascular outcomes consisting of 71,595 asymptomatic patients (65% male)5. Overall, 29,312 (41%) patients did not have any evidence of CAC. In a mean follow-up of 50 months, 154/29,312 (0.47%) patients without CAC suffered a cardiovascular event during follow-up as compared to 1,749/42,283 (4.14%) patients with CAC. The cumulative relative risk ratio was 0.15 (95% CI=0.11-0.21, p<0.001).5 Interestingly, in almost same duration of follow-up in our study (49 months) we also found similar all CHD events (0.5%) as reported by Sarwar et al5.
While available outcome data for CAC scoring in almost every study has shown CAC to be an independent and incremental predictor of future events over conventional risk factors, the distinction between zero scores and low scores is often not reported or recognized.14,15 As treatment in an intermediate risk population is most uncertain, CAC can theoretically direct intensified treatment for a high score and lifestyle management for a zero score in persons at intermediate risk. Prospective evaluation of an algorithm that evaluates the safety of withholding lipid therapy in persons with zero scores has not yet been performed requiring larger populations as well longer term follow-up.16
Large, long-term mortality studies have also been reported for CAC. A study by Shaw et al reported 5-year follow up of 10,377 persons.17 Those with scores 0-10 had a 5 year survival of 99.4%. That study did not evaluate zero scores compared to low scores. Budoff et al more recently reported on 25,253 persons with mean follow up of 6.8 years, with survival of 99.7% for persons with zero scores. 18 However, neither study reported on non-fatal cardiovascular events, or reported cardiovascular mortality separate from all-cause mortality. Recently Blaha et al specifically demonstrated that score of zero was associated with excellent survival with all-cause mortality rates of 0.87 per 1000 person-years (<1% 10-year risk, or <0.1% per year), with individuals with low CAC scores (1-10) had a nearly 2-fold increased mortality compared to those with no CAC, suggesting that patients with low CAC represent a distinct risk group6. While recent scientific statements and expert consensus documents support the idea that persons with low scores are at low risk of cardiovascular events, this study suggests that a distinction must be made between those with presence of any calcium and those with no detectable calcium.15 Furthermore, while elevated CAC scores have outperformed carotid IMT as a predictor of CHD events in MESA, 19 the use of IMT in this population with zero or low calcium scores may be useful, as trends were for a higher IMT in those persons. Individuals without CHD events with zero vs. low scores had IMT of 0.89 vs. 1.02 mm respectively, which increased among participants who suffered incident CHD events to were 1.19 and 1.52 mm respectively.
Our study confirms the recent finding of a robust increased risk in individuals with low positive CAC, demonstrating that presence and absence of CAC are distinct profiles. In our study we noted almost a threefold increase in all and hard CHD events as compared to only 2 fold increase seen with minimal CAC reported for all cause mortality reported by Blaha et al. A stronger association noted in our study may be due to the fact that the recent study only assessed all cause mortality without including the cause of death and, as such may have attenuated the association due to deaths unrelated to atherosclerotic disease. However, caution must be applied in obese patients and those patients with noisy scans. These patients may be more likely to have small scores on CAC algorithms that look for any pixel greater than 130, thus giving a ‘false-positive’ result of a low score. These scores rarely exceed 10.
