Abstract
The Cornelia de Lange syndrome (CdLS) (OMIM# 122470) is a dominantly inherited multisystem developmental disorder. The phenotype consists of characteristic facial features, hirsutism, abnormalities of the upper extremities ranging from subtle changes in the phalanges and metacarpal bones to oligodactyly and phocomelia, gastroesophageal dysfunction, growth retardation, and neurodevelopmental delay. Prevalence is estimated to be as high as 1 in 10,000. Recently, mutations in NIPBL were identified in sporadic and familial CdLS cases. To date, mutations in this gene have been identified in over 45% of individuals with CdLS. NIPBL is the human homolog of the Drosophila Nipped-B gene. Although its function in mammalian systems has not yet been elucidated, sequence homologs of Nipped-B in yeast (Scc2 and Mis4) are required for sister chromatid cohesion during mitosis, and a similar role was recently demonstrated for Nipped-B in Drosophila. In order to evaluate NIPBL role in sister chromatid cohesion in humans, metaphase spreads on 90 probands (40 NIPBL mutation positive and 50 NIPBL mutation negative) with CdLS were evaluated for evidence of precocious sister chromatid separation (PSCS). We screened 50 metaphases from each proband and found evidence of PSCS in 41% (compared to 9% in control samples). These studies indicate that NIPBL may play a role in sister chromatid cohesion in humans as has been reported for its homologs in Drosophila and yeast.
Keywords: cornelia de Lange syndrome, CdLS, NIPBL, Nipped-B, precocious sister chromatid separation, PSCS
Introduction
The Cornelia de Lange syndrome (CdLS), also termed the Brachmann de Lange syndrome (BDLS) (OMIM# 122470), is a dominantly inherited genetic multisystem developmental disorder. The phenotype consists of characteristic facial features (Fig. 1), hirsutism, abnormalities of the upper extremities (ranging from subtle changes in the phalanges and metacarpal bones to oligodactyly and phocomelia), gastroesophageal dysfunction, growth retardation, and neurodevelopmental delay [Ireland and Burn, 1993; Jackson et al., 1993]. Other frequently seen findings include ptosis, myopia, cryptorchidism, hypospadias, pyloric stenosis, congenital diaphragmatic hernias, cardiac septal defects, seizures, and hearing loss. The mental retardation seen in CdLS, although typically moderate to severe, does display a wide range of variability with IQs ranging from 30 to 86 with an average of 53.
Fig. 1.
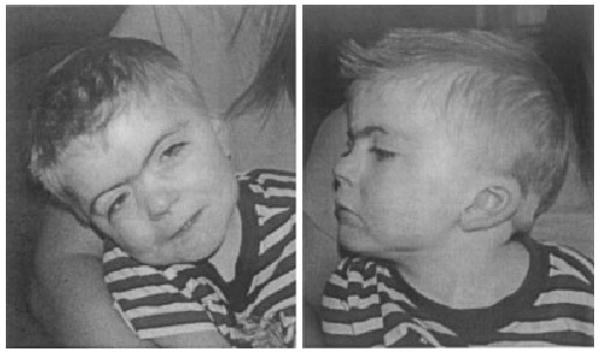
Full face and profile of a child with typical facial features of Cornelia de Lange syndrome. Note ptosis, synophrys, long eyelashes, depressed nasal bridge with upturned nasal tip, posterior-rotated ears, thin upper lip with down-turned corners and a small chin.
