Abstract
Dickkopf-like 1 (DkkL1) is related to the Dickkopf gene family, a group of proteins that are characterized as secreted antagonists of Wingless (Wnt) signal transduction proteins. DkkL1 mRNA is found in preimplantation mouse embryos and in developing neural tissue, but in adults it is found primarily in the testes. In an effort to elucidate its function, the distribution of DkkL1 protein in mouse testis and mature sperm was analyzed by immunohistochemistry and immuno-blotting techniques. DkkL1 first appeared in the developing spermatocytes in seminiferous tubules as early as Stage XII, coincident with the appearance of DkkL1 mRNA. Surprisingly, however, DkkL1 localized to the developing acrosome in spermatocytes and spermatids and to the acrosome in mature sperm. Furthermore, DkkL1 was N-glycosylated in the testis, but it did not appear to be excreted, and the DkkL1 in mature sperm was no longer N-glycosylated, suggesting that additional post-translational modifications occurred during the final stages of spermatogenesis. These results identify a member of the Dickkopf family as a novel acrosomal protein that may be involved in acrosome assembly or function, a unique role for a secreted signaling molecule.
Keywords: sperm, acrosome, glycoprotein, Wnt-signaling
INTRODUCTION
Mammals express four highly conserved TEAD transcription factors that share the same DNA binding domain, but are expressed in a variety of different tissues where, in the presence of a transcriptional co-activator, they activate different genes (reviewed in Vassilev et al., 2001). However, only one of these genes, Tead2, is expressed in mouse embryos during the first 7 days of development (Kaneko et al., 1997; Wang and Latham, 2000). Thus, Tead2 appears to play a unique role at the beginning of mammalian development by allowing preimplantation embryos and embryonic stem cells to utilize Tead-dependent promoters and enhancers (Martinez-Salas et al., 1989; Melin et al., 1993; Kaneko et al., 1997).
While searching for Tead2 regulatory elements, a novel single copy gene formerly called Soggy (Sgy), since renamed Dickkopf-like 1 (DkkL1) by the mouse genome project (www.informatics.jax.org), was discovered only 3.8 kb upstream of the Tead2 mRNA start site where it is transcribed in the direction opposite to Tead2 (Kaneko and DePamphilis, 2000). Thus, DkkL1 and Tead2 regulatory elements lie in unusually close proximity to one another. In fact, the same locus exists in the human genome except that the two mRNA start sites are separated by only 1.5 kb.
These two closely spaced, divergently transcribed genes provide a unique paradigm for differential regulation of gene expression during mammalian development (Kaneko and DePamphilis, 2000; Kaneko et al., 2004). During mouse embryogenesis, both Tead2 and DkkL1 transcription are activated during the onset of zygotic gene expression at the 2-cell stage and continue until the blastocyst stage. However, with the onset of embryonic stem (ES) cell differentiation, DkkL1 expression is repressed, while Tead2 expression is stimulated. DkkL1 mRNA is again detected around day 15 in the developing dorsal root ganglia and in the cartilage primordium of the nasal septum (Krupnik et al., 1999), but in adult mice, DkkL1 mRNA is detected at high levels only in the testes, where it is localized to developing spermatocytes, and at low levels only in lymphocytes (Krupnik et al., 1999; Kaneko and DePamphilis, 2000; Kaneko et al., 2004). The “National Center for Biotechnology Information” database for expressed sequence tags reveals that DkkL1 mRNA has been detected in fetal eye and neural tissue, spermatocytes, trophoblast and placenta, and various tumors in mice and humans. These results are in keeping with observations that cultured cell lines express either DkkL1 or Tead2, but not both, and that most cells and tissues express Tead2 (Kaneko et al., 2004).
Virtually nothing is known about the biological role of DkkL1 protein, although its relation to the Dickkopf (Dkk) gene family suggest that it too may regulate tissue development (Krupnik et al., 1999). Dkk proteins are secreted proteins, some of which are known to antagonize Wingless (Wnt) function by binding to Wnt proteins. Wnt proteins are secreted glycoproteins that mediate cell proliferation and fate determination in both adults and embryos and have important roles in both development and tumorigenesis (reviewed in Nelson and Nusse, 2004). Activation of the Wnt signaling pathway results in accumulation of β-catenin in the cell nucleus, where it can activate transcription.
In an effort to elucidate the role of DkkL1 in mammalian development, we examined the cellular location of DkkL1 mRNA and protein in mouse testis, the one site where sufficient DkkL1 protein could be detected with the available antibodies to allow accurate histochemical analysis. The results revealed that DkkL1 protein rapidly associates with the acrosome during spermatogenesis and remains there in mature sperm, albeit in an altered form. Given the characteristics of other Dkk proteins and the diverse expression pattern of DkkL1 mRNA, we were surprised at this result, because of the highly specialized nature of the acrosome.