The reasons of increased risk with minimal CAC can be explained in part by a recent study assessing the prevalence of noncalcified coronary artery plaque (NCAP) as a function of CAC. Compared to individuals with no CAC, individuals with low CAC (1-10) had marked increased rate of NCAP (65% vs. 7%) including NCAP causing >50% luminal obstruction (9% vs. 1%).This could be secondary to the arbitrary threshold used to separate speckled calcification signal from background noise in CT imaging. Individuals with very low calcium scores represent those who accumulated an amount of calcified plaque (among the total pool) enough to surpass the 130 HUs threshold in at least four contiguous voxels. Because the amount of calcified plaque is proportional to total plaque burden, those enabled to surpass the arbitrary limits may have more plaque and be at greater risk than the average of those individuals below the chosen attenuation threshold. Based on our findings that individuals with minimal CAC have a significantly higher likelihood of CHD events as compared to those without any CAC, we suggest that clinicians and future investigators consider these groups group as separate entities. In addition our study findings also support Blaha et al notion that diabetes, as well as smoking, are associated with CHD in individuals with no CAC5. The potential mechanisms may include presence of underlying non-calcified components, rapid development of atherosclerosis, and plaque destabilization. However, it must be kept in mind that while the relative risk of events is higher in the presence of low CAC, the absolute event rate remains low. Interestingly, there was a significant excess risk seen in those taking lipid lowering medications. This is probably due to the indication for treatment, namely, high lipid levels and supports the idea that the events are occurring in people with a large risk factor burden, e.g. smokers, diabetics or people with unfavorable lipid profiles.
Limitations of this study reflect the very small number of events that occurred among zero and low scores in this population. It was only possible to adjust for age and gender in the models assessing relationship of risk factors for CHD outcomes in presence of absent or minimal calcification. In addition, the risk factors which have not shown significant relationship with CHD outcome in these subgroups, may quite possibly predict adverse events in long term follow-up. The multi-ethnic nature of the study and population-based recruitment in MESA allow for some generalizability, however, there were age restrictions for participation in MESA (45-84 years at entry) and a limited duration of follow up. Most importantly, because this cohort was asymptomatic at baseline, so data cannot be extrapolated to individuals with symptoms or known CAD. The minimum lesion size used to define a calcified plaque was set conservatively large in MESA to reduce variability among scanners resulting from differences in the signal-to-noise ratio. This difference from the usual clinical scanning protocols may have a mild effect on the absolute values of calcium measurements. There is a possibility that some of the events in those with CAC=0 may be in individuals who are misclassified as no CAC due to image quality issues or reader error in addition to reading method used due to the four voxel requirement (Agatston et al9 used 2 voxels), or missed calcific lesions which are less ‘bright’ due to the fixed cutpoint of 130 Hounsfield units required as discussed above Despite this, the event rates among those individuals who were classified as zero in this study were very low, and consistent with other outcome studies.
In conclusion, individuals with zero or minimal calcification who had cardiovascular events were more likely to be diabetic and smokers as compared to those who did not have any event, and a trend was present for increased carotid IMT. As expected, annual event rates were very low (<0.1%/year) for those persons with scores of zero. The risk of hard and all CHD was approximately 3-fold higher in the CAC range of 1-10, so these two categories of CAC may warrant different consideration for risk reduction strategies.
Table 4.
Risk Factors for Hard CHD Events in Participants with CAC=0 and CAC 1-10
| |
CAC=0 (n=3415) |
CAC 1-10 (n=508) |
||||
|---|---|---|---|---|---|---|
| No Event (n=3405) mean or N (%) | Event (n=10) mean or N (%) | HR*(95% CI) | No Event (n=502) Mean or N (%) | Event (n=6) Mean or N (%) | HR*(95% CI) | |
