Abstract
Cell mediated immune responses have been thought to be important in the control of HPV infections. We examined cell-mediated immune responses to HPV 16 E6 and E7 in the peripheral blood using IFN-γ enzyme-linked ImmunoSpot (ELISpot) assay in women with HPV 16 infection who showed clearance and compared these women to women with HPV 16 persistence. Women participating in a longitudinal study of cervical HPV were recruited once cervical HPV 16 infection was detected by PCR. Four groups of women were examined; 1) persistent 2) intermittent 3) transient and 4) cleared. Ninety-six samples from 55 women were compared. Comparing IFN-γ ELISpot to HPV 16 clearance, of 10 women with recent persistence, none had response to either E6 or E7; 14/24 women with recent clearance had E6 and 8/24 had E7 response. Women with intermittent persistence behaved similar to clearance group than recent persistors: 50% were positive to E6 and 20% to E7. In summary, anti E6 responses appeared critical in the immediate control of HPV and in some women an immune tolerance eventually develops if HPV is not eliminated soon after infection.
Keywords: CMI, IFN-γ, ELISpot, HPV 16 E6 and E7, persistent infection, clearance
Introduction
Human Papillomavirus (HPV), a sexually transmitted infection, is strongly associated with the development of cervical cancer. (1) Although over 30 genital types have been described, HPV 16 is responsible for 50-60% of cervical cancers. (2) However, HPV 16 infection in the genital tract does not necessarily lead to malignant disease; most women appear to clear the infection spontaneously within the first 48 months of detection of the virus. (3) In contrast, persistent detection of HPV 16 DNA in women with or without abnormal cytology is associated with an increased risk for the development of cervical cancer. (1, 4) Strong evidence suggests that HPV specific cell-mediated immune (CMI) response is pivotal in clearing HPV. (5) However, the target antigen remains to be elucidated. HPV 16-E6 and E7 are both considered prime targets, since these oncoproteins are expressed early in infection and increased expression is seen with increasing level of dysplasia. (6) Previously, using chromium release cytotoxic T- lymphocyte (CTL) assays, we showed that CMI responses to HPV 16- E6 and E7 were detectable in the peripheral blood of women with HPV 16 infection and clearance was associated with detection of CMI responses to HPV 16 E6 only. (7) Chromium release assays, however, are expensive, labor intensive, complicated by high background, and require a substantial number of cells. In this study, we examined the association between detection of IFN-γ-producing CTL by ELISpot and clearance of HPV 16. More specifically, we examined the chance of detecting a CMI response and type of infections (transient vs. established) and amount of time since clearance.
Materials and Methods
Study Population
The subjects in this study were recruited from participants in a longitudinal study of HPV. The first wave of recruitment occurred between 1990 and 1994. (3, 8) A second wave of recruitment occurred between 2000 and 2002. Subjects from the 1990 cohort were recruited if found HPV positive on routine screening and were exited in 2000 if they had evidence of HPV clearance for at least two years prior to being exited (i.e. women with at least 6 consecutive negative HPV tests, obtained at approximately 4 month intervals). Subjects with HPV persistence were retained in study. Women recruited into the second wave (2000 cohort) had unknown HPV status at the time of recruitment. Women in both cohorts were recruited if sexually active less than 5 years, were between 13 and 24 years of age and had no history of ablative treatment to the cervix. The subjects have been monitored by cervical HPV DNA testing by PCR, cytology, and colposcopy every 4 months. Women who tested positive from cervical samples for HPV 16 detected by PCR, were asked to participate in the immunology study. In 2002, IFN-γ ELISpot was initiated. Subjects from the 1990 cohort had their prevalent or incident infection several years before entering this study. Many of the subjects from the 2000 cohort were enrolled into the study within 2-4 months after detection of HPV 16 for the first blood draw. Once in the study, 60 cc's of blood was drawn at every 4 month visit whether there was evidence of clearance or not since the sustainability of the response was of interest. In order to examine the specificity of the IFN-γ ELISpot, 10 HPV 16 naïve women were recruited from the cohort (defined both by absence of cervical HPV DNA detection and negative HPV 16 serology testing). All subjects gave written consent as approved by the University of California, San Francisco Human Subject Review Board.