The acrosome is a vesicle-like structure composed of several compartments at the anterior end of a sperm. The acrosome contains proteases and hydrolases that are exocytotically released enabling the sperm to penetrate the egg’s thick extracellular matrix (zona pellucida). A great deal of work has gone into determining the proteins that are responsible for sperm recognition of the zona pellucida and vice versa. In the mouse, zona pellucida glycoprotein 3 (ZP3) is the sperm-binding protein (Wassarman et al., 1999) while the sperm protein, sp56, is the likely receptor for ZP3 (Bleil and Wassarman, 1990; Kim et al., 2001). However, there are several other proteins that also are implicated in binding and fusion to the egg, such as ADAMs (a disintegrin and a metalloprotease) on sperm and integrins on the egg (Talbot et al., 2003). Much is known about the physical structure of the acrosome (reviewed in Yoshinaga and Toshimori, 2003), but the mechanisms involved in acrosome assembly and function remain elusive. Thus, the results presented here not only identify a novel acrosomal protein, but a protein that is a potential regulator of tissue development and therefore, may be involved in acrosome assembly as well as sperm—egg interaction.
MATERIALS AND METHODS
Western Immuno-Blotting
Testis lysates were prepared with testes from CD-1 mice (Charles River), homogenized in LDS Sample Buffer (Invitrogen, Carlsbad, CA), then repeatedly sonicated and boiled. Sperm were collected from the cauda epididymides of CD-1 mice. The cauda epididymides were isolated, several incisions were made in the tissue and the sperm were allowed to swim out into PBS for several minutes. The sperm were then spun down, the PBS was aspirated and the pellet was denatured using LDS Sample Buffer with 5% β-Mercaptoethanol, followed by repeated rounds of both sonication and boiling. Other tissues were collected from adult CD1 males and females (ovaries only), and lysates prepared as above.
SDS—PAGE was performed using NuPAGE 4%–12% Bis-Tris gels (Invitrogen). The samples were then transferred to Immobilon-P membranes (Millipore, Billerica, MA) and blotted. The membranes were blocked with 5% powdered milk in PBS, and then incubated with 0.2 μg/ml polyclonal goat anti-mouse Soggy-1 (DkkL1) antibody (R&D Systems, Minneapolis, MN), 1 μg/ml monoclonal rat anti-mouse Soggy-1 (DkkL1) antibody (R&D Systems), or with 0.5 μg/ml goat anti-Lamin B (Santa Cruz Biotechnology, Santa Cruz, CA, sc-6217). The goat antibodies were detected using a peroxidase-conjugated rabbit anti-goat IgG secondary antibody (Pierce Biotechnology, Rockford, IL) and the rat antibody was detected with a peroxidase-conjugated goat anti-rat IgG secondary, followed by electro-chemiluminescence using SuperSignal West Dura substrates (Pierce Biotechnology).
Immunohistochemistry
Testes were collected from CD-1 males and fixed for 4 hr in 10% formalin, then embedded in paraffin. Six micrometer serial sections were prepared by American Histolabs (Gaithersburg, MD). Paraffin embedded tissues were deparaffinized and rehydrated through a graded series of ethanol prior to staining. The sections were quenched with hydrogen peroxide, boiled in 0.1M EDTA, pH 8.0, and blocked with CAS Block (Zymed Laboratories, San Francisco, CA). The polyclonal goat anti-mouse Soggy-1 (DkkL1) antibody (R&D Systems) was used at 1 μg/ml, and detected with a biotinylated rabbit anti-goat secondary antibody (Zymed Laboratories), followed by Streptavidin-peroxidase (Zymed Laboratories). Goat IgG (Zymed Laboratories) was used as a negative control. FE-J1 (Developmental Studies Hybridoma Bank), MON8017 (Monosan) and the actin antibody, JLA20 (Developmental Studies Hybridoma Bank), are mouse IgM antibodies. They were detected with a biotinylated goat anti-mouse IgM secondary antibody (Zymed Laboratories), followed by Streptavidin-peroxidase. All staining was visualized with 3,3′-diaminobenzidine (DAB Substrate Kit for Peroxidase, Vector Laboratories, Burlingame, CA). Harris’ Modified Hematoxylin was used as a counter-stain. The samples were dehydrated through a graded series of ethanol and mounted.
Immunofluorescence
Sperm were collected from cauda epididymides of CD-1 males, as above. After collection in PBS, the samples were spread onto silanted microscope slides (KD Medical), dried, and fixed in 2% paraformaldehyde with 0.1% Triton X-100. The slides were then permeabilized and blocked with 1% BSA, 0.1M glycine in PBS. Fixed samples were rehydrated in phosphate buffered saline (PBS), boiled in 0.1M EDTA pH 8.0, blocked with CAS Block (Zymed Laboratories) and incubated with the polyclonal anti-mouse Soggy-1 (DkkL1) primary antibody at 1 μg/ml. The anti-Soggy1 (DkkL1) antibody was detected with FITC-conjugated donkey anti-goat IgG (Jackson Immunoresearch, West Grove, PA). The slides were mounted with the Slow Fade Light Anti-fade Kit with DAPI (Molecular Probes, Eugene, OR).
For the double immunofluorescence, samples were blocked again between application of the first secondary antibody and application of the second primary antibody. The second primary antibodies were also used at 1 μg/ml and included FE-J1 (Developmental Studies Hybridoma Bank), MON 8017 (MONOSAN), and anti-Sp56 (QED Bioscience, San Diego, CA). FE-J1 and MON8017 were detected with TRITC-conjugated goat anti-mouse IgM (Jackson Immunoresearch) and the Sp-56 antibody was detected with Cy3-conjugated goat anti-mouse IgG (Jackson Immunoresearch). After detection of the second primary antibody, slides were mounted with the Slow Fade Light Anti-fade Kit with DAPI.
Deglycosylation
Testis and sperm lysates were prepared as above, with the exception that the denaturing buffer used was and Glycoprotein Denaturing Buffer (New England Biolabs, Beverly, MA) at 2.5 ×. After denaturing the samples, they were treated with NP-40, 10× G7 buffer and Peptide: N-glycosidase-F (PNGaseF) (New England Biolabs). The lysates were incubated at 37°C overnight. The samples were then subjected to SDS—PAGE followed by Western immuno-blotting, as above.
RESULTS
DkkL1 First Appears in Developing Spermatocytes
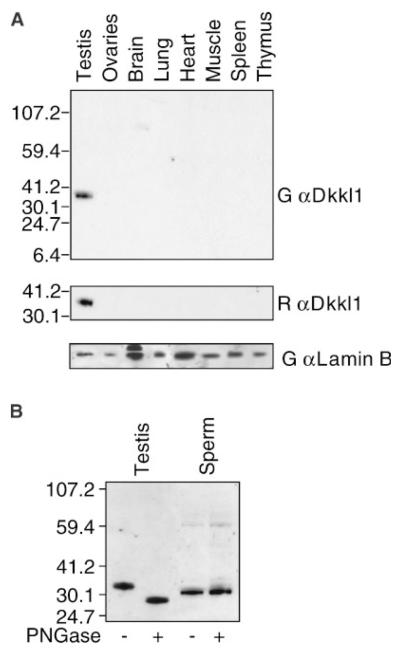
Previous studies from our lab using Northern blotting-hybridization to detect RNA concluded that mouse DkkL1 mRNA is produced at high levels only in the adult testes (Kaneko and DePamphilis, 2000). These studies were extended using two different antibodies directed against DkkL1 protein (Soggy) to detect DkkL1 in various mouse tissues by Western immuno-blotting. In agreement with DkkL1 mRNA expression, these antibodies detected only one major band in the testis lysate which migrated at approximately 34 kD (Fig. 1A). DkkL1 was not detected in any of the other tissues tested, including spleen despite the presence of DkkL1 mRNA in lymphocytes (Kaneko and DePamphilis, 2000).
Fig. 1.
Western immuno-blotting of DkkL1 in mouse tissues and sperm. A: Protein lysates from adult mouse tissues were Western immuno-blotted with two DkkL1 antibodies, a polyclonal goat anti-DkkL1 (top) and a monoclonal rat anti-DkkL1 (middle). A loading control stained with goat anti-Lamin antibody is shown on the bottom. B: Lysates from testis and sperm were treated with PNGaseF to remove N-linked carbohydrate residues. Untreated samples were run next to the deglycosylated lysates to detect a shift in the migration pattern. All molecular weight markers are kD.
DkkL1 Undergoes Post-Translational Modification During Spermatogenesis
Previous data on human DKKL1 protein showed that when expressed exogenously in vivo, the protein is post-translationally modified by N-linked glycosylation (Krupnik et al., 1999). Treatment of the protein lysate with a glycosidase resulted in a decrease in the apparent molecular weight by 5–10 kD. In vitro transcribed and translated mouse DkkL1 migrated at an approximate molecular weight of 30 kD (Kaneko and DePamphilis, 2000) but DkkL1 produced in the testis migrated at approximately 34 kD. To determine if endogenous DkkL1 is N-glycosylated in the testes, we treated the testis lysate with Peptide: N-glycosidase-F (PNGaseF). PNGaseF is an enzyme that releases specifically those carbohydrates in glycoproteins that are linked to asparagines (N) (Maley et al., 1989; Plummer and Tarentino, 1991). Digestion with this enzyme reduced the size of testis DkkL1 from ~34 to ~29 kD, similar to the size of the protein produced in vitro, revealing that endogenous DkkL1 synthesized in developing spermatocytes is a glycoprotein containing predominantly, if not exclusively, N-linked carbohydrates (Fig. 1B).
To determine if DkkL1 is present in mature sperm and if it carries the same post-translational modification as in developing spermatocytes, lysates of mature sperm were collected from the cauda epididymides and analyzed in parallel with lysates from the testis. By contrast to DkkL1 from testes, DkkL1 from both untreated and PNGaseF treated sperm lysates migrated the same, revealing that DkkL1 is not N-glycosylated in mature sperm (Fig. 1B). Furthermore, the DkkL1 from the sperm migrated at a molecular weight intermediate to the glycosylated and deglycosylated testis form, suggesting that DkkL1 has other post-translational modifications in the mature sperm.
Localization of DkkL1 to Developing Spermatocytes
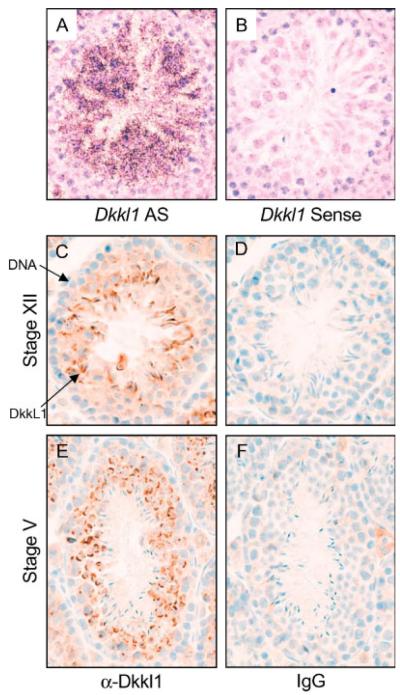
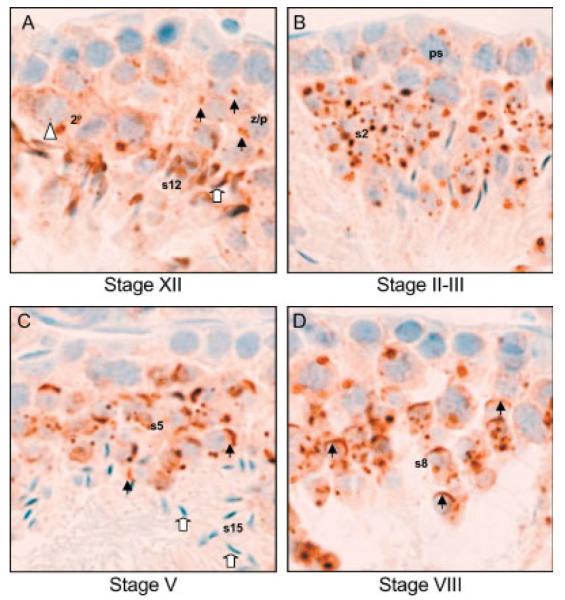
In situ hybridization to detect RNA previously revealed that DkkL1 mRNA was present specifically in the developing spermatocytes of the mouse testis and not in Leydig or Sertoli cells (Kaneko and DePamphilis, 2000 and Fig. 2A). Consistent with this conclusion, DkkL1 was detected by immunohistochemistry only in developing spermatocytes and spermatids in seminiferous tubules (Fig. 2C,E). Moreover, DkkL1 first appeared in Stage XII tubules, specifically in zygotene/pachytene spermatocytes (Fig. 3A). Stage XII tubules are characterized by the presence of step 12 spermatids (containing the longest nucleus of any seen during mouse spermatogenesis), secondary spermatocytes, and zygotene/pachytene spermatocytes (containing rounded nuclei packed with densely stained chromatin) (Russel et al., 1990). As spermatocyte development continues, DkkL1 is found in puddles in the pachytene spermatocytes of all stage tubules (Stage II–III, V, and VIII tubules, Fig. 3) and then in crescent shaped structures characteristic of acrosomes in early step spermatids (Stage V and VIII, Fig. 3C,D). It is important to note that spermatogenesis is cyclical; the process is not continuous between cell types in a particular stage; rather the cells must progress through all stages, moving closer to the lumen as they differentiate.
Fig. 2.
Expression of mDkkL1 mRNA and DkkL1 protein in adult mouse testis sections. In situ hybridization of a testis section with a 35S-labeled mDkkL1 anti-sense probe (A) or control mDkkL1 sense probe (B). The sections were counterstained with hematoxylin and eosin. The in situ hybridization data are republished (Kaneko and DePamphilis, 2000) for comparison with the following IHC staining. Paraffin embedded serial sectioned testes were stained for the presence of DkkL1 protein using a polyclonal goat anti-DkkL1 antibody (C and E) or with goat IgG as a control (D and F). The antibody was detected with a biotinylated rabbit anti-goat IgG secondary and visualized with DAB, resulting in a reddish-brown positive signal. The sections were counterstained with hematoxylin to visualize the nuclei (blue). (C) and (D) are Stage XII tubules and (E) and (F) are Stage V tubules, as judged by Periodic Acid Schiff staining of neighboring sections (data not shown).
Fig. 3.
Immunohistochemical detection of DkkL1 protein in developing spermatocytes and spermatids in testes. Sections shown are at 3 × magnification from Figure 2. A: A Stage XII tubule showing the first appearance of detectable DkkL1 protein in the zygotene/pachytene spermatocytes. The black arrows indicate the DkkL1 signal in the zygotene/pachytene spermatocytes (z/p). The white triangle points to the unstained nucleus of a secondary spermatocyte (2°), in which DkkL1 can also be seen. The white arrow points to a step 12 spermatid (s12) where DkkL1 can be seen in the forming spermatid head. B:A Stage II–III tubule showing puddling of DkkL1 protein in pachytene spermatocytes (ps) and acrosomic vesicles in step 2 or 3 spermatids (s2). C: By Stage V, DkkL1 appears in a crescent shape around the edge of the nuclei, indicated by black arrows, in step 5 spermatids (s5), but step 15 spermatids (s15), in which the nuclei has condensed, no longer stain positive. D: In step 8 spermatids (s8), found in Stage VIII, DkkL1 is found in a thin band spreading almost half way around the edge of the nuclei (black arrows).
Although DkkL1 was readily detected in the developing spermatocytes, it was not evident in the mature spermatids in sections through seminiferous tubules beyond step 12 (Fig. 3), yet we detected the protein in mature sperm by Western immuno-blotting. With more extensive epitope recovery (longer boiling in EDTA), we were able to detect DkkL1 in later spermatids in sections of both semiferous tubules and epididymides, but at the cost of tissue integrity (data not shown). Nuclear condensation in the spermatid head likely masks the antigen more thoroughly, requiring more rigorous exposure to detect it.
DkkL1 Localizes to the Acrosome in Mature Sperm
To further explore localization of DkkL1, mature sperm were isolated from the cauda epididymides and subjected to indirect immunofluorescence with DkkL1 (Soggy) antibody. DNA in the sperm heads was visualized with DAPI stain contained in the mounting medium. The two images were then merged and over-layed with a negative light microscopy image of the sperm. These results revealed that DkkL1 was localized specifically to the crescent shaped acrosome at the apex of the sperm head; it was not detected on the DNA, the midpiece, or the sperm tail (Fig. 4).
Fig. 4.
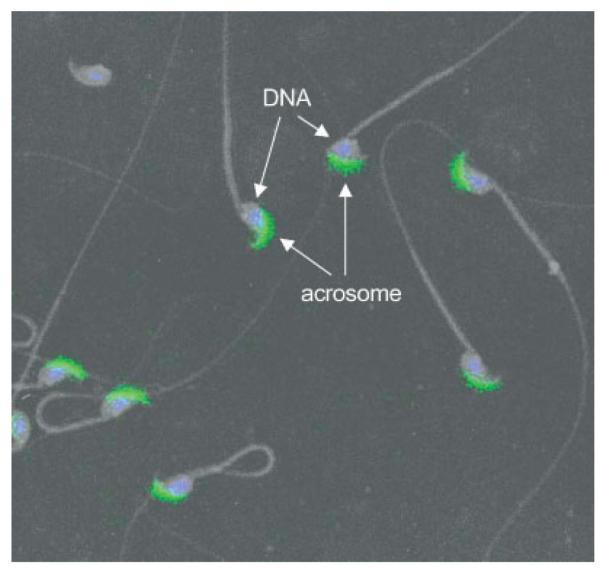
Immunofluorescence detection of DkkL1 protein in mature sperm. Mature sperm collected from the cauda epididymides of adult mice were spread on slides, the slides were stained for DkkL1 protein and visualized with immunofluorescence of the FITC-coupled secondary antibody. The nuclei were recognized by the incorporation of DAPI. Shown is a merged image of DAPI and FITC (DkkL1 staining) over-layed with a negative of the light microscopic image of the sperm. The DAPI staining localizes to the nuclei of the sperm head and the DkkL1 staining localizes to the acrosome.
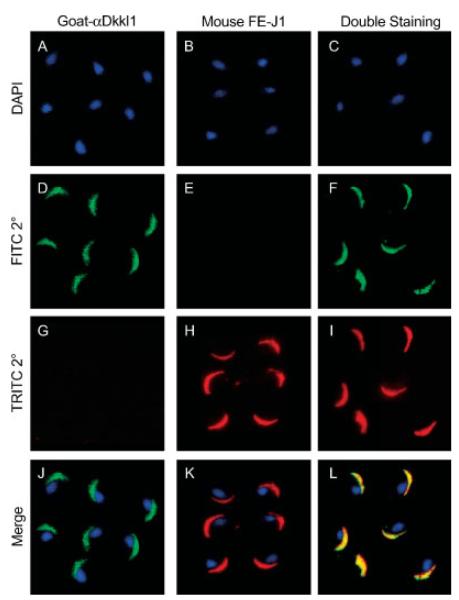
To confirm that the acrosome was indeed the structure to which DkkL1 protein was localized, sperm were subjected to double immunofluorescence staining. Sperm were first treated with the polyclonal DkkL1 (Soggy) antibody then with the FITC-coupled secondary antibody (Fig. 5, green staining). This was followed by treatment with one of three antibodies known to recognize the acrosome: FE-J1 (Fig. 5, red staining), an antibody that recognizes glycoproteins found in the acrosome (Fenderson et al., 1984; Geijsen et al., 2004); MON8017, which recognizes both human and mouse intra-acrosomal proteins (Chladek et al., 2000); and an antibody against Sp56, an acrosomal matrix protein (Kim et al., 2001; Kim and Gerton, 2003) that is involved in egg recognition (Cohen and Wassarman, 2001). The second set of antibodies was detected with either TRITC-coupled (FE-J1 and MON8017) or Cy3-coupled (Sp56) secondary antibody. The DkkL1 stain was completely superimposed on the acrosome stained by FE-J1 (Fig. 5, bottom right, yellow staining), MON 8017 and the Sp56 antibodies (data not shown) confirming that DkkL1 was associated with the acrosome in mature sperm.
Fig. 5.
Immunofluorescence detection of DkkL1 protein in mature sperm compared with immunofluorescence detection of a previously reported acrosomal protein. Mature sperm collected from cauda epididymides of adult mice and spread on slides were stained for DkkL1 protein and/or with the anti-acrosomal antibody FE-J1. All images are composites of several sperm heads from two fields, pasted together to present a larger number of sperm in a smaller field. Sperm in the left column were stained only for the presence of DkkL1, sperm in the middle column only with FE-J1. Sperm in the far right column were double stained with both antibodies. The top row of images shows DAPI staining of the nuclei (A, B, and C). The second row shows the emission from the FITC-coupled secondary antibody recognizing DkkL1 (D and F) and serves as a control for the FE-J1 staining (E). The third row shows a control for the DkkL1 staining (G) and the emission from the TRITC-coupled secondary antibody recognizing the FE-J1 antibody (H and I). The bottom row shows the overlay of the above three fields in each column. (J) DAPI and DkkL1 staining, (K) DAPI and FE-J1 staining, and (L) the overlap of DkkL1 staining (green) and FE-J1 staining (red) together producing yellow.
DISCUSSION
The results presented here identify a new protein, DkkL1, that is involved with formation of the acrosome during mammalian spermatogenesis. DkkL1 mRNA in adult mice first appears in spermatocytes (Kaneko and DePamphilis, 2000 and Fig. 2A), and DkkL1 protein first accumulates in zygotene spermatocytes as they transit to pachytene spermatocytes in Stage XII seminiferous tubules (Fig. 3A). Pachytene spermatocytes then progress to step 1 spermatids (Stage I) as they differentiate. In pachytene spermatocytes and early step spermatids, DkkL1 is found in discrete puddles. These are presumed to be acrosomic vesicles, because later in spermiation, DkkL1 is restricted specifically to the acrosome (Figs. 3C,D and 5), a crescent shaped structure at the apex of mouse sperm that plays a vital role in fertilization. Both DkkL1 and FE-J1, a previously identified acrosomal antigen (Fenderson et al., 1984), co-localize to this structure (Fig. 5). During spermato-genesis, DkkL1 undergoes N-linked glycosylation. This presumably occurs shortly after translation, because unmodified DkkL1 protein was never detected in lysates of mouse testes. Noteworthy is the fact that DkkL1 is no longer N-glycosylated in mature sperm, although it is modified in other, as yet undefined, ways (Fig. 1B). Analysis of the DkkL1 amino acid sequence suggests the presence of three phosphorylation sites and a single myristoylation site, in addition to the N-glycosylation site. However, all of these sites occur throughout the eukaryotic kingdom with high frequency. One of these modifications may account for the migration pattern detected in sperm lysates.
The specific localization of DkkL1 to the acrosome was unexpected, because the Dickkopf proteins are involved in regulation of signal transduction, and they are considered to be secreted ligands. DkkL1 is most closely related to Dkk-3 [also known as Reduced Expression in Immortalized Cells (REIC)], with the greatest homology in the N-terminal region. However, this region of Dkk-3 has little similarity to the other Dkks (Krupnik et al., 1999). In humans, both DKKL1 and DKK3 are glycoproteins, and both are secreted upon over-expression in human cell lines, presumably due to the presence of signal peptides in the N-terminus (Krupnik et al., 1999). It is unclear how DkkL1 remains sequestered in the acrosome despite the presence of a signal peptide (frequently found in secreted proteins) and the absence of definable sites for a GPI-anchor (a common post-translational modification that tethers a protein to lipid membranes). DKK3 is found down-regulated in a number of tumors (Kurose et al., 2004) and upon overexpression in osteosarcoma cells is able to suppress invasion and motility in vitro suggesting that repression of hDkk3 may correlate with metastasis (Hoang et al., 2004). The decreased motility observed in osteosarcoma cells upon overexpression correlates with relocalization of β-catenin from the nucleus to the cell membrane. Whether DKK3 exerts its effects directly through the Wnt pathway or circumvents it to affect β-catenin localization remains unclear.
The role of DkkL1 in acrosomal function remains speculative. Nevertheless, data presented here, together with the properties of Dickkopf proteins, suggest several possibilities. While DkkL1 may be secreted from certain cells, at least some of it is clearly not secreted from developing spermatocytes. Therefore, DkkL1 may act as an internal signal. For example, DKK3 suppresses motility when overexpressed in tumor cell lines (Hoang et al., 2004). Since DkkL1 expression is highest in developing spermatocytes, perhaps DkkL1 suppresses motility in developing spermatids, thus preventing their premature migration into the seminiferous tubule’s lumen and spermiation. DKK3 is also an effector of Wnt signaling (Caricasole et al., 2003), and Wnt signaling has clear roles in axon guidance (reviewed in Zou, 2004). Thus, DkkL1 may have a role in guidance of sperm through either the seminiferous tubules or the epididymis, or in fertilization.
Glycosylation plays an important role in both sperm development and in sperm—egg binding (reviewed in Tulsiani, 2003; Yoshinaga and Toshimori, 2003; Wassarman et al., 2004). Glycosylation of acrosomal proteins is necessary for proper trafficking through the Golgi complex to the developing acrosome during spermiogenesis, but modifications to these glycoproteins continue within the acrosome as it matures. One interpretation of our results is that DkkL1 is glycosylated in order to direct its localization to the acrosome, but then further modified to direct its proper localization within the acrosome.
An alternative hypothesis is that instead of being active in mature sperm, DkkL1 may facilitate formation of the acrosome during spermatogenesis. DkkL1 may be deglycosylated and further modified to inactivate it once this role is complete. The latter possibility is suggested by the presence of DkkL1 protein in pachytene spermatocytes, well before acrosome formation from Golgi vesicles is believed to commence (Yoshinaga and Toshimori, 2003).
Finally, it is noteworthy that although DkkL1 is expressed almost exclusively in the testis of adult animals, its expression is not restricted to that tissue. Blastocysts (day 3.5) consist of the inner cell mass, from which embryonic stem (ES) cells can be derived, surrounded by a cell monolayer (the trophoblast) that will become the placental tissue (Stewart, 2000). Preimplantation embryos express DkkL1 mRNA at levels equivalent to those of Tead2 (Kaneko and DePamphilis, 2000; Kaneko et al., 2004). Differential regulation of these genes becomes evident upon formation of two distinct lineages within the blastocysts. Furthermore, DkkL1 expression is repressed when ES cells begin to differentiate whereas Tead2 expression is stimulated (Kaneko et al., 2004). DkkL1 and Tead2 become differentially expressed upon differentiation, when most cells appear to express one of the two genes, but not both. In fact, the trophoblast may preferentially express DkkL1 over Tead2. Hence, DkkL1 may play a role in implantation by facilitating trophoblast cells in degrading (“hatching”) the zona pellucida (“egg shell”) so that the embryo can implant into the uterine endometrium. Thus, DkkL1 may play a role in penetration through the zona pellucida from the outside by sperm, and from the inside by the trophectoderm. These and other hypotheses will ultimately be challenged by analysis of homozygous null mice, the generation of which is ongoing.
ACKNOWLEDGMENTS
M.J.K. thank J. Boyle for her support and members of the laboratory for their helpful discussions.
Footnotes
Publisher's Disclaimer: Published 2005 Wiley-Liss, Inc.
REFERENCES
- Bleil JD, Wassarman PM. Identification of a ZP3-binding protein on acrosome-intact mouse sperm by photoaffinity crosslinking. Proc Natl Acad Sci USA. 1990;87:5563–5567. doi: 10.1073/pnas.87.14.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricasole A, Ferraro T, Iacovelli L, Barletta E, Caruso A, Melchiorri D, Terstappen GC, Nicoletti F. Functional characterization of WNT7A signaling in PC12 cells: Interaction with A FZD5 × LRP6 receptor complex and modulation by Dickkopf proteins. J Biol Chem. 2003;278:37024–37031. doi: 10.1074/jbc.M300191200. [DOI] [PubMed] [Google Scholar]
- Chladek D, Peknicova J, Capkova J, Geussova G, Tepla O, Madar J. Use of human sperm protein monoclonal antibodies in the diagnosis of sperm pathology and selection of a suitable assisted reproduction method for fertilization. Ceska Gynekol. 2000;65:28–32. [PubMed] [Google Scholar]
- Cohen N, Wassarman PM. Association of egg zona pellucida glycoprotein mZP3 with sperm protein sp56 during fertilization in mice. Int J Dev Biol. 2001;45:569–576. [PubMed] [Google Scholar]
- Fenderson BA, O’Brien DA, Millette CF, Eddy EM. Stage-specific expression of three cell surface carbohydrate antigens during murine spermatogenesis detected with monoclonal antibodies. Dev Biol. 1984;103:117–128. doi: 10.1016/0012-1606(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- Kaneko KJ, DePamphilis ML. Soggy, a spermatocyte-specific gene, lies 3.8 kb upstream of and antipodal to TEAD-2, a transcription factor expressed at the beginning of mouse development. Nucleic Acids Res. 2000;28:3982–3990. doi: 10.1093/nar/28.20.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko KJ, Cullinan EB, Latham KE, DePamphilis ML. Transcription factor mTEAD-2 is selectively expressed at the beginning of zygotic gene expression in the mouse. Development. 1997;124:1963–1973. doi: 10.1242/dev.124.10.1963. [DOI] [PubMed] [Google Scholar]
- Kaneko KJ, Rein T, Guo ZS, Latham K, DePamphilis ML. DNA methylation may restrict but does not determine differential gene expression at the Sgy/Tead2 locus during mouse development. Mol Cell Biol. 2004;24:1968–1982. doi: 10.1128/MCB.24.5.1968-1982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Gerton GL. Differential release of soluble and matrix components: Evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev Biol. 2003;264:141–152. doi: 10.1016/j.ydbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kim KS, Cha MC, Gerton GL. Mouse sperm protein sp56 is a component of the acrosomal matrix. Biol Reprod. 2001;64:36–43. doi: 10.1095/biolreprod64.1.36. [DOI] [PubMed] [Google Scholar]
- Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, Chang B, Duong T, Goodearl AD, Gearing DP, Sokol SY, McCarthy SA. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- Kurose K, Sakaguchi M, Nasu Y, Ebara S, Kaku H, Kariyama R, Arao Y, Miyazaki M, Tsushima T, Namba M, Kumon H, Huh NH. Decreased expression of REIC/Dkk-3 in human renal clear cell carcinoma. J Urol. 2004;171:1314–1318. doi: 10.1097/01.ju.0000101047.64379.d4. [DOI] [PubMed] [Google Scholar]
- Maley F, Trimble RB, Tarentino AL, Plummer TH., Jr Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem. 1989;180:195–204. doi: 10.1016/0003-2697(89)90115-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Salas E, Linney E, Hassell J, DePamphilis ML. The need for enhancers in gene expression first appears during mouse development with formation of a zygotic nucleus. Genes Dev. 1989;3:1493–1506. doi: 10.1101/gad.3.10.1493. [DOI] [PubMed] [Google Scholar]
- Melin F, Miranda M, Montreau N, DePamphilis ML, Blangy D. Transcription enhancer factor-1 (TEF-1) DNA binding sites can specifically enhance gene expression at the beginning of mouse development. EMBO J. 1993;12:4657–4666. doi: 10.1002/j.1460-2075.1993.tb06154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer TH, Jr., Tarentino AL. Purification of the oligosaccharide-cleaving enzymes of Flavobacterium meningosepticum. Glycobiology. 1991;1:257–263. doi: 10.1093/glycob/1.3.257. [DOI] [PubMed] [Google Scholar]
- Russel LD, Ettlin RA, Sinha-Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Cache River Press; Clearwater, FL: 1990. [Google Scholar]
- Stewart CL. Oct-4, scene 1: The drama of mouse development. Nat Genet. 2000;24:328–330. doi: 10.1038/74129. [DOI] [PubMed] [Google Scholar]
- Talbot P, Shur BD, Myles DG. Cell adhesion and fertilization: Steps in oocyte transport, sperm-zona pellucida interactions, and sperm-egg fusion. Biol Reprod. 2003;68:1–9. doi: 10.1095/biolreprod.102.007856. [DOI] [PubMed] [Google Scholar]
- Tulsiani DR. Glycan modifying enzymes in luminal fluid of rat epididymis: Are they involved in altering sperm surface glycoproteins during maturation? Microsc Res Tech. 2003;61:18–27. doi: 10.1002/jemt.10313. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Latham KE. Translation of maternal messenger ribonucleic acids encoding transcription factors during genome activation in early mouse embryos. Biol Reprod. 2000;62:969–978. doi: 10.1095/biolreprod62.4.969. [DOI] [PubMed] [Google Scholar]
- Wassarman P, Chen J, Cohen N, Litscher E, Liu C, Qi H, Williams Z. Structure and function of the mammalian egg zona pellucida. J Exp Zool. 1999;285:251–258. [PubMed] [Google Scholar]
- Wassarman PM, Jovine L, Litscher ES, Qi H, Williams Z. Egg-sperm interactions at fertilization in mammals. Eur J Obstet Gynecol Reprod Biol. 2004;115(Suppl 1):S57–S60. doi: 10.1016/j.ejogrb.2004.01.025. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K, Toshimori K. Organization and modifications of sperm acrosomal molecules during spermatogenesis and epididymal maturation. Microsc Res Tech. 2003;61:39–45. doi: 10.1002/jemt.10315. [DOI] [PubMed] [Google Scholar]
- Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 2004;27:528–532. doi: 10.1016/j.tins.2004.06.015. [DOI] [PubMed] [Google Scholar]