Abstract
BACKGROUND
Recent studies have demonstrated the significant prognostic value of stress CMR myocardial perfusion imaging (CMRMPI). Apart from characterizing reversible perfusion defect (RevPD) from flow-limiting coronary stenosis, CMR late enhancement imaging (LGE) is currently the most sensitive method in detecting subendocardial infarction (MI). We therefore tested the hypothesis that, characterization of these 2 processes from coronary artery disease (CAD) by CMR can provide complementary prognostic values.
Methods and Results
We performed CMRMPI followed by LGE imaging on 254 patients referred with symptoms of myocardial ischemia. At a median follow up of 17 months, 49 cardiac events (MACE) occurred including 12 cardiac deaths, 16 acute myocardial infarction (MI), and 21 cardiac hospitalizations. RevPD and LGE both maintained a > 3-fold association with cardiac death or acute MI (Death/MI) when adjusted to each other and to the effects of patient age and gender (adjusted HR 3.31, P=0.02 and 3.43, P=0.01, respectively). In patients without a history of MI who had negative RevPD, LGE presence was associated with >11-fold hazards increase to Death/MI. Patients with neither RevPD nor LGE had a 98.1% negative annual event rate for Death/MI. For association with MACE, RevPD was the strongest multivariable variable in the best overall model (HR 10.92, P<0.0001).
Conclusions
CMR imaging provides robust risk-stratification of patients who presents with symptoms of ischemia. Characterization of RevPD and LGE by CMR provides strong and complementary prognostic implication towards cardiac death or acute MI.
Keywords: Magnetic Resonance, Myocardial Ischemia, Perfusion, Mortality, Infarction
INTRODUCTION
Recent data have demonstrated that cardiac magnetic resonance myocardial perfusion imaging (CMRMPI) provides strong prognosis for cardiac events in patients suspected to have myocardial ischemia. In a study of 513 patients with symptoms of suspected ischemia, Jahnke et al. reported strong prognostic association of CMRMPI results with cardiac death or nonfatal myocardial infarction (MI).1 Apart from characterizing reversible perfusion defect reflecting hemodynamically significant CAD, late gadolinium enhancement (LGE) imaging offers the most sensitive method in detecting subendocardial MI. It is unclear whether combining CMRMPI and LGE imaging provide complementary diagnostic and prognostic value important to patient care. In this study, we hypothesize that since CMRMPI and LGE describe different aspects of CAD, combining the diagnostic information of CMRMPI and LGE can provide incremental prognostic association with adverse cardiac events during follow-up.
METHODS
Patient Population
We studied 264 patients (156 males, mean age 56±13 years) referred to undergo CMRMPI. Presenting signs/symptoms were categorized by the Diamond and Forrester Criteria2 included typical angina (n=37), atypical angina (n=129), and non-anginal symptoms (n=88). We determined the age and gender-specific pretest likelihood of CAD by the combined Diamond/Forrester and CASS registry recommended by the ACC/AHA 2002 guideline.3-5 Patients who had a history of CAD at the time of CMR referral were not excluded. Patients were excluded from performing CMR by: 1) acute chest pain consistent with unstable angina, 2) decompensated heart failure, 3) hemodynamic instability, 4) a history of contraindication to vasodilator use, or 5) metallic hazards. Medical history was obtained immediately before the CMR. Since June 2006, patients with renal dysfunction were also excluded if serum glomerular filtration rate within prior 30 days was ≤30 ml/min/1.73m2. Hypertension, hypercholesterolemia, diabetes, family history of premature CAD, and coronary risk factors were defined by published criteria.6 Significant smoking was defined by current smoking or prior tobacco use of > 10 pack-years. Patients provided informed consent prior to the CMR. Institutional ethics committee of our institution approved the study for patient follow-up.