Recently, mutations in NIPBL were identified in sporadic and familial CdLS cases. To date, mutations in this gene have been identified in over 45% of individuals with CdLS [Gillis et al., 2004]. NIPBL is the human homolog of the Drosophila Nipped-B gene. Although its function in mammalian systems has not been elucidated, Nipped-B has been shown to be an essential regulator of cut, Ultrabithorax, and Notch receptor signaling in Drosophila. Sequence homologs of Nipped-B in yeast (Scc2 and Mis4) are required for sister chromatid cohesion during mitosis, and a similar role was recently demonstrated for Nipped-B in Drosophila [Rollins et al., 2004]. In order to evaluate NIPBL's role in sister chromatid cohesion in humans, metaphase spreads on a large cohort of mutation positive and mutation negative probands with CdLS were evaluated for evidence of precocious sister chromatid separation (PSCS). PSCS was seen in a significant number of CdLS probands when compared to unaffected controls. These studies indicate that NIPBL may play a role in sister chromatid cohesion in humans as has been reported for its homologs in Drosophila and yeast. The identification of PSCS in individuals with CdLS may be a helpful diagnostic aid, as NIPBL mutational analysis is currently only available on a research basis and mutations are identified in only ∼45% of affected probands.
Materials and Methods
Cornelia de Lange Syndrome Patients
All individuals enrolled in the study were ascertained by the authors as having a diagnosis of CdLS. All patients and family members were enrolled in the study under an IRB-approved protocol of informed consent at The Children's Hospital of Philadelphia.
Chromosomal Analysis and Evaluation for PSCS
Metaphase spreads were prepared for the 90 CdLS probands and 90 controls without clinical features of CdLS, or other conditions known to be associated with PSCS, from either whole blood (cultured in RPMI 1640 with 15% fetal bovine serum and phytohemagglutinin for 72 hr) or lymphoblastoid cell lines (transformed with Epstein–Barr Virus and harvested during the log phase). Cells were arrested at metaphase with 0.8 μg/ml Colchicine (SIGMA-ALDRICH) for 20 min at 37°C, hypotonized with 0.075 M KCL at room temperature and fixed with three parts methanol: 1 part acetic acid. The slides were stained with Wright's Stain (Fisher Scientific). Ten proband slides were C-banded (constitutive heterochromatin). A minimum of 50 metaphases were microscopically examined and scored for PSCS. PSCS was diagnosed when the sister chromatids were completely separated and no connection at the centromere was seen [Plaja et al., 2003]. A metaphase was scored as positive for PSCS if all or the majority of sister chromatids in the metaphase spread demonstrated sister chromatid separation. A positive PSCS score was recorded for any individual with at least one metaphase per slide demonstrating PSCS.
Mutational Analysis Using Conformation Sensitive Gel Electrophoresis (CSGE)
Conformation sensitive gel electrophoresis (CSGE) was carried out using standard protocols [Ganguly et al., 1993]. Oligonucleotide primer sequences and PCR conditions used for amplification of all exons of the NIPBL gene are as previously reported [Gillis et al., 2004]. PCR products corresponding to all altered migration patterns (shifts) were purified using QIAquick® PCR purification kit, (QIAGEN Sciences) and sequenced on an ABI 377 sequencer.
Results
In order to evaluate NIPBL's role in sister chromatid cohesion in humans, metaphase spreads on 90 CdLS probands (40 NIPBL mutation positive and 50 NIPBL mutation negative) were evaluated for evidence of PSCS (Table I). We screened a minimum of 50 metaphases from each proband and found evidence of PSCS in 37 of 90 probands (41%) (Fig. 2). Of these 37 probands with PSCS, 16 (43%) were mutation positive and 21 (57%) mutation negative. Of the 53 probands without evidence of PSCS, 24 (45%) were mutation positive and 29 (54%) were mutation negative. Ninety control slides were screened and 8 (9%) demonstrated evidence of PSCS. Both severe and mild CdLS phenotypes were seen in the PSCS positive and negative groups. Missense, frameshift, and nonsense mutations have been seen in both groups. Two of the patients with PSCS have the same missense mutation: R2298H in exon 40 in a highly conserved amino acid residue. An average of 2.05 metaphases (4%) (range: 2%–10%) were found to have PSCS in the CdLS probands where as in the PSCS positive control samples an average of 1.125 metaphases (2%) (range 2%–4%) were found to have PSCS. All PSCS positive control samples had only one PSCS per sample except for one sample that had two. Results of PSCS screening from the CdLS cohort with NIPBL mutation status are listed in (Table II). Additionally, several metaphase spreads from individuals with CdLS demonstrated some evidence of breakage (Fig. 2I).
TABLE I.
Observed Instances of PSCS in Metaphase Spreads From CdLS Probands and Controls
| CdLS probands (N = 90) | |||||
|---|---|---|---|---|---|
| NIPBL mutation positive (N = 40) | NIPBL mutation negative (N = 50) | Controls (N = 90) | |||
| PSCS positive | PSCS negative | PSCS positive | PSCS negative | PSCS positive | PSCS negative |
| 16/40 (40%) | 24/40 (60%) | 21/50 (42%) | 29/50 (58%) | 8/90 (9%) | 82/90 (91%) |
| Total PSCS positive: 37/90 (41%)* | Total PSCS positive: 8/90 (9%)* | ||||
Chi-square test demonstrates a P-value = 0.0000003.
Fig. 2.

Metaphase spreads in individuals with CdLS and unaffected controls. A: Control metaphase spread stained with giemsa. B: Control C-stained metaphase. C–E: Metaphase spread from three individuals with CdLS stained with giemsa demonstrating PSCS. Note separated sister chromatids and centromeres present in practically all sister chromatids. F–H: C-stained metaphases from individuals with CdLS demonstrating the premature division and separation of the centromeres in the majority of sister chromatids. I: A metaphase form an individual with CdLS demonstrating apparent chromatid breaks (arrowheads).
TABLE II.
Characteristics of PSCS-Positive Individuals With CdLS
| Proband # | PSCS (%) | Male(M)/Female(F) | Mutation |
|---|---|---|---|
| 1 | 6 | M | p.A1246G |
| 2 | 6 | F | Negative |
| 3 | 4 | M | g.3057–3060delTAGA |
| 4 | 6 | M | p.R1723X |
| 5 | 4 | F | Negative |
| 6 | 6 | F | Negative |
| 7 | 4 | F | Negative |
| 8 | 2 | M | Negative |
| 9 | 4 | F | Negative |
| 10 | 6 | F | Negative |
| 11 | 4 | F | g.961delA |
| 12 | 2 | F | g.1669-1670insC |
| 13 | 4 | M | p.R2298H |
| 14 | 2 | F | Negative |
| 15 | 2 | F | Negative |
| 16 | 6 | M | p.R832X |
| 17 | 2 | F | Negative |
| 18 | 2 | F | Negative |
| 19 | 4 | F | g.2479–2480delAG |
| 20 | 2 | M | Negative |
| 21 | 2 | M | Negative |
| 22 | 2 | M | p.R2298H |
| 23 | 4 | M | g.6763 + 5 G > T |
| 24 | 6 | M | Negative |
| 25 | 4 | F | g.4069–4070insG |
| 26 | 4 | M | p.G2381A |
| 27 | 10 | F | Negative |
| 28 | 8 | M | Negative |
| 29 | 2 | F | Negative |
| 30 | 6 | F | g.7825–7826insG |
| 31 | 6 | F | Negative |
| 32 | 6 | F | p.S1459X |
| 33 | 6 | F | Negative |
| 34 | 4 | F | Negative |
| 35 | 2 | F | Negative |
| 36 | 4 | M | g.5721–5725delTGAAA |
| 37 | 2 | M | p.R479X |
Discussion
In eukaryotic cells, replicated DNA molecules remain physically connected from their synthesis in S phase until they are separated during anaphase. This phenomenon, called sister chromatid cohesion, is essential for the temporal separation of DNA replication and mitosis and for the equal separation of the duplicated genome. Sister chromatids in metaphase chromosomes are physically connected until their separation during anaphase [Nasmyth et al., 2000, 2001]. The “glue” that holds sister chromatids together is a protein complex called “cohesin.” Cohesin is a four-member protein complex composed of a heterodimer of SMC1 and SMC3 associated with SCC1 and SCC3 proteins [Hagstrom and Meyer, 2003]. Several other proteins are involved in the loading, unloading, maintenance, and function of this complex. NIPBL, the gene that results in CdLS when mutated, codes for a protein that appears to be involved in the loading and unloading of the cohesin complex onto and off of the chromosomes [Rollins et al., 2004]. The inability to properly regulate the loading and unloading of the cohesin complex, which is regularly spaced on mammalian chromosomes at approximately 20 kb intervals, in CdLS appears to disrupt long-range enhancer–promoter interactions and likely is the cause of the main phenotypic findings in this disorder [Krantz et al., 2004; Rollins et al., 2004]. This study investigated whether or not the role of the cohesin complex in sister chromatid cohesion was also affected by alterations of NIPBL in CdLS probands. PSCS is a phenomenon whereby separate and splayed chromatids, with discernible centromeres, are seen and involves all or most chromosomes of a metaphase [Kajii and Asamoto, 2004]. It involves not only the centromere but also the entire sister chromatids of almost all mitotic chromosomes in a given metaphase [Kajii and Ikeuchi, 2004]. PSCS has been described in a number of conditions including Roberts syndrome [German, 1979], Fanconi Anemia, Ataxia Teleangiectasia [Mehes and Buhler, 1995], Alzheimer disease [Moorhead and Heyman, 1983; Spremo-Potparevic et al., 2004], Tuberous Sclerosis [Scappaticci et al., 1988], and Variegated Aneuploidy [Kajii et al., 1998; Plaja et al., 2001, 2003]. Recently, mutations in the BUB1B gene were found to be a cause of multiple variegated aneuploidy [Hanks et al., 2004]. BUB1B encodes BUBR1, a key protein in the mitotic spindle checkpoint. PSCS has also been described in association with cancer, such as Wilms tumor [Mehes et al., 2002] and breast cancer [Rao et al., 1996]. PSCS has been seen in spontaneous abortions [Keser et al., 1996] and also in normal individuals after exposure to genotoxic chemicals [Major et al., 1999]. PSCS has also been reported to be present in a low percentage (less than 2%–3%) of normal individuals [Dominguez and Rivera, 1992; Kajii and Ikeuchi, 2004].
Due to the role-played by the yeast homologs of NIPBL (Scc2, Rad21, Mis4) in sister chromatid cohesion and the evidence that a similar cohesion abnormality is seen in Drosophila [Rollins et al., 2004], we hypothesized that a similar phenomenon may be present in individuals with CdLS. In studying a minimum of 50 metaphase spreads in each of 90 CdLS individuals and 90 control subjects, we found a significant increase in PSCS (41%) in the CdLS samples over control samples (9%) (P-value 0.0000003). The presence or absence of PSCS in CdLS did not appear to be influenced by the presence or absence of an identified mutation in NIPBL or by the age or sex of the individuals with CdLS.
The finding of several metaphases in some of the CdLS probands demonstrating apparently increased breakage (not observed in any of the controls) (Fig. 2I) indicates that there may be some predisposition to chromosomal fragility in CdLS probands; however, this has not been formally assessed as part of this study and warrants further investigation.
It is of interest that Roberts syndrome, an autosomal recessive condition that has phenotypic overlap with CdLS consisting of craniofacial anomalies, tetraphocomelia, cardiac septal defects with growth and cognitive retardation that has been classically associated with PSCS, was recently found to be caused by mutations in the ESCO2 gene [Vega et al., 2005]. The ESCO2 gene is a member of a conserved protein family required for the establishment of sister chromatid cohesion during the S phase of the cell cycle. This discovery implicates disruption of the cohesion complex in another human developmental disorder, with involvement of organ systems in a strikingly conserved pattern to that seen in CdLS.
The identification of PSCS in individuals with CdLS may be of value diagnostically. Presently mutations in NIPBL are identified in approximately 45% of individuals with a clear diagnosis of CdLS [Gillis et al., 2004; Krantz et al., 2004; Tonkin et al., 2004]. There has not been any clear evidence that there are other loci for a CdLS gene at this time and it may be that the low mutation detection rate is complicated by the large size of the NIPBL gene and incomplete characterization of the entire coding region [Gillis et al., 2004]. The development of an auxiliary test, such as screening for PSCS, could help with supporting a diagnosis in individuals with CdLS in whom an NIPBL mutation has not been identified, or in whom testing was not performed, as NIPBL mutational analysis is not clinically available as of the writing of this manuscript. Further work is needed to determine whether PSCS can be in induced in all individuals with CdLS by altering culture or pellet preparation conditions (i.e., tonicity of media, temperature) without affecting the rate of this phenomenon in unaffected individuals.
Acknowledgments
We would acknowledge the generous contributions of the families studied in this report as well as the support of the CdLS Foundation.
Grant sponsor: National Institutes of Health (NICHD) (to IDK); Grant number: RO1 HD039323; Grant sponsor: NIDDK (to NBS); Grant number: RO1 DK53104.
References
- Dominguez MG, Rivera H. C-anaphases: A mitotic variant. Ann Genet. 1992;35(3):183–185. [PubMed] [Google Scholar]
- Ganguly A, Rock MJ, Prockop DJ. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: Evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci USA. 1993;90(21):10325–10329. doi: 10.1073/pnas.90.21.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J. Roberts' syndrome. I. Cytological evidence for a disturbance in chromatid pairing. Clin Genet. 1979;16(6):441–447. doi: 10.1111/j.1399-0004.1979.tb01354.x. [DOI] [PubMed] [Google Scholar]
- Gillis LA, McCallum J, Kaur M, DeScipio C, Yaeger D, Mariani A, Kline AD, Li HH, Devoto M, Jackson LG, Krantz ID. NIPBL mutational analysis in 120 individuals with Cornelia de Lange syndrome and evaluation of genotype-phenotype correlations. Am J Hum Genet. 2004;75(4):610–623. doi: 10.1086/424698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom KA, Meyer BJ. Condensin and cohesin: More than chromosome compactor and glue. Nat Rev Genet. 2003;4:520–534. doi: 10.1038/nrg1110. [DOI] [PubMed] [Google Scholar]
- Hanks S, Coleman K, Reid S, Plaja A, Firth H, Fitzpatrick D, Kidd A, Mehes K, Nash R, Robin N, Shannon N, Tolmie J, Swansbury J, Irrthum A, Douglas J, Rahman N. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet. 2004;36(11):1159–1161. doi: 10.1038/ng1449. [DOI] [PubMed] [Google Scholar]
- Ireland M, Burn J. Cornelia de Lange syndrome—Photo essay. Clin Dysmorphol. 1993;2(2):151–160. [PubMed] [Google Scholar]
- Jackson L, Kline AD, Barr MA, Koch S. de Lange syndrome: A clinical review of 310 individuals. Am J Med Genet. 1993;47(7):940–946. doi: 10.1002/ajmg.1320470703. [DOI] [PubMed] [Google Scholar]
- Kajii T, Asamoto A. Prenatal diagnosis of a heterozygous carrier of premature chromatid separation (PCS) trait. Am J Med Genet A. 2004;126(4):432. doi: 10.1002/ajmg.a.20615. [DOI] [PubMed] [Google Scholar]
- Kajii T, Ikeuchi T. Premature chromatid separation (PCS) vs. premature centromere division (PCD) Am J Med Genet A. 2004;126(4):433–434. doi: 10.1002/ajmg.a.20612. [DOI] [PubMed] [Google Scholar]
- Kajii T, Kawai T, Takumi T, Misu H, Mabuchi O, Takahashi Y, Tachino M, Nihei F, Ikeuchi T. Mosaic variegated aneuploidy with multiple congenital abnormalities: Homozygosity for total premature chromatid separation trait. Am J Med Genet. 1998;78(3):245–249. doi: 10.1002/(sici)1096-8628(19980707)78:3<245::aid-ajmg7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Keser I, Luleci G, Gunduz G. Premature centromere division in three unrelated families. Ann Genet. 1996;39(2):87–90. [PubMed] [Google Scholar]
- Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJ, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li HH, Devoto M, Jackson LG. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet. 2004;36(6):631–635. doi: 10.1038/ng1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major J, Jakab MG, Tompa A. The frequency of induced premature centromere division in human populations occupationally exposed to genotoxic chemicals. Mutat Res. 1999;445(2):241–249. doi: 10.1016/s1383-5718(99)00129-1. [DOI] [PubMed] [Google Scholar]
- Mehes K, Buhler EM. Premature centromere division: A possible manifestation of chromosome instability. Am J Med Genet. 1995;56(1):76–79. doi: 10.1002/ajmg.1320560117. [DOI] [PubMed] [Google Scholar]
- Mehes K, Kajtar P, Kosztolanyi G. Association of nonsyndromic Wilms tumor with premature centromere division (PCD) Am J Med Genet. 2002;112(2):215–216. doi: 10.1002/ajmg.10661. [DOI] [PubMed] [Google Scholar]
- Moorhead PS, Heyman A. Chromosome studies of patients with Alzheimer disease. Am J Med Genet. 1983;14(3):545–556. doi: 10.1002/ajmg.1320140319. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Peters JM, Uhlmann F. Splitting the chromosome: Cutting the ties that bind sister chromatids. Science. 2000;288(5470):1379–1385. doi: 10.1126/science.288.5470.1379. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Peters JM, Uhlmann F. Splitting the chromosome: Cutting the ties that bind sister chromatids. Novartis Found Symp. 2001;237:113–133. doi: 10.1002/0470846666.ch10. discussion 133–138, 158–163. [DOI] [PubMed] [Google Scholar]
- Plaja A, Vendrell T, Smeets D, Sarret E, Gili T, Catala V, Mediano C, Scheres JM. Variegated aneuploidy related to premature centromere division (PCD) is expressed in vivo and is a cancer-prone disease. Am J Med Genet. 2001;98(3):216–223. doi: 10.1002/1096-8628(20010122)98:3<216::aid-ajmg1091>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Plaja A, Mediano C, Cano L, Vendrell T, Sarret E, Farran I, Sanchez MA. Prenatal diagnosis of a rare chromosomal instability syndrome: Variegated aneuploidy related to premature centromere division (PCD) Am J Med Genet A. 2003;117(1):85–86. doi: 10.1002/ajmg.a.10810. [DOI] [PubMed] [Google Scholar]
- Rao NM, Joshi NN, Shinde SR, Advani SH, Ghosh SN. Premature separation of centromere and aneuploidy: An indicator of high risk in unaffected individuals from familial breast cancer families? Eur J Cancer Prev. 1996;5(5):343–350. doi: 10.1097/00008469-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Rollins RA, Korom M, Aulner N, Martens A, Dorsett D. Drosophila nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol Cell Biol. 2004;24(8):3100–3111. doi: 10.1128/MCB.24.8.3100-3111.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scappaticci S, Cerimele D, Tondi M, Vivarelli R, Fois A, Fraccaro M. Chromosome abnormalities in tuberous sclerosis. Hum Genet. 1988;79(2):151–156. doi: 10.1007/BF00280555. [DOI] [PubMed] [Google Scholar]
- Spremo-Potparevic B, Zivkovic L, Djelic N, Bajic V. Analysis of premature centromere division (PCD) of the X chromosome in Alzheimer patients through the cell cycle. Exp Gerontol. 2004;39(5):849–854. doi: 10.1016/j.exger.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet. 2004;36(6):636–641. doi: 10.1038/ng1363. [DOI] [PubMed] [Google Scholar]
- Vega H, Waisfisz Q, Gordillo M, Sakai N, Yanagihara I, Yamada M, van Gosliga D, Kayserili H, Xu C, Ozono K, Wang Jabs E, Inui K, Joenje H. Roberts syndrome is caused by mutations in ESCO2, a human homolog of yeast ECO1 that is essential for the establishment of sister chromatid cohesion. Nat Genet. 2005;37:468–470. doi: 10.1038/ng1548. [DOI] [PubMed] [Google Scholar]