PCR analysis
The cervicovaginal lavage samples were tested for 37 HPV genotypes using PCR amplification with the PGMY09/11 primer system, as previously described. (8, 9) Amplified product was tested via a reverse line blot assay (Roche Molecular Diagnostics, Inc., Alameda, CA) for the presence of a positive Beta-globin signal and for the following HPV types: 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 and CP6108. Samples which were negative for Beta-globin or were positive for 3 or more HPV types were re-amplified and retested. In addition, 5% of samples chosen at random were run in duplicate on each PCR plate.
IFN-γ ELISpot
Blood was collected in 10 cc heparinzed tubes. The tubes from each of the sites were aerated by lying horizontally on a rotating rocker at ambient temperature until ground transport the next morning for immediate processing. All bloods were processed within 20-24 hours of blood draw. In house testing showed that the helper T lymphocytes (CD4) and cytotoxic T lymphocytes (CD8) yield was not significantly decreased if processed within 24 hours; however, monocytes (CD14) yield was reduced to 50%, eight hours post blood collection (data not shown). To measure IFN-γ release, MAHA plates (Millipore, Bedford, MA) were coated with 50ul of mouse IgG1 anti-human IFN-γ monoclonal antibody (1-D1K, 20ug/ml in 0.1M NaHCO3 buffer; pH 9.5, Mabtech, Stockholm, Sweden), incubated at 4°C overnight. The next day, coated wells were washed 4 x with PBS (Gibco /Life Technologies, Gaithersburg, MD), blocked with 50ul of RPMI 1640/5% heat inactivated pooled human serum (Omega Scientific, Cleveland, Ohio) and incubated in humidified 37°C incubator for at least an hour. Peripheral blood mononuclear cells (PBMCs) from 20ml heparinized blood were isolated by Ficoll–Hypaque gradient centrifugation (Amersham Pharmacia Biotech, Piscataway, NJ), washed, counted and re-suspended in RPMI 1640/10% fetal calf serum (FCS). One hundred ul (3×105/well) of PBMC was added to each well in triplicates.
Monocytes were positively selected from the remaining PBMCs using CD14+ magnetic beads (Milteny Biotec, Bergisch Gladbach, Germany) and the purity of the monocyte selection was verified by fluorescence activated cell sorter (FACS). 1.2 × 106 monocytes were added to each of three 15ml conical tube. The monocytes were washed with RPMI 1640 /1% FCS, centrifuged and pelleted. The monocytes were then infected with recombinant vaccinia virus expressing HPV16-E6 or E7 or WR,[a non recombinant vaccinia virus derived from Western Reverse (WR) strain, used as negative control], at a multiplicity of infection (MOI) of 10. The infection was carried out in a humidified 37°C incubator for 1 hour with shaking every 20 minutes. At the end of one-hour incubation, the virally infected monocytes were resuspended in 500 ul of RPMI 1640/1%FCS and added in triplicate to the plate with 100ul (1×105cells) per well of either CD14+E6-Vac, CD14+E7-Vac, or CD14+WR-Vac. The positive control wells were stimulated with PHA (10ug/ml). All the wells including the positive and negative controls received 5ul/well (20U/ml) of rIL2 and 5ul/well (5ug/ml) of rIL7 (R&D Systems, Minneapolis, MN). Following 18 hour incubation in humidified 37°C, the plates were washed 4 times with PBS/0.05% Tween-20 (Sigma, St. Louis, MO). Fifty ul/well (4ug/ml) of biotinylated anti-human IFN-γ monoclonal detection antibody (7-B6-1 Mabtech, Stockholm, Sweden) was added to each well and the plates were incubated for 2 hours at 37°C prior to 4x wash with PBS/0.1% Tween-20. Avidin-bound biotinylated horseradish peroxidase (Vector laboratories, Burlingame, CA) (50ul/well) was added to each well, and the plates were incubated at 37°C for an hour. The plates were washed with PBS/0.1% Tween-20 followed by addition of 50ul/well of DAB (Invitrogen, Carlsbad, CA). Spot forming colonies (SFC) were counted using a dissecting microscope. The assay was considered invalid if the SFC in PHA wells were less than 500 spots and was considered positive if the average number of SFC in HPV antigen wells were 3 standard deviations (SD) above the background (average of triplicates).
Since we use whole E6 and E7 protein for antigen stimulation, we expected the primary source of the IFN-γ in the assay would be CD8+ cells. Because of the volume of blood required and cost, we were unable to perform CD4+ and CD8+ cell selection on all assays. However, we did perform a cell selection assay for one subject with a previously know positive result. CD4+ and CD8+ T cell populations were magnetically isolated using MACS micro beads (Milteny Biotec, Bergisch Gladbach, Germany) verified by FACS analysis. The assays were performed as described above in triplicates with enriched CD4+ cells, enriched CD8+ cells and intact PBMCs. The CD8+ enriched populations had two times as many SFCs/106 than the CD4+ enriched populations (for E6: 150 vs. 80 SFCs, respectively and for E7: 430 vs. 240 SFCs, respectively). The SFC for intact PBMC for E6-vac and E7-vac was 250 and 480 respectively. This suggested that although both CD4 and CD8 contribute to IFN -γ production, CD8 cells are the primary producer.
To check for specificity, we tested 10 women who were negative for HPV 16 by both serology and cervical DNA by PCR. The serology was performed in Dr. Ray Viscidi's laboratory, Johns Hopkins University, as previously described. (10, 11) None of the negative control women had responses to either E6 or E7.
Data analysis
Initially, we defined women with a pattern of clearance as those with 2 or more consecutive negative tests by PCR with no subsequent positive test (++−−−). Clearance was then further categorized into 3 groups: 1) Recent Clearance (RC) defined as those who had ELISpot test results within 4-8 months after HPV 16 detection and had an established infection (defined as at least 2 positive tests for HPV 16 followed by at least 2 negative tests (++−−), 2) Clearance after Transient Infection (TI) defined as those who had recent clearance but only after a single positive HPV 16 test (−+−), 3) Past Clearance defined as those who had the first available ELISpot test result at 20 months or greater (range 20-104) after clearance was documented (−+++−−−−−−−−). Likewise, Persistence was initially defined as 2 or more consecutive HPV 16 positive tests by PCR, with no subsequent negative tests. Persistence was then further categorized into: 1) Recent Persistence (RP) defined as at least 2 sequential cervical HPV positive tests with no intermittent negative tests (e.g. −−+++) and 2) Intermittent Positive (IP) defined as having cervical HPV positive tests intermittently over the observed period (e.g. −−++−++−−−++). The mean number of months over which cervical HPV tests were positive consecutively for the RP group was 8.1 months (range 4-20) and the mean number of months over which cervical HPV positive tests were detected intermittently for the IP group was 61 months (range 17-15). Comparison between the IFN-γ ELISpot results and women who cleared HPV 16 infection versus women with persistent HPV 16 infection were performed by Chi-square analysis or by Fishers' exact test. For the comparison of clearance vs. persistence, any positive IFN-γ ELISpot test among all samples obtained during follow-up in this comparison was considered a positive response for that subject. Only subjects who had adequate number of follow-up visits to categorize them were included.
Results
Comparison of IFN-γ ELISpot result to E6 and E7 and HPV clearance
Among the 104 women meeting the eligibility criteria (details in Materials and Methods section), the mean number of assays per person was 3.65 (range 1-6). When we compared women with the initial definitions of persistence or clearance (see methods), we found that among the 20 women, who had HPV 16 persistence, 5 (25%) were positive for E6 and 2 (10%) were positive for E7. In comparison, among the 84 women who cleared their infection, 35 (41%) were positive for E6 (p=0.17) and 17 (20%) were positive for E7 (p=0.29).
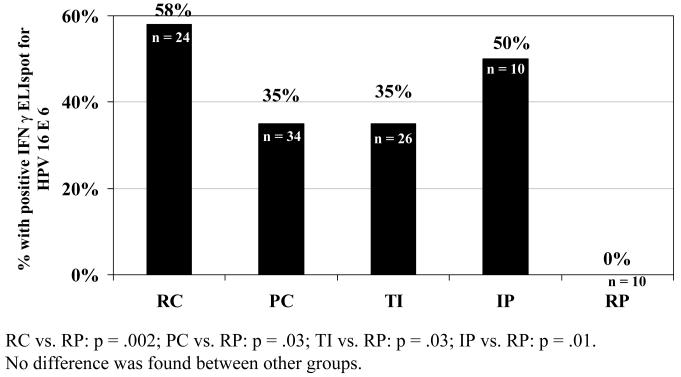
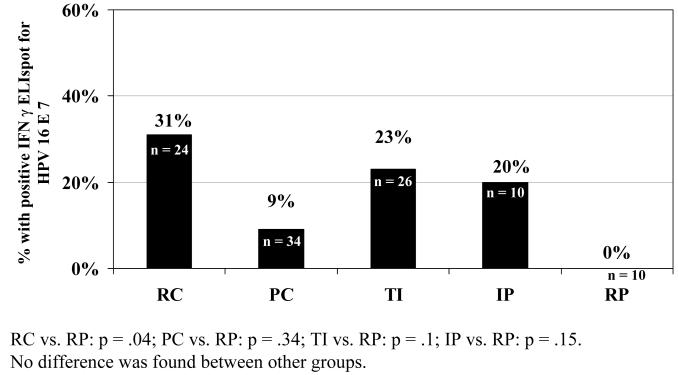
We next examined the more precise definitions of clearance or persistence based on specific patterns described in the methods. We found that the two definitions of persistence (RP and IP) were different in their responses to E6. Among the 10 women in the IP group 5 of the 10 had responses to E6 and 2 to E7 (p=0.01 and p=0.15, respectively) compared to 10 women in the RP group, with no response to either E6 or E7. When we examined difference among the 3 clearance groups, no statistical differences were found. However, there was a higher tendency for the RC group to show a detectable response to E6 (figure 1) than either the PC or TI group. All three clearance groups were more likely to have CMI responses to E6 than RP group. Among the 24 women in the RC group 58% had a response to E6 and 33 % to E7 (p= 0.002 and=0.04, respectively, compared to the RP). Among the 34 women in the PC group, 35% had a response to E6 and 9% to E7 (p=0.002 and p=0.34 respectively). Among the 26 women with TI, 35% responded to E6 and 23% to E7 (p= 0.03 and p=0.1 respectively). No differences were seen between any of the 3 clearance groups and the IP group.
Figure 1.
Response to IFN-g ELISpot HPV16 E6 among groups of women with evidence of HPV16: recent clearance (RC), past clearance (PC), clearance after transient infection (TI), intermittent positive (IP) and recent persistence (RP)
Discussion
Our results indicate that IFN-γ ELISpot responses to HPV 16 E6 and possibly to E7 are important to clearance of HPV 16. We note that the comparison between initial classifications of the persistent and clearance groups was not significant. However, when the analysis examined a more refined definition of HPV persistence, clear associations were found. As we and others have previously reported the comparison was most dramatic for E6 (12, 22). We find the consistent association with E6 interesting, specifically since E7 has been used as the target antigen in several therapeutic vaccines for CIN (13-15). E7 has been favored as a target antigen since it was reported to be more highly conserved than E6 in pre-cancer and cancer tissues (16).
Our findings suggest that E6 is more critical in the early stages of HPV infection. Welters, et al also have shown that not only successful control of HPV 16 infection is mediated by E6-specific memory T-cells but also suggested that observed IFN-γ producing T cells circulating in the peripheral blood, play a role in protection against persistent HPV infection and associated development of malignancies. (22) Unlike most investigations that are limited to women with CIN, our study was comprised of healthy young women without disease and who were well characterized as to when they experienced clearance. The association with early infection was underscored by the fact that the strongest association with E6 was in women who had recently been infected and cleared compared to those who continued to show persistence. It may be that the reported associations with E7 and CIN clearance suggest that CMI responses to E7 become more important in the later natural history.
Although the obvious comparison groups are women who have evidence of clearance vs. persistence, we had the opportunity in this analysis to study specific subgroups including women with transient infections, women with intermittent HPV 16 detection and women who had cleared some time in the past. The findings for those with transient infections with HPV were less dramatic than those with established infections. It has been postulated that transient infections often represent semen contamination or viral infections that are not yet established in the basal cell layer. (12, 17) Our findings suggest that some of these transient infections were not established and no antigenic stimulation was present. It may also be that innate immune factors (i.e. natural killer cells, toll-like receptors) were more important in eliminating the initial infection resulting in rapid clearance. (18) Onda et al showed similar findings for antibody response in that HPV seroconversion using VLP-based ELISA assays were less common in women with transient infection.(19)
One of the most interesting findings in this study was the detection of responses in women who had intermittent positive tests over periods of time. This group looked similar to the clearance groups. The detection of responses in subjects with intermittent HPV DNA detection may suggest that a certain immune tolerance develops with ongoing persistence despite detectable adaptive immune responses. This group may also represent women repeated re-infections. Hence, immune response in these individuals would be expected. On the other hand, the CMI detected may be adequate to dampen viral replication periodically so that HPV DNA detection falls below the level of detection during certain periods. The same phenomenon could also explain the absence of disease progression to HSIL in these subjects (data not shown).
Limitations to the study are the small cohort size and the potential insensitivity of the assay due to the localized nature of the infection. The small sample size may have resulted in our missing greater associations with E7. The difficulty in detection of CMI responses in the peripheral blood is not surprising since HPV 16 is a localized cervicovaginal infection and consequently the number of circulating memory cells hypothesized would be small. It may also be that antigens other than E6 or E7 are important in viral control and these differ by stage of infection. (20, 21) In addition, the responses we detected were not sustainable. As we found in our previous study using chromium release assay, IFN-γ ELISpot often required a fair number of visits to identify a single positive. (7) Women in this study who were positive had a mean of 3.65 assay visits. The positive IFN-γ ELISpot, like the chromium release assay, did not directly coincide with the clearance visit (data not shown) (7). It is also worth noting that 40% of the women who had recently cleared HPV 16 had no detectable response by the IFN-γ ELISpot. The lack of sustainable response by the IFN-γ ELISpot was also illustrated by the lower detected response rate among those who had the assay performed years away from the point of clearance. This should not be surprising since the antigen important to the immune response has long been eliminated in those who cleared in the past and the remaining circulating memory cells are likely even lower than initial infection.
Conclusion
In summary, our data suggest that early in the natural history of HPV 16 infections, CMI responses to E6 and to a lesser extend to E7 are important for clearance. Our data also suggest that an immune tolerance may develop among some women with persistent infection. This appeared to result in intermittent positive tests without the development of disease. The role of these responses to E6 in women with long-term persistence is not clear. However, it appears to be protective against the development of high-grade squamous intraepithelial lesion.
Figure 2.
Response to IFN-g ELISpot HPV16 E7 among groups of women with evidence of HPV16: recent clearance (RC), past clearance (PC), clearance after transient infection (TI), intermittent positive (IP) and recent persistence (RP)
Acknowledgments
This work was supported in part by National Cancer Institute #R01CA51323 and #R01CA54053, NIH Grant #M01 RR01271, Maternal and Child Health Bureau Training Grant #MCJ000978, NCI K07 CA75974, and the Arkansas Biosciences Institute, the major component of the Tobacco Settlement Proceeds Act of 2000.
The authors acknowledge the technical contributions of Bernadeth Sibunga and Annamaria Vafiadis for HPV testing and chromium release CTL assays, Dr. Ray Viscidi for HSV serologic testing, Anthony Kung in manuscript preparation and Roche Diagnostics for their generous donation of materials for HPV DNA testing.
References
- 1.Kjaer SK, van den Brule AJ, Bock JE, et al. Human papillomavirus--the most significant risk determinant of cervical intraepithelial neoplasia. Int J Cancer. 1996;65:601–606. doi: 10.1002/(SICI)1097-0215(19960301)65:5<601::AID-IJC8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz N, Bosch FX, De Sanjose S, et al. Epidemiological Classification of Human Papillomavirus Types Associated with Cervical Cancer. NEJM. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 4.Wallin KL, Wiklund F, Luostarinen T, et al. A population-based prospective study of Chlamydia trachomatis infection and cervical carcinoma. Int J Cancer. 2002;101:371–374. doi: 10.1002/ijc.10639. [DOI] [PubMed] [Google Scholar]
- 5.Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8:209–220. doi: 10.1128/CDLI.8.2.209-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus I, Molden T, Erno LE, Skomedal H, Karlsen F, Hagmar B. Human papillomavirus oncogenic expression in the dysplastic portio; an investigation of biopsies from 190 cervical cones. Br J Cancer. 2004;90:1407–1413. doi: 10.1038/sj.bjc.6601691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa M, Stites D, Patel S, Scott M, Hills N, Palefsky J, Moscicki AB. Persistence of Human Papillomavirus 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigen. J Infect Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 8.Moscicki AB, Hills N, Shiboski S, et al. Risks for incident human papillomavirus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995–3002. doi: 10.1001/jama.285.23.2995. [DOI] [PubMed] [Google Scholar]
- 9.Gravitt P, Peyton CL, Alessi TQ, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viscidi RP, Ahdieh-Grant L, Clayman B, et al. Serum Immunoglobulin G Response to Human Papillomavirus Type 16 Virus- Like Particles in Human Immunodeficiency Virus (HIV)-Positive and Risk-Matched HIV-Negative Women. J Infect Dis. 2003;187:194–205. doi: 10.1086/346052. [DOI] [PubMed] [Google Scholar]
- 11.Wang SS, Schiffman M, Shields TS, et al. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10000 women in Costa Rica. Br J Cancer. 2003;89:1248–1254. doi: 10.1038/sj.bjc.6601272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostrow RS, Za Chow KR, Nijmura M, Okagaki T, Muller S, Bender M, Faras AJ. Detection of Human Papillomavirus DNA in human semen. Science. 1986;231:731–733. doi: 10.1126/science.3003908. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann AM, Nieland JD, Jochmus I, et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3) Int J Cancer. 2007 doi: 10.1002/ijc.23022. [DOI] [PubMed] [Google Scholar]
- 14.Roman LD, Wilczynski S, Muderspach LI, et al. A phase II study of Hsp-7 (SGN-00101) in women with high-grade cervical intraepithelial neoplasia. Gynecol Oncol. 2007;106:558–566. doi: 10.1016/j.ygyno.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Massa S, Franconi R, Brandi R, Muller A, Mett V, Yusibov V, Venuti A. Anti-cancer activity of plant-produced HPV16 E7 vaccine. Vaccine. 2007;25:3018–3021. doi: 10.1016/j.vaccine.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Zehbe I, Wilander E, Delius H, Tommasino M. Human papillomavirus 16 E6 variants are more prevalent in invasive cervical carcinoma than the prototype. Cancer Res. 1998;58:829–833. [PubMed] [Google Scholar]
- 17.Kyo S, Inoue M, Koyama M, Fujita M, Tanizawa O, Hakura A. Detection of high-risk human papillomavirus in the cervix and semen of sex partners. J Infect Dis. 1994;170:682–685. doi: 10.1093/infdis/170.3.682. [DOI] [PubMed] [Google Scholar]
- 18.Woodworth CD. HPV Innate Immunity. Front Biosci. 2002;7:d2058–2071. doi: 10.2741/A898. [DOI] [PubMed] [Google Scholar]
- 19.Onda T, Carter JJ, Koutsky LA, et al. Characterization of IgA response among women with incident HPV 16 infection. Virology. 2003;312:213–221. doi: 10.1016/s0042-6822(03)00196-x. [DOI] [PubMed] [Google Scholar]
- 20.Bontkes HJ, de Gruijl TD, Bijl A, et al. Human papillomavirus type 16 E2-specific T-helper lymphocyte responses in patients with cervical intraepithelial neoplasia. J Gen Virol. 1999;80:2453–2459. doi: 10.1099/0022-1317-80-9-2453. [DOI] [PubMed] [Google Scholar]
- 21.Davidson EJ, Sehr P, Faulkner RL, et al. Human papillomavirus type 16 E2- and L1-specific serological and T-cell responses in women with vulval intraepithelial neoplasia. J Gen Virol. 2003;84:2089–2097. doi: 10.1099/vir.0.19095-0. [DOI] [PubMed] [Google Scholar]
- 22.Welters MJ, de Jong A, van den Eeden SJ, et al. Frequent display of Human Papillomavirus type 16 E6-specific memory T-helper cells in the healthy population as witness of previous viral encounter. Cancer Research. 2003;63::636–641. [PubMed] [Google Scholar]