Vasodilator Stress CMR Perfusion and LGE Imaging Protocol
Patients underwent CMR supine in a 1.5T scanner (Signa CV/i, General Electric Healthcare) with an 8-element cardiac phased-array receiver coil. Cine steady-state free-precession (typical repetition time, 3.4 ms; echo time, 1.2 ms; temporal resolution, 40-50 ms; in-plane spatial resolution approximately 1.5×2.0 mm) was used for imaging left ventricular (LV) size and function in parallel short-axis (slice thickness 8 mm with 0 mm spacing) and 3 radial long-axis views. Patients were instructed to refrain from caffeine, tobacco, and medications such as aminophylline for 24 hours before CMR and kept in a > 4-hour fasted state. For CMRMPI, a T1-weighted notched saturation fast gradient-echo (typical TR=6 msec, TE=2.5ms, FOV 32–40 cm) was acquired while a first-pass bolus of contrast (gadolinium-DTPA, Magnevist, Berlex, Wayne, New Jersey) was injected (0.075-0.1 mmol/kg at 5 ml/s) during peak vasodilatation. Adenosine was the vasodilating agent of choice but dipyridamole was used in 30 cases (11%) when requested by the referral physicians. Adenosine was administered intravenously at 140 mcg/kg/min over six minutes and CMRMPI was acquired during the last minute of the infusion. Dipyridamole was infused at 0.56 mg/kg/min over 4 minutes with an additional dose of 0.28 mg/kg/min in order to achieve a 10% heart rate increase from baseline within a 3-minute interval after the infusion. CMRMPI was acquired when a 10% heart rate increase was observed or 3 minutes beyond dipyridamole infusion. While most CMRMPI were performed with 6-9 short-axis locations acquired over every other heartbeat, early in the study period CMRMPI was acquired from 4-5 slice locations over every heartbeat. At least 10 minutes after the stress CMRMPI, we acquired resting CMRMPI with an additional bolus of gadolinium (0.075-0.1 mmol/kg at 5 ml/s) using matching slice locations and pulse sequence. When dipyridamole was used, rest perfusion was performed first followed by stress perfusion > 10 minutes later. LGE imaging was performed using a previously described inversion recovery pulse sequence7 at locations matching cine function, starting at 10 minutes after the second CMRMPI. We optimized the inversion time (200 to 300 ms) to achieve a signal intensity of < 10 in the anteroseptal wall. The total CMR scan time was approximately 50 minutes.
CMR Image Analysis
All images were analyzed with specialized software (CineTool 5.43, GE Healthcare) blinded to outcome. Epicardial and endocardial borders at end-systole and end-diastole were manually traced to determine the LV ejection fractions (LVEF), end-diastolic volume (LVEDV), end-systolic volume (LVESV), and end-diastolic LV myocardial mass and indexed to body surface area when appropriate.8 LVEF was calculated using the standard Simpson’s rule.9 For CMRMPI, two readers (RYK, KES) jointly interpreted rest and stress CMRMPI (side-by-side display) in a subsequent session, blinded to clinical information, patient outcome, or cine LV function and LGE data. Using the AHA/ACC 17-segment nomenclature10, segmental perfusion was interpreted as normal or abnormal. Each segmental perfusion was scored based on the transmural extent of any perfusion defect (0=no defect, 1=1-25%, 2=26-50%, 3=51-75%, and 4=76-100%). CMRMPI of the apical cap (segment 17) could not be assessed due to the short-axis acquisition and this segment was treated as missing. A perfusion defect is significant only if it persisted beyond peak myocardial enhancement. When uncertainty of this existed, we derived signal intensity versus time curve from a remote region to determine the time frame of peak myocardial enhancement. Presence of a reversible perfusion defect (RevPD) was defined by any segmental worsening of the transmural score during stress by ≥ 1 compared to at rest. Summed stress (SSS) and rest scores (SRS) were calculated by summing up the transmural scores of all 16 segments from stress and rest CMRMPI, respectively, and their difference yielded the summed difference score (SDS). Perfusion defects that were not reversible and did not demonstrate any LGE in the same segment were considered artifacts. In a session separate from CMRMPI reading, any segmental presence of endocardial LGE (LV apical cap included) consistent with MI was recorded using the same AHA/ACC 17-segment nomenclature. Infarct mass was quantified using a semi-automated algorithm using signal intensity > 2 SD above the mean signal intensity of a remote myocardial region.11-13
For both CMRMPI and LGE, corresponding coronary assignments were based on the same AHA/ACC 17-segmental nomenclature.14 RevPD in left anterior descending (RevPDLAD), circumflex(RevPDLCx), and right coronary artery (RevPDRCA) territories were all binary variables (presence or absence of RevPD). A similar coronary assignment was used for LGE (LGELAD, LGELCx, and LGERCA, respectively).
Quantitative Coronary Angiography
Coronary angiography was performed at the discretion of the attending cardiologists. An experienced interventional cardiologist performed quantitative coronary angiographic (QCA) analysis blinded to the patients’ history and clinical outcome. Two orthogonal views of each BARI-defined segment was used to detect significant stenosis (QCAStenosis) which was defined by ≥ 70% luminal narrowing in the more severe view (≥ 50% for left main stenosis).
Follow-Up of Clinical Events
At least 6 months after the CMR, we contacted patients either by telephone or mailed questionnaire, hospital chart review, and/or correspondence with the patient’s physicians. We also obtained institutional approval to search an electronic data registry regarding patient hospitalization after the CMR. For patients who could not be contacted, we referenced patient survival and cause of death from the Social Security Death Index and any available death certificates provided by the Commonwealth of Massachusetts. We considered the following major adverse cardiac events (MACE): cardiac death, new acute MI, unstable angina hospitalization, and coronary revascularization performed beyond 30 days. We further defined cardiac death or new acute MI (Death/MI) as an endpoint of interests. Death was considered cardiac if it was preceded by acute MI, acute or exacerbation of cardiac failure, or documented fatal arrhythmia. Any other unexpected death without a non-cardiac cause was also considered cardiac. New MI was defined by hospitalization with symptoms consistent with acute MI and elevation of serum troponins of > 2-fold in a temporal profile consistent with acute MI. When a patient experienced > 1 event, the first event was chosen. While patients who died from non-cardiac causes were censored at the time of death, early (within 30 days after CMR) coronary revascularizations were not used as censoring events. CMR results including RevPD and LGE were made available to the ordering physicians on the day of the CMR.
Statistical Analyses
Prognostic Association of RevPD and LGE with Death/MI and MACE
Demographic data were compared by Student-t or Fisher exact test, with regards to RevPD presence. Kaplan-Meier distributions, for Death/MI, stratified by the presence of RevPD and LGE, were compared by log-rank tests, respectively. To assess for any prognostic implication of the extent and severity of RevPD, we categorized SDS into tertiles and assessed the unadjusted HR within each SDS tertile to MACE. Presence of LGE and presence of LGE without a history of MI were coded as 2 separate variables in any model selection. To build the best final model for Death/MI and MACE, respectively, we performed multivariable Cox proportional-hazards regression analysis using a stepwise-forward selection using P=0.01 as the criteria for model entry or stay. In addition, in order to determine if RevPD and LGE could provide complementary prognostic association with MACE, we built a model that included patient age, gender, and LV systolic function, and then entered both RevPD and LGE into the model. This approach would determine if RevPD and LGE could maintain prognostic significance not only adjusted to each other, but also adjusted to important clinical risk markers such as age, gender, and left ventricular function.
Detection of QCA Stenosis or Death/MI
We also evaluated the association of RevPD by CMR to angiographic diagnosis of QCAStenosis in the first 12 months after CMR. Since verification bias existed in subsequent referral to coronary angiography, we used clinical events (cardiac death or new MI) within the first 12 months after CMR as an arbiter in patients who did not undergo invasive angiography. Sensitivities and specificities by RevPD and LGE to detect QCAStenosis or Death/MI combined at 12 months were calculated. We first determined, after adjustment to age, gender, and left ventricular systolic function, if RevPD and LGE provided complementary association with (QCAStenosis/MACE12months). We performed stepwise forward logistic regression to select the strongest set of covariates that formed the best final model in predicting QCAStenosis or MACE within the first 12 months after CMR (QCAStenosis/MACE12months). Models were compared by model likelihood chi-square (LRχ2). Receiver operator characteristics (ROC) curves were constructed from these respective models and were compared using c-statistics. To prevent overfitting, the ratio of number of events to covariates was kept to be 5 or higher in all models. In each final multivariable model, the validity of the proportional-hazards assumption was tested by adding a time-dependent interaction variable for each of the covariates in the model. For all analyses, a P value < 0.05 was used to define statistical significance. All analyses were performed with SAS 9.1 (SAS Institute, Cary, NC) for Windows.
RESULTS
Patient Characteristics
Out of the initial 264 patients, 10 patients were excluded due to technical problems including 6 cases of claustrophobia and 4 cases of inability to complete adenosine infusion due to intolerable side effects. Table 1 summarizes the baseline characteristics and the CMR findings of the remaining 254 (150 males, mean age 58±13 years) which formed the study cohort. Patients in the cohort carried an intermediate pre-test coronary risk profile: 57%, 25%, and 22% had a history of hypertension, diabetes, and prior infarction, respectively. There were no complications except one case of self-terminated atrial flutter during vasodilator infusion. Patients experienced an average of 29% increase in heart rate and a 6% drop in systolic blood pressure during stress. Seventy-four patients (29%) demonstrated RevPD by CMR. While the average LVEF and LVEDV index of the study cohort were within normal range, patients with RevPD were older, had more coronary risk factors, higher pre-test CAD likelihood, lower LVEF, and higher ventricular mass and LVEDV index.
Table 1.
| All patients (N=254) |
RevPD Absent (N=180) |
RevPD Present (N=74) |
P-value | ||
|---|---|---|---|---|---|
| Clinical Characteristics | Age, years | 58 ± 13 | 55 ± 14 | 63 ± 11 | <0.0001 |
| Female, % | 41 | 46 | 30 | 0.02 | |
| Body Mass Index, kg/m2 | 29 ± 6 | 28 ± 6 | 29 ± 6 | 0.80 | |
| Cardiac Risk Factors, number | 2.2 ± 1.4 | 2 ± 1 | 3 ± 1 | <0.0001 | |
| Hypertension, % | 57 | 48 | 77 | <0.0001 | |
| Diabetes, % | 25 | 17 | 45 | <0.0001 | |
| Hyperlipidemia, % | 61 | 52 | 82 | <0.0001 | |
| Tobacco use, % | 11 | 8 | 18 | 0.04 | |
| Family Hx Coronary Artery Disease, % | 29 | 28 | 31 | 0.76 | |
| Pre-test Likelihood CAD, % | 29 ± 22 | 25 ± 21 | 40 ± 28 | <0.0001 | |
| Hx MI, % | 22 | 10 | 51 | <0.0001 | |
| Any prior Coronary Intervention | 26 | 14 | 55 | <0.0001 | |
| Hx Percutaneous Intervention, % | 18 | 8 | 31 | <0.0001 | |
| Hx Coronary Bypass Surgery, % | 11 | 7 | 22 | <0.01 | |
| Medication use | Aspirin, % | 56 | 47 | 87 | <0.0001 |
| Beta blocker, % | 53 | 44 | 76 | <0.0001 | |
| ACE inhibitor, % | 37 | 28 | 61 | <0.0001 | |
| Calcium channel blocker, % | 18 | 16 | 23 | 0.15 | |
| HMG-CoA reductase, % | 62 | 54 | 82 | <0.0001 | |
| ECG Findings | Resting heart rate, bpm | 69 ± 14 | 70 ± 14 | 74 ± 14 | 0.89 |
| Sinus rhythm, % | 95 | 97 | 97 | 0.99 | |
| QRS >120 msec, % | 8 | 8 | 10 | 0.80 | |
| Left bundle branch block, % | 5 | 3 | 8 | 0.12 | |
| Left ventricular hypertrophy, % | 1 | 0 | 3 | 0.09 | |
| Significant Q-waves, % | 10 | 6 | 20 | 0.0008 | |
| CMR Measurements | LVEF, % | 58 ± 11 | 60 ± 9 | 52 ± 14 | <0.0001 |
| LV Mass, grams | 121 ± 36 | 115 ± 32 | 135 ± 41 | <0.0001 | |
| LVEDV index, ml/m2 | 83 ± 22 | 79 ± 18 | 92 ± 28 | <0.0001 | |
| LVESV index, ml/m2 | 37 ± 20 | 32 ± 14 | 47 ± 28 | <0.0001 | |
| MI by LGE Imaging, % | 28 | 12 | 67 | <0.0001 | |
| LGE mass, grams# | 11.0 ± 12.4 | 9.8 ± 10.5 | 11.7 ± 13.2 | 0.56 | |
| LGE size, % of LV mass# | 12.1 ± 13.7 | 11.6 ± 13.9 | 12.3 ± 13.8 | 0.84 | |
| LGE mass without Hx. MI, grams£ | 7.2 ± 8.2 | 6.5 ± 7.5 | 7.7 ± 8.9 | 0.71 | |
| LGE without Hx. MI, %LV mass£ | 8.0 ± 1.0 | 6.8 ± 7.3 | 8.8 ± 1.2 | 0.63 | |
Based on N=70 patients with presence of LGE
Based on N=28 patients with LGE but without a history of MI
Association of Presence and Extent of RevPD and LGE with Death/MI and MACE
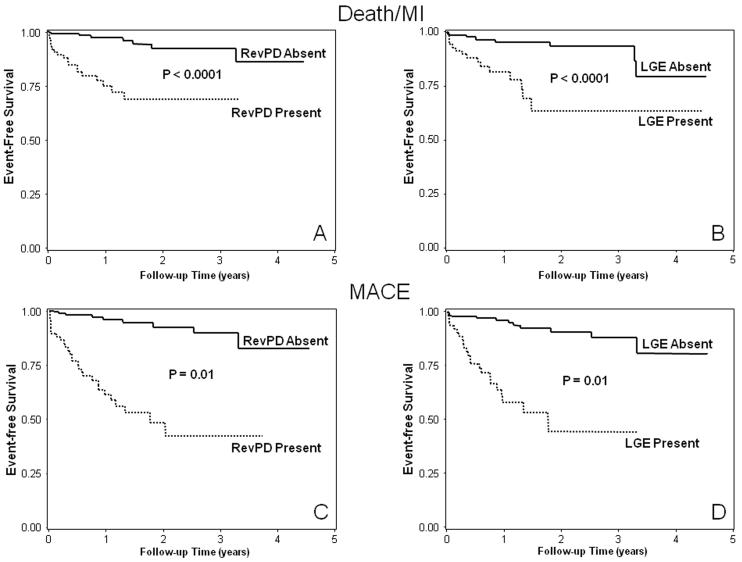
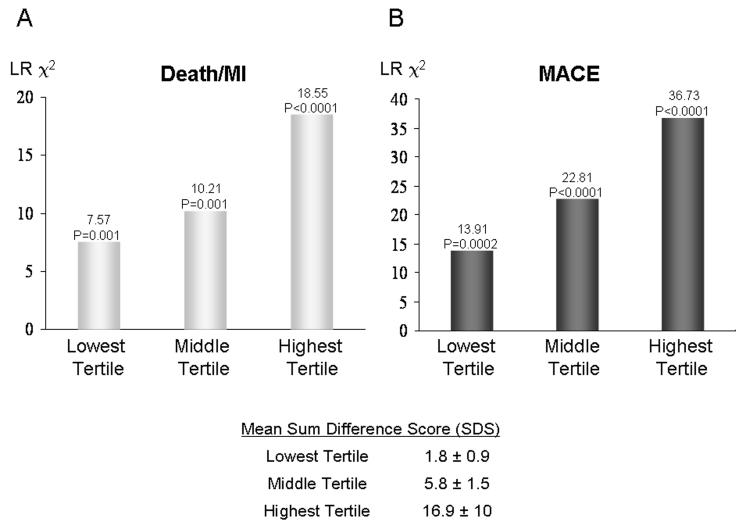
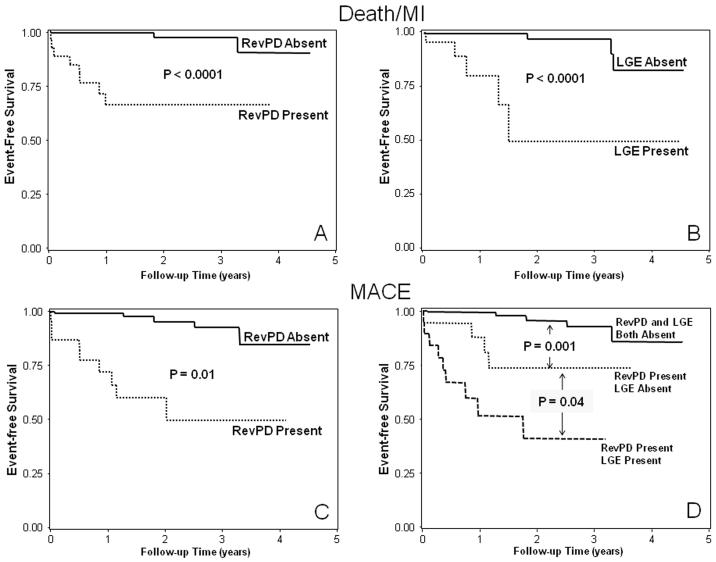
Clinical follow-up was successful in all 254 patients (100%). After a median follow up of 17 months (8 months to 4.7 years), there were 49 events including 12 cardiac deaths, 16 acute MI, 19 unstable anginal hospitalizations, and 2 cases of late coronary revascularization. Another 6 patients died from non-cardiac causes and were censored on the day of death. By univariable analysis (Table 2), presence of RevPD and LGE both demonstrated strong association with Death/MI (HR 6.88 and 5.31, respectively, both P<0.0001) and with MACE (HR 10.92 and 8.09, respectively, both P<0.0001). The Kaplan-Meier curves corresponding to these associations are shown on figures 1A-B and figures 1C-D, for Death/MI and MACE, respectively. By quantitative analysis, the myocardial extent of RevPD and infarct mass by LGE were also strong predictors of both Death/MI and MACE. SDS demonstrated significant association with Death/MI and was the strongest univariable predictor for MACE (LRχ2 36.94, P<0.0001). For every SDS gained hazards to MACE on average increased by 8%. The model LRχ2 for association with Death/MI (figure 2A) and MACE (figure 2B) became progressively higher from the lowest to the highest SDS tertile (compared to patients outside of the tertile of interest) with a mean SDS that ranged from 1.8 (lowest tertile) to 16.9 (highest tertile), indicated a progressive stronger association of increasing SDS with Death/MI and MACE, respectively.
Table 2.
| Death/MI (n=28) |
MACE (n=49) |
|||||||
|---|---|---|---|---|---|---|---|---|
| HR | HR 95% CI | LRχ2 | P-value | HR | HR 95% CI | LRχ2 | P-value | |
| Clinical Characteristics | ||||||||
| Age, years | 1.05 | 1.01-1.08 | 6.77 | 0.01 | 1.06 | 1.03-1.10 | 15.04 | 0.0001 |
| Female | 1.51 | 0.71-3.22 | 1.14 | 0.29 | 0.92 | 0.47-1.80 | 0.05 | 0.82 |
| Body mass index (Kg/m2) | 0.95 | 0.88-1.03 | 1.71 | 0.19 | 1.00 | 0.94-1.05 | 0.03 | 0.87 |
| Number of cardiac risk factors | 1.54 | 1.19-1.99 | 11.04 | <0.001 | 1.74 | 1.39-2.17 | 23.62 | <.0001 |
| Hypertension | 2.79 | 1.17-6.63 | 5.38 | 0.02 | 4.82 | 2.00-11.6 | 12.29 | <0.001 |
| Diabetes | 3.51 | 1.64-7.5 | 10.50 | 0.001 | 1.64 | 0.83-3.21 | 2.04 | 0.15 |
| Hyperlipidemia | 1.39 | 0.62-3.1 | 0.65 | 0.42 | 2.70 | 1.19-6.17 | 5.59 | 0.02 |
| Heavy Tobacco Use | 3.48 | 1.50-8.07 | 8.43 | <0.01 | 2.10 | 0.92-4.81 | 3.10 | 0.08 |
| Family hx CAD | 0.72 | 0.27-1.93 | 0.44 | 0.51 | 1.28 | 0.62-2.64 | 0.46 | 0.50 |
| Pre-test likelihood CAD (%) | 1.01 | 0.99-1.03 | 1.90 | 0.17 | 1.03 | 1.02-1.04 | 27.94 | <0.0001 |
| Hx MI | 2.60 | 1.16-5.81 | 5.41 | 0.02 | 4.56 | 2.36-8.80 | 20.38 | <0.0001 |
| Any prior coronary intervention | 1.58 | 0.70-3.53 | 1.21 | 0.27 | 5.77 | 2.96-11.25 | 26.39 | <0.0001 |
| Hx percutaneous intervention | 2.03 | 0.88-4.65 | 2.78 | 0.10 | 5.02 | 2.63-9.58 | 23.95 | <0.0001 |
| Hx coronary bypass surgery | 0.35 | 0.05-2.59 | 1.06 | 0.30 | 3.03 | 1.42-6.45 | 8.22 | <0.01 |
| Medication Use | ||||||||
| Aspirin | 1.09 | 0.51-2.36 | 0.05 | 0.82 | 3.64 | 1.52-8.76 | 8.36 | <0.01 |
| Beta blocker | 1.81 | 0.83-3.93 | 2.22 | 0.14 | 3.27 | 1.53-6.95 | 9.44 | <0.01 |
| ACE inhibitor | 2.39 | 1.13-5.07 | 5.20 | 0.02 | 1.72 | 0.90-3.27 | 2.69 | 0.10 |
| Calcium channel blocker | 1.89 | 0.82-4.32 | 2.25 | 0.13 | 1.60 | 0.78-3.32 | 1.62 | 0.20 |
| Statin | 2.21 | 0.83-5.87 | 2.54 | 0.11 | 2.06 | 0.94-4.51 | 3.25 | 0.07 |
| ECG Findings | ||||||||
| Resting heart rate > 100 bpm | 2.33 | 0.31-17.45 | 0.68 | 0.41 | ** | ** | ** | ** |
| Non-sinus rhythm | 4.34 | 1.02-18.51 | 3.94 | 0.05 | 2.11 | 0.50-8.81 | 1.04 | 0.31 |
| QRS>120 msec | 0.77 | 0.18-3.25 | 0.13 | 0.72 | 0.78 | 0.24-2.55 | 0.17 | 0.68 |
| QTc >400 msec | 1.78 | 0.83-3.79 | 2.22 | 0.14 | 1.96 | 1.02-3.78 | 4.04 | 0.04 |
| Left bundle branch block | 1.86 | 0.44-7.91 | 0.70 | 0.40 | 1.33 | 0.32-5.55 | 0.15 | 0.70 |
| Right bundle branch block | ** | ** | ** | ** | 0.32 | 0.04-2.38 | 1.23 | 0.27 |
| Meet criteria of LV hypertrophy | 7.42 | 0.99-55.66 | 3.80 | 0.05 | ** | ** | ** | ** |
| Significant Q Waves | 2.39 | 0.90-6.34 | 3.09 | 0.08 | 0.99 | 0.30-3.22 | 0.00 | 0.98 |
| Resting ST changes | 2.18 | 0.82-5.76 | 2.45 | 0.12 | 1.81 | 0.70-4.66 | 1.51 | 0.22 |
| Resting T wave inversions | 1.53 | 0.62-3.8 | 0.85 | 0.36 | 1.39 | 0.61-3.18 | 0.62 | 0.43 |
| Any ECG abnormality | 2.04 | 0.93-4.47 | 3.21 | 0.07 | 1.87 | 0.94-3.73 | 3.17 | 0.08 |
| CMR Results | ||||||||
| LVEF, per 10% | 0.55 | 0.42-0.73 | 18.45 | <0.0001 | 0.71 | 0.56-0.90 | 7.90 | <0.01 |
| LV Mass, grams | 1.01 | 1.00-1.02 | 5.75 | 0.02 | 1.00 | 0.99-1.01 | 0.00 | 0.98 |
| LVEDD, mm | 1.00 | 0.99-1.00 | 0.05 | 0.83 | 1.00 | 0.99-1.00 | 0.05 | 0.83 |
| LVEDVi, per 10 ml/m2 | 1.32 | 1.14-1.53 | 13.05 | <0.001 | 1.12 | 0.97-1.30 | 2.52 | 0.11 |
| LVESVi, per 10 ml/m2 | 1.37 | 1.21-1.55 | 23.95 | <0.0001 | 1.19 | 1.05-1.34 | 7.80 | 0.01 |
| Resting regional LV dysfunction | 4.78 | 2.22-10.29 | 15.98 | <0.0001 | 6.76 | 3.42-13.35 | 30.32 | <0.0001 |
| Resting perfusion defect | 4.27 | 2.00-9.09 | 14.12 | <0.001 | 3.17 | 1.62-6.20 | 11.37 | <0.001 |
| Stress perfusion defect | 5.09 | 2.24-11.59 | 15.02 | 0.0001 | 8.04 | 3.76-17.17 | 28.94 | <0.0001 |
| Presence of RevPD | 6.88 | 2.87-16.5 | 18.69 | <0.0001 | 10.92 | 4.96-24.06 | 35.21 | <0.0001 |
| Summed stress score (SSS) | 1.05 | 1.02-1.08 | 10.71 | 0.001 | 1.07 | 1.04-1.09 | 35.61 | <.0001 |
| Summed rest score (SRS) | 1.16 | 1.08-1.25 | 15.25 | <0.0001 | 1.13 | 1.05-1.22 | 11.35 | <0.001 |
| Summed difference score (SDS) | 1.05 | 1.01-1.09 | 5.89 | 0.02 | 1.08 | 1.06-1.11 | 36.94 | <0.0001 |
| Presence of LGE | 5.31 | 2.35-11.98 | 16.17 | <0.0001 | 8.09 | 3.90-16.79 | 31.47 | <0.0001 |
| LGE mass, grams | 1.04 | 1.02-1.07 | 9.52 | <0.01 | 1.03 | 1.00-1.06 | 4.04 | 0.04 |
| LGE per 10 % of LV mass | 1.47 | 1.13-1.9 | 8.37 | <0.01 | 1.34 | 1.03-1.73 | 4.73 | 0.03 |
| Presence of LGE without Hx MI | 3.47 | 1.50-8.05 | 8.42 | <0.01 | 3.78 | 1.77-8.06 | 11.85 | <0.001 |
| LGE mass, grams | 1.10 | 1.04-1.17 | 11.75 | <0.001 | 1.09 | 1.03-1.17 | 7.68 | 0.01 |
| LGE per 10 % of LV mass | 1.94 | 1.19-3.14 | 7.15 | <0.01 | 1.88 | 1.13-3.12 | 5.85 | 0.02 |
Hazards could not be estimated due to low events
Figure 1.
Kaplan-Meier curves from the entire cohort (n=254) for Death/MI (top) and MACE (bottom) stratified by RevPD (A and C) and LGE (B and D), respectively.
Figure 2.
Progressive stronger prognostic association of increasing SDS tertiles with Death/MI(A) and MACE(B).
Best Final Models for Death/MI and MACE
Table 3A demonstrates the best final model for Death/MI by stepwise forward selection which included LVESV index and heavy tobacco use. Table 3B demonstrates the best final model for MACE. The only variables selected include RevPD and pre-test CAD likelihood (Diamond/Forrester and Cass criteria). RevPD was the strongest multivariable predictor for MACE. Adjusted to pre-test likelihood of CAD, RevPD maintained a >8-fold hazards increase for MACE (adjusted HR 8.61, P<0.0001).
Table 3.
| A) Death/MI | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) |
P-value | Chi-Square | Model LR Chi-Square, P value |
| LVESVi, per 10 ml/m2 | 1.36 (1.19-1.55) | <0.0001 | 19.77 | 25.73, P<0.0001 |
| Heavy Tobacco Use | 3.65 (1.57-8.49) | 0.003 | 9.06 |
| B) MACE | ||||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) |
P-value | Chi-Square | Model LR Chi-Square, P value |
| Pre-test CAD Likelihood (%) | 1.02 (1.01-1.03) | 0.0001 | 15.06 | 59.12, P<0.0001 |
| RevPD | 8.61 (3.85-19.29) | <0.0001 | 27.38 |
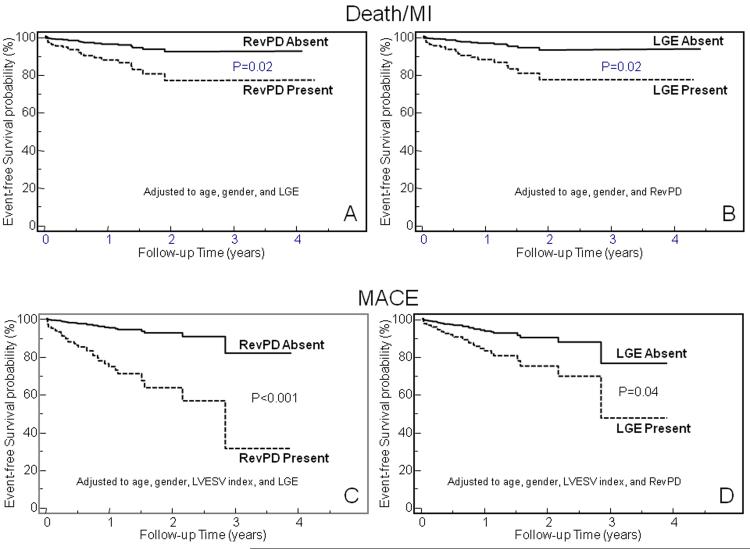
Complementary Prognostic Roles of RevPD and LGE Adjusted to Patients Age and Gender
When RevPD and LGE were both entered into a model that included age and gender, RevPD and LGE each maintained strong association with Death/MI. RevPD maintained a 3.3-fold hazards increase to Death/MI when adjusted to the effects of age, gender, and LGE, whereas LGE maintained a 3.4-fold hazards increase to Death/MI when adjusted to the effects of age, gender and RevPD (P=0.02 and 0.01, respectively). This similar pattern was also observed in the association with MACE. RevPD maintained a 5.5-fold hazards increase to MACE when adjusted to the effects of age, gender, and LGE (P=0.0004), whereas LGE maintained a 2.7-fold hazards increase to MACE when adjusted to the effects of age, gender and RevPD (P=0.04). Figure 3 illustrates the Kaplan-Meier event-free survival curves stratified by RevPD or LGE after these adjustments. The complementary prognostic roles of RevPD and LGE demonstrated by these models were not altered by early revascularization (within the first 30 days after CMR). When early revascularization was used as a stratification factor and adjustment made to patient age, gender and LGE presence, RevPD maintained a close to 3-fold hazard to Death/MI (adjusted HR 2.85, P=0.05) and a > 4-fold hazard to MACE (adjusted HR 4.35, P=0.003). On the other hand, with early revascularization stratified and patient age, gender, and RevPD adjusted, LGE maintained a > 3-fold hazard to Death/MI and to MACE (adjusted HR 3.70, P=0.01 and 3.27, P=0.02, respectively). While LVESVI is a known robust risk marker to MACE, when it was added into the multivariable model for MACE, the respective prognostic association of RevPD and LGE were not affected: RevPD maintained a 6.3-fold whereas LGE maintained a 2.7-fold hazards increase to MACE, when each of them was adjusted to all other variables in the model (P=0.0002 and 0.04, respectively). In 179 patients (70%) where LGE was absent, RevPD presence indicated a 17-fold and a 14-fold increase in hazards to Death/MI and MACE, respectively, adjusted to age and gender (P=0.0005 and <0.0001, respectively). By stepwise forward selection, RevPD was the strongest multivariable predictor of Death/MI and of MACE in patients without LGE. In 180 patients (71%) where RevPD was absent, LGE presence indicated a 13-fold and a 9-fold increase in hazards to Death/MI and MACE, respectively, adjusted to age and gender (P=0.0002 and 0.002, respectively). There were 3 patients without LGE but had resting perfusion defects that were not reversible. None of these 3 patients experienced MACE.
Figure 3.
Adjusted Kaplan-Meier curves for Death/MI (top) and MACE (bottom) stratified by RevPD and LGE, respectively. Adjusted to the effects of age, gender, and LGE, RevPD maintained strong association with Death/MI(A) and MACE(C), respectively. On the other hand, adjusted to the effects of age, gender, and RevPD, LGE maintained significant association with Death/MI(B) and MACE(D), respectively. Note that for association with MACE, LVESV index was also entered into the model for effect adjustment.