| Age | 58 | 61 | 1.04 (0.98-1.10) | 62 | 62 | 1.01 (0.93-1.10) |
| Male | 1244 (37) | 5 (50) | 1.84 (0.53-6.38) | 244 (48) | 5 (83) | 5.36 (0.62-46.41) |
| Race | ||||||
| White | 1122 (33) | 5 (50) | ref | 208 (41) | 2 (33) | ref |
| Chinese | 399 (12) | 0 (0) | NA | 63 (13) | 0 (0) | NA |
| African American | 1070 (31) | 2 (20) | 0.40 (0.08-2.06) | 129 (26) | 2 (33) | 1.63 (0.23-11.61) |
| Hispanic | 814 (24) | 3 (30) | 0.84 (0.84-3.53) | 102 (20) | 2 (33) | 2.04(0.29-14.52) |
| Hypertension | 1194 (35) | 5 (50) | 1.63 (0.44-6.00) | 222 (44) | 3 (50) | 1.51 (0.29-7.92) |
| LDL cholesterol (per 1mg/dl) | 116 | 116 | 1.00 (0.98-1.02) | 121 | 127 | 1.01 (0.98-1.03) |
| HDL cholesterol (per 1mg/dl) | 53 | 50 | 0.99 (0.94-1.04) | 50 | 49 | 1.01 (0.95-1.08) |
| Lipid lowering meds | 361 (11) | 2 (20) | 1.84 (0.38-8.84) | 103 (21) | 3 (50) | 5.79 (1.06-31.12) |
| Cigarette Smoking | ||||||
| Never | 1902 (56) | 2 (20) | ref | 257 (51) | 1 (17) | ref |
| Former | 1039 (31) | 6 (60) | 4.93 (0.98-24.87) | 173 (35) | 2 (35) | 2.25 (0.20-25.36) |
| Current | 449 (13) | 2 (20) | 4.79 (0.65-35.11) | 72 (14) | 3(50) | 4.93 (1.14-121.94) |
| Diabetes | 361 (11) | 2 (20) | 1.88 (0.39-9.04) | 64 (13) | 0 (0) | NA |
| Family history of heart attack | 1198 (37) | 5 (50) | 1.77 (0.51-6.17) | 207 (43) | 4 (67) | 3.47 (0.62-19.07) |
| Internal carotid thickness ( per 1 mm) | 0.89 | 1.19 | 2.51 (0.95-6.67) | 1.02 | 1.52 | 2.64 (1.15-6.06) |
| Peripheral arterial disease (ABI<0.9) | 10 (0.3) | 0 (0) | NA | 6 (1) | 0 (0) | NA |
age and gender adjusted
Acknowledgement
This research was supported by R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 3. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final Report. NIH Publication No. 02-5215. September 2002. [PubMed]
- 4.Budoff MJ, Nasir K, McClelland RL, Detrano R, Wong N, Blumenthal RS, Kondos GT, Kronmal RA. Coronary calcium predicts events better with absolute calcium scores than age-gender-race percentiles – The Multi-Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2009;53:345–52. doi: 10.1016/j.jacc.2008.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffman U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2:675–88. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 10.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified Coronary Artery Plaque Measurement With Cardiac CT in Population-Based Studies: Standardized Protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly: The Cardiovascular Health Study. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 12.O'Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly: The Cardiovascular Health Study. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 13.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 14.Gopal A, Budoff MJ. Coronary Calcium Scanning. Am Heart Hops J. 2006;4:43–50. doi: 10.1111/j.1541-9215.2006.04682.x. [DOI] [PubMed] [Google Scholar]
- 15.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JAC, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of Coronary Artery Disease by Cardiac Computed Tomography, A Scientific Statement From the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114(16):1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]; Roberts WC, Jones AA. Quantitation of coronary arterial narrowing at necropsy in sudden coronary death: analysis of 31 patients and comparison with 25 control subjects. Am J Cardiol. 1979;44:39–45. doi: 10.1016/0002-9149(79)90248-0. [DOI] [PubMed] [Google Scholar]
- 16.Naghavi M, Falk E, Hecht HS, et al. From Vulnerable Plaque to Vulnerable Patient-Part III: Executive Summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force Report. Am J Cardiol. 2006 Jul 17;98(2 Suppl 1):2–15. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Shaw LJ, Raggi P, Schisterman E, et al. Prognostic Value of Cardiac Risk Factors and Coronary Artery Calcium Screening for All-Cause Mortality. Radiology. 2003;28:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 18.Budoff MJ, Shaw LJ, Liu ST, et al. Long-Term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Kronmal R, Detrano RC, O'Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, Tracy RP, Watson KE, Burke GL. Coronary Artery Calcification Compared with Carotid Intima-Media Thickness in Prediction of Cardiovascular Disease Incidence: The Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168(12):1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]