Abstract
Adolescents have been shown to have the highest rates of HPV infection. The cause of this is likely a combination of sexual risk behavior and biologic vulnerability. Not surprisingly, the frequent nature of HPV in this age group also results in frequent abnormal cytology. Most HPV and its associated abnormal cytology are transient with frequent clearance of HPV and the lesion. These findings have resulted in new strategies for adolescents with abnormal cytology, which include observation. For cytologic atypical squamous cells of undetermined significance (ASC-US) or low-grade squamous intraepithelial lesions (LSIL), adolescents should be followed with cytology at one-year intervals up to two years before referral for colposcopy is necessary. For biopsy proven cervical intraepithelial neoplasia (CIN 1), management is similar with yearly cytology indefinitely or until high (H)SIL or CIN 2/3 develops. CIN 2/3 in compliant adolescents can be managed with 6-month cytology and colposcopy.
Keywords: Adolescence; human papillomavirus; low grade and high-grade squamous intra-epithelial lesions; cervical intra-epithelial neoplasia I, II and III
Introduction
Clearly, cytology screening programs have resulted in decreased cancer rates worldwide. However, the cost of these current programs in the U.S. and other developed countries reach into the millions.1 Cytology was first used to detect early cervical invasive cancer and targeted adult women at the age of cervical cancer. Epidemiology studies began to unfold the natural history of cervical cancer showing that cancers were preceded by the development of pre-invasive lesions which if treated, could prevent cancer development. Consequently, programs expanded referral diagnosis to encompass a much broader group of abnormalities. With the development of new molecular techniques, human papillomavirus (HPV) was defined as the causative agent.2 Epidemiology studies embraced these molecular techniques in the 1980's and quickly showed that HPV was common in sexually active women and extremely common in adolescents. In particular, studies targeted “at risk” youth defined as those with multiple partners, pregnant and infected with sexually transmitted infections which showed unprecedented rates of both HPV and abnormal cytology. These findings, with all good intention, were interpreted as uncovering an unrecognized glacier of women at risk for cancer. The numbers of young women finding their way into colposcopy clinics begin to escalate. Several important epidemiologic findings began to elucidate the natural history of HPV underscoring its benign nature as well as its oncogenic potential. These studies were critical in assisting the more recent guidelines for triage and treatment of HPV-associated disease in adolescents. This Chapter discusses the prevalence and natural history of HPV, cytologic squamous intraepithelial lesions (SIL) and histologic cervical intraepithelial neoplasia (CIN) in adolescents as well as biologic factors associated with vulnerability to HPV and its consequences in this age group. Last, the Chapter covers new guidelines in the U.S. based on these observations.
Adolescents and HPV
Repeated studies have shown that adolescents remain one of the highest risk groups for HPV infection. A recent meta-analysis of studies throughout the world show that most countries demonstrate the same pattern with a peak in women under 25 years of age and a steady decline afterwards.3 Underscoring the vulnerability of young women to cervical HPV infection, studies of young women who recently initiate sexual intercourse show that half will acquire HPV within 2–3 years.4–6 Rates of HPV in adolescents, however, do vary; some populations show rates as low as 5% in adolescents with no decline or increase over time.3 The high rates of HPV reported in most adolescent populations have been attributed to either sexual behavior or biologic vulnerability. It remains unclear whether adolescents are more vulnerable to HPV because of their risk behaviors or if there is a true biologic vulnerability. Likely, both contribute.
Certainly, risks for HPV in adolescents are similar to those of adult women and include new sexual partners and lack of condom use.5,7,8 Most studies show that adolescents have more sexual partners than adult women and are worse users of condoms.9 One study showed herpes simplex virus (HSV) infection as an independent predictor of HPV acquisition as well.7 It is plausible that inflammation associated with HSV may contribute to the risk but presence of HSV may also reflect risky behavior.
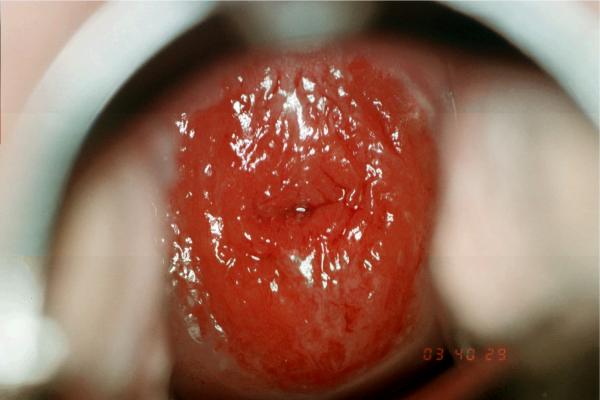
Structurally the adolescent cervix is different from the adult's in that is has greater areas of immaturity described as a predominance of columnar and metaplastic epithelium. (Figure 1) This topography starts during embryologic development.10 In a brief review, the cervix is initially lined by Müllerian columnar epithelium and later replaced by urogenital squamous epithelium from the vagina towards the endocervical os in utero. This results in an abrupt squamous-columnar junction located on the ectocervix in the neonate. This junction remains intact until puberty when hormonal changes trigger uncommitted generative cells of the columnar epithelium to transform themselves into squamous epithelium in a process referred to as squamous metaplaisa. Eventually, the replacement results in a new squamo-columnar junction occurring well into the os as seen in older women. This area of transition is referred to as the transformation zone. This area is also known as the site most vulnerable for cancer development.
Figure 1.
Typical adolescent cervix. The cervix is primarily covered by a mixture of columnar and metaplastic tissue.
It is thought that this epithelium may itself be vulnerable to HPV. First, columnar epithelium is a single layer thick, hence, basal cells which is the presumed target for HPV are quite accessible. An example of the fragility of this area is the common presence of blood when Pap smears are obtained in adolescents with large areas of ectopy. Studies of HPV comparing age groups show that incident infections remain more common in young women even when controlling for recent sexual behavior. Munoz et al11 examined the incidence of HPV in women who were normal cytologically and HPV negative at entry. The incidence of HPV was highest in adolescents aged 15–19 years of age with a cumulative incidence of 17% at 1 year and 35.7% at 3 years. The rates declined with age: for women in the 20–24 year old group, the 3 year incident rate was 24.1% and for women 45 years and older it fell to 8.1%. Although this data supports the notion that adolescents may be biologically vulnerable, it may also suggest that the male sexual partner of the older women is less likely to carry HPV decreasing the chance of infection. Because of either behavior or biologic vulnerability, repeated infections in adolescents and young women are also common12,13
Second, the process of metaplasia itself may support viral replication. HPV requires cell replication and differentiation for it to complete its life cycle.14 Metaplasia, by definition is a process of cell replication and differentiation and hence perfect environment for HPV replication. Hence, exposure to HPV during times of active metaplasia is more likely to result in an established infection. In one study, adolescents with evidence of active metaplasia were more likely to show LSIL if infected with HPV.15 The high rates of squamous metaplasia in young women are likely the explanation for the high rates of LSIL seen in this population. LSIL is found most commonly in adolescents16. This is supported by the observation that some countries see a second peak in HPV prevalence in women over 55 years of age, yet LSIL does not reflect this second peak with rates remaining under 1% in the older women.16
Differences in immune responses may also explain these differences. Unfortunately, little is known about the adult let alone the adolescent cervical mucosal immune response to HPV and questions remain as to whether these differ or not from adults. One study found that levels of IL-10 were much higher in adolescents with large areas of ectopy compared to those with mature cervixes.17 IL-10 is considered a Th-2 type cytokine which may favor HPV infection and persistence. Hormonal differences may also play a role.18 Since adolescents have frequent anovulatory menstrual cycles, unopposed estrogen may also have effects on the immune response. The consequences of these high rates of HPV in adolescents may be concerning. Several studies have shown that initiating sex at a young age is a risk for cervical cancer.19,20 Whether the risk is reflective of a high risk partner or whether it's related to a biologic risk remains unknown. Other factors such as C. trachomatis infections may play a role in increasing adolescent vulnerability. C. trachomatis which is also most common in the adolescent age group has been shown to enhance HPV persistence.21
Natural history of SIL and CIN
Since cytology and histology are overlapping but unique entities, studies that reflect SIL and CIN outcomes will be discussed separately.
SIL
Although some insist that all HPV infections result in LSIL, the rates of LSIL are in general much lower than those found for HPV DNA detection.3,16 Certainly, LSIL is the manifestation of HPV replication and protein expression. However, studies have found that risk factors for LSIL are different than those for HPV acquisition.7 An example is cigarette smoking which is commonly associated with SIL but not HPV acquisition7 These findings may be explained if the risk factor is associated with acceleration of the lesion causing it to become larger quicker. Certainly, larger lesions are more likely to be detected by cytology than smaller lesions.
Not surprisingly, the natural history of LSIL parallels that of HPV with rapid regression in the majority of cases. As with HPV, over 90% of LSIL has been shown to regress in adolescent and young women populations within 3 years.22–24 These observations differ from adult studies where regression rates are much lower25. Most likely, many of the LSIL detected in adults reflect persistent infections with underlying CIN 2 or 3, helping to explain these differences.
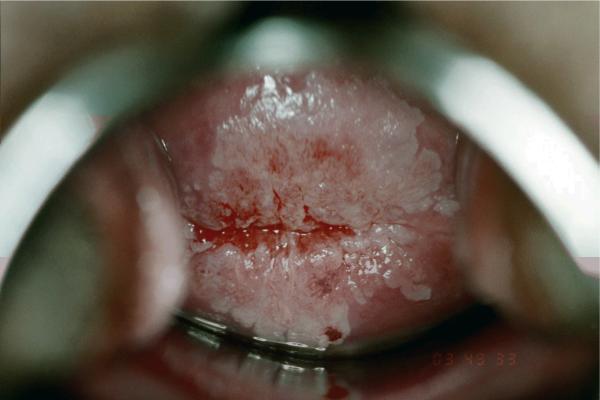
HSIL is also a reflection of HPV infection. However, as it represents CIN 2/3 lesions, it is thought to be further along the natural history of HPV. On the other hand, studies have shown that HSIL arises as rapidly as LSIL, possibly bypassing LSIL development26,27 Interestingly, the rate of HSIL in adolescents is similar to those found in older women. Mount & colleagues16 reported that 0.7% of cytologies from 15–19 year olds had HSIL compared to 0.8% of women aged 20–29 years and 0.7% in 30–39 year-olds. Some speculate that these HSIL cases are just “bad” cases of HPV with more cellular changes than seen in LSIL. Certainly, the reproducibility of HSIL is less than desirable28 One study found that only 50% of adolescents referred to colposcopy for HSIL had confirmed CIN 2 or 3 suggesting that many of HSIL are “overcalls.”29. For those who perform colposcopy on adolescents, colposcopic interpretation can be challenging since atypical squamous metaplasia (Figure 2), a common finding in this age group, has similar features as CIN misguiding the colposcopist to biopsy metaplastic tissue instead of neoplastic.30
Figure 2.
Atypical squamous metaplastic tissue in adolescent (after application of 3% acetic acid)
Natural history of CIN 1, 2 and 3
Although cytology and colposcopy have their limitations, they both reflect current tools which guide providers to obtain histology. Histology remains the gold standard for diagnosis. Unfortunately, because of its limitations, even histology is not perfect. Providers have long known that experience and number of areas biopsied increases the chance of CIN 2 or 3 diagnosis.31 The reproducibility of CIN 1, 2 and 3 is also problematic. All of these diagnosis often have less than 50% agreement between pathologists28. Most studies agree however that over 80% of CIN 1 diagnosis are likely to regress across all ages28 CIN 2 regression rates are more controversial. Syrjanen25 made observations that CIN 2 behaves more similar to CIN 1 than CIN 3. A recent study of adolescents based on chart review reported that 65% of adolescents with CIN 2 showed regression over 18 month period23 Most studies show that CIN 2 is more common in adolescents with HSIL than CIN 3.16,29,32 Most importantly, the incidence of invasive cancer in women under among 20 years of age in the U.S. is rare with only 0–3 cases reported per million women in this age group.33 The low rates of invasive cancer suggest that even in those with CIN 2 or 3 diagnosis, progression to cancer as an adolescent is rare. On the other hand, the incidence of invasive cancer sees its first rise at 25 years of age, supporting more aggressive triage starting at age 25 years. Interestingly, cancer rates in U.S. adolescent age groups have been stable over the last few decades despite the lowering of the age of sexual debut.
Screening
One of the strategies to avoid overtreatment and overreferral in adolescents is to avoid obtaining the Pap smear which triggers intervention. Several groups including the American Cancer Society recommend initiating cervical cytology screening after 3 years of the onset of vaginal intercourse but no later than 21 years of age.2 These recommendation were based on the notion that HPV is commonly acquired after sexual intercourse is initiated, most of these infections are likely to be transient, and cancer development during this short period almost never occurs34,35.
Management of Abnormal Cervical Cytology
The overall rational for changes in management of abnormal cytology36,37 in adolescents was based primarily on the following: 1) Because HPV is commonly acquired shortly after the onset of sexual intercourse, adolescents have high rates of HPV and its associated LSIL; 2) Most of these infections and their corresponding LSIL will spontaneously regress; 3) Adolescents frequently have multiple partners or serial monogamy resulting in frequent new infections; 4) The rare CIN 3 that does occur is unlikely to progress to cancer during this age period.36,37 Consequently, with the comings and goings of HPV during this age period, observation remains our best mode of surveillance. Another rational for conservative management is the risk of the procedure vs the perceived benefit. Studies have shown that preterm delivery, low birthweight and premature rupture of membranes are risks of cervical excisional procedures.38 These risks are of concern in adolescents particularly since the age group is at increased risk of repeated HPV infections, repeated cytologic abnormalities and consequently, repeated treatments.
HPV testing
Given the current FDA approved test for HPV, which is not type specific, HPV testing for any reason (ASC-US, LSIL follow-up) is not recommended in adolescents. (www.asccp.org)
Rationale: The repeat acquisition of HPV appears to be extremely common, specifically in non-monogamous young women. Most of these infections are transient. Hence, HPV detection (with or without abnormal cytology) in young women is likely to reflect this transient infection. A study by Boardman et al39 showed that over three-quarters of adolescents with ASC-US were positive for high-risk HPV type. Certainly, these high rates of infection negate the cost-effectiveness of using high-risk HPV DNA testing as triage in adolescents. Rather, future studies should focus on strategies that identify women with type-specific HPV persistence.40 Although HPV persistence is key to the development of HSIL and invasive cervical cancers, the length of persistence in this group requiring referral is yet to be established.
ASC-US and LSIL
Recommendations for ASC-US or LSIL include repeat cytology at 12-month intervals for two years. (www.asccp.org) During the two years of follow-up, a threshold of HSIL or greater is recommended before referral to colposcopy. After two years, a threshold of ASC-US or greater is recommended before referral to colposcopy. Since HPV testing is not recommended, triage for ASC-US using HPV testing is no longer recommended for this age group. If HPV testing is accidentally obtained, ASC-US/HR HPV positive is treated identical to ASC-US or LSIL. HPV testing for follow up is not recommended. (www.asccp.org)
Rationale: ASC-US and LSIL have similar natural histories and therefore management guidelines for these have been combined. The justification for bypassing the requirement to colposcopy in adolescents with LSIL is based on natural history studies of cytologic LSIL as well as histologic CIN 1. Prevalence studies of LSIL in adolescents show that these are predominantly CIN 1 lesions. This is quite different from adult women where LSIL on screening reveal higher rates of CIN 2 or 3 on referral to colposcopy. Follow-up by cytology is recommended up to two years based on the observation that only 60% of LSIL regressed at one year and 92% regressed by 3 years.22
ASC-US/suggestive of HSIL and HSIL
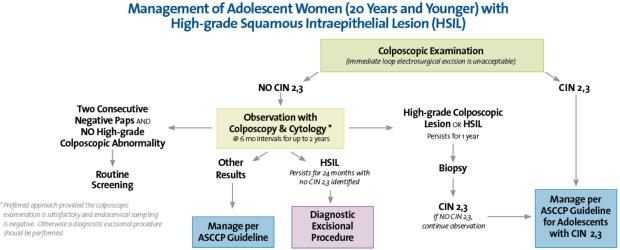
There have been no changes in recommendations and they remain similar to adults. Immediate triage to colposcopy with biopsy is recommended for both ASC-US cannot exclude high grade SIL (ASC-H) and HSIL. The main difference from adults is that immediate excisional treatment for HSIL is an option for adult women, but it is not warranted in adolescents. In the case of HSIL, if the biopsy does not show CIN 2 or 3, it is suggested to observe the adolescent with colposcopy and cytology at 6-month intervals up to 2 years. If HSIL persists by cytology or there is colposcopic persistence at one year, then repeat biopsy is recommended. If HSIL on cytology persists at the end of 2 years with or without CIN 2,3 diagnosis, a diagnostic excisional procedure is recommended at that time. The exception to this rule is the absence of a satisfactory examination. For adolescents with an unsatisfactory examination or the endocervical sampling is positive for HSIL, a diagnostic excisional procedure is recommended. Two consecutive negative Paps and no high-grade abnormality visible on colposcopy is criteria for a return to routine screening. This is summarized inFigure 3.
Figure 3.
Management of Adolescent Woman (20 Years and Younger) with High-Grade Squamous Intraepithelial Lesion (HSIL). From 2006 Consensus Guidelines for the Management of Women with Abnormal Cervical Cancer Screening Tests, used by permission of the American Society for Colposcopy and Cervical Pathology.
ASC-US/HSIL is treated similar to adults with immediate referral to colposcopy. If no CIN 2,3 is identified, cytology at 6 month intervals is recommended. If the repeat Pap is ASC-US or greater then the adolescents should be referred back to colposcopy. If the repeat Paps at 6 and 12 month are negative, the adolescent may go back to routine screening. HPV testing in follow-up of ASC-H is not recommended in adolescents.
Rationale: Because a significant proportion of HSIL in adolescents is likely to be CIN 2 or less, referral to colposcopy rather than immediate treatment is justified. One study found that only 54% of LEEP specimens in adolescents referred for histologic or cytologic HSIL had confirmed CIN 2.41 This finding suggest that many of the lesions in adolescents, whether CIN 1, 2, or 3 regress spontaneously. On the other hand, this information may suggest that many HSILs are simply overcalled.
Histologic CIN 1
CIN 1 is considered benign in adolescents as well as adult women. In adolescents, treatment of CIN 1 parallels that of ASC-US/LSIL for adolescents. Treatment of CIN 1 among adolescents is considered unwarranted. (www.asccp.org)42 It is recommended that cytology should be obtained at 12-month intervals. HSIL on repeat cytology at one year warrants rereferral. At 24 month follow-up, ASC-US or greater should be referred back to colposcopy. Two consecutive negative Pap tests are criteria for return to routine screening. If repeat biopsies are performed in follow-up, as long as CIN 1 remains the histologic diagnosis, observation is warranted. This is true for those with endocervical CIN1.
Rational: CIN 1 remains a benign reflection of HPV. Repeated diagnosis of CIN 1 in young women may reflect new HPV infections rather than persistent. Because of its benign nature, treatment of CIN 1 in adolescents remain unwarranted.
Histologic CIN 2, 3
Recommendation for treatment of adults or adolescents is either excisional procedure or ablative. For those with unsatisfactory colposcopy, excisional therapy is recommended. If the examination is satisfactory, either ablative or exclisional therapy is recommended. Some suggest that focal LEEPs or cryotherapy are more suitable for adolescents with smaller lesions since both of these have lower rates of complications. Complications of excisional procedures include pelvic inflammatory disease which underscores the importance of screening for sexually transmitted infections prior to treatment.43
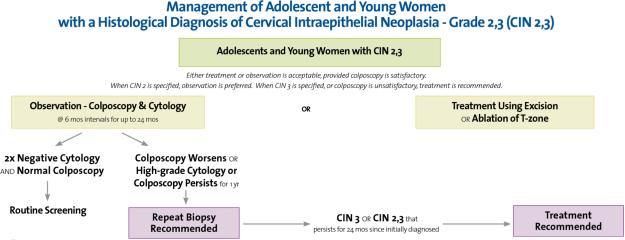
The new treatment guidelines for CIN 2,3 have an additional option for adolescents. It is acceptable to not treat adolescents with CIN 2 or lesions diagnosed CIN 2/3 and consider observation. It is suggested that this recommendation be applied to those who are considered reliable candidates for follow-up. (www.asccp.org) Observation is similar to that described for HSIL without CIN 2,3. Follow-up with colposcopy and cytology are recommended at 6-month intervals. If the CIN 2,3 lesion persist by colposcopy or cytology (HSIL) at one year, repeat biopsy is recommended. If the lesion progresses to CIN 3 or greater or persistence of CIN 2 or greater at 2 years, treatment is recommended. Treatment is always recommended for a CIN 3 diagnosis. The recommendations are summarized inFigure 4.
Figure 4.
Management of Adolescent and Young Women with a Histological Diagnosis of Cervical Intraepithelial Neoplasia - Grade 2, 3 (CIN 2, 3). From 2006 Consensus Guidelines for the Management of Women with Cervical Intraepithelial Neoplasia or Adenocarcinoma in situ, used by permission of the American Society for Colposcopy and Cervical Pathology.
Rationale: CIN 2, by many pathologists, is considered an equivicable diagnosis and in reflection often is re-categorized as a CIN 1 or CIN 3. CIN 2 in adolescents is thought to reflect a lesion more similar to CIN 1 than CIN 3 since CIN 3 lesions are rare in adolescents and cervical cancers extremely rare. Consequently, with time, lesions that would spontaneously regress will have the opportunity. In the same vein, lesions that are destined to progress are unlikely to undergo significant progression within relatively short time periods. With close surveillance, the lesions can be diagnosed as persistent and still be treated in a timely and preventive manner. Observation, however, is for those adolescents where compliance is assured. The recommendations include CIN 2 or CIN 2/3 since in the latter case, the histologic diagnosis of CIN 2 and 3 are often not distinguished on a pathology report. Because CIN 2 lesions are more common than CIN 3 in adolescent girls, it is recommended that lesions diagnosed as CIN 2/3 be treated similar to CIN 2.
In summary, the new management guidelines for adolescents with abnormal cytology and histology strongly favor observation. These guidelines are based on evidence that show that HPV- associated lesions in adolescents are likely to regress and persistent lesions have a low probability of progression to cancer during adolescence. The guidelines recommend discussing the risks of treatment versus the risks of progression with the adolescent. Since recurrent HPV infections are common in young women, follow-up strategies using the current HPV tests is not recommended in adolescents. Current recommendations are for both vaccinated and unvaccinated adolescents.
Acknowledgments
Funding Support: Dr. Moscicki is supported in part by NIH/NCI 2 R37 CA51323 and NCI 3 R01 CA87905-05S1
REFERENCES
- 1.Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi S, Herrero R, Clifford GM, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119:2677. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 4.Moscicki AB, Ma Y, Holland C, et al. Cervical ectopy in adolescent girls with and without human immunodeficiency virus infection. J Infect Dis. 2001;183:865. doi: 10.1086/319261. [DOI] [PubMed] [Google Scholar]
- 5.Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 6.Brown DR, Shew ML, Qadadri B, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005;191:182. doi: 10.1086/426867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 8.Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 9.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data. 2005;362:1. [PubMed] [Google Scholar]
- 10.Moscicki AB, Singer A. The cervical epithelium during puberty and adolescence. In: Jordan JA, Singer A, editors. The Cervix. Second Edition Blackwell; Malden: 2006. p. 81. [Google Scholar]
- 11.Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 12.Mendez F, Munoz N, Posso H, et al. Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005;192:1158. doi: 10.1086/444391. [DOI] [PubMed] [Google Scholar]
- 13.Rousseau MC, Pereira JS, Prado JC, et al. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 14.Doorbar J, Foo C, Coleman N, et al. Characterization of events during the late stages of HPV16 infection in vivo using high-affinity synthetic Fabs to E4. Virology. 1997;238:40. doi: 10.1006/viro.1997.8768. [DOI] [PubMed] [Google Scholar]
- 15.Moscicki AB, Burt VG, Kanowitz S, et al. The significance of squamous metaplasia in the development of low grade squamous intraepithelial lesions in young women. Cancer. 1999;85:1139. doi: 10.1002/(sici)1097-0142(19990301)85:5<1139::aid-cncr18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Mount SL, Papillo JL. A Study of 10,296 Pediatric and Adolescent Papanicolaou Smear Diagnoses in Northern New England. Pediatrics. 1999;103:539. doi: 10.1542/peds.103.3.539. [DOI] [PubMed] [Google Scholar]
- 17.Hwang LY, Ma Y, Moscicki AB. Factors that influence the rate of epithelial maturation in the cervix of healthy young women. Journal of Adolescent Health. 2008;42:2. doi: 10.1016/j.jadohealth.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott ME, Ma Y, Farhat S, et al. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 19.Green J, Berrington de Gonzalez A, Sweetland S, et al. Risk factors for adenocarcinoma and squamous cell carcinoma of the cervix in women aged 20–44 years: the UK National Case-Control Study of Cervical Cancer. Br J Cancer. 2003;89:2078. doi: 10.1038/sj.bjc.6601296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sierra-Torres CH, Tyring SK, Au WW. Risk contribution of sexual behavior and cigarette smoking to cervical neoplasia. Int J Gynecol Cancer. 2003;13:617. doi: 10.1046/j.1525-1438.2003.13392.x. [DOI] [PubMed] [Google Scholar]
- 21.Samoff E, Koumans EH, Markowitz LE, et al. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol. 2005;162:668. doi: 10.1093/aje/kwi262. [DOI] [PubMed] [Google Scholar]
- 22.Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 23.Moore K, Cofer A, Elliot L, et al. Adolescent cervical dysplasia: histologic evaluation, treatment, and outcomes. Am J Obstet Gynecol. 2007;197:141 e1. doi: 10.1016/j.ajog.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 25.Syrjanen K, Kataja V, Yliskoski, et al. Natural History of Cervical Human Papillomavirus Lesions Does Not Substantiate the Biologic Relevance of the Bethesda System. Obstet Gynecol. 1992;79:675. [PubMed] [Google Scholar]
- 26.Woodman CB, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 27.Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 28.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 29.Case AS, Rocconi RP, Straughn JM, Jr., et al. Cervical intraepithelial neoplasia in adolescent women: incidence and treatment outcomes. Obstet Gynecol. 2006;108:1369. doi: 10.1097/01.AOG.0000245448.19446.81. [DOI] [PubMed] [Google Scholar]
- 30.Coppelson M, Pixley E, Reid B. Colposcopy: A Scientific and Practical Approach to the Cervix and Vagina in Health and Disease. Charles C. Thomas; Springfield, IL: 1978. [Google Scholar]
- 31.Gage JC, Hanson VW, Abbey K, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108:264. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 32.Sadeghi SB, Hsieh EW, Gunn SW. Prevalence of cervical intraepithelial neoplasia in sexually active teenagers and young adults. Am J Obstet Gynecol. 1984;148:726. doi: 10.1016/0002-9378(84)90555-6. [DOI] [PubMed] [Google Scholar]
- 33.Chan PG, Sung HY, Sawaya GF. Changes in cervical cancer incidence after three decades of screening US women less than 30 years old. Obstet Gynecol. 2003;102:765. doi: 10.1016/s0029-7844(03)00696-3. [DOI] [PubMed] [Google Scholar]
- 34.Ries LAG, Eisner MP, Kosary CL. SEER Cancer Statistics Review, 1973–1999. National Cancer Institute; Bethesda: 2002. [Google Scholar]
- 35.Ries LAG, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2004. National Cancer Institute; Bethesda, MD: 2007. [Google Scholar]
- 36.Wright TC, Jr., Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 37.Wright TC, Jr., Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 38.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, et al. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 39.Boardman LA, Stanko C, Weitzen S, et al. Atypical squamous cells of undetermined significance: human papillomavirus testing in adolescents. Obstet Gynecol. 2005;105:741. doi: 10.1097/01.AOG.0000157126.12678.a6. [DOI] [PubMed] [Google Scholar]
- 40.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325:572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perlman SE, Lubianca JN, Kahn JA. Characteristics of a group of adolescents undergoing loop electrical excision procedure (LEEP) J Pediatr Adolesc Gynecol. 2003;16:15. doi: 10.1016/s1083-3188(02)00209-7. [DOI] [PubMed] [Google Scholar]
- 42.American Society for Colposcopy and Cervical Pathology 2007 www.ASCCP.org.
- 43.Hillard PA, Biro FM, Wildey L. Complications of cervical cryotherapy in adolescents. J Reprod Med. 1991;36:711. [PubMed] [Google Scholar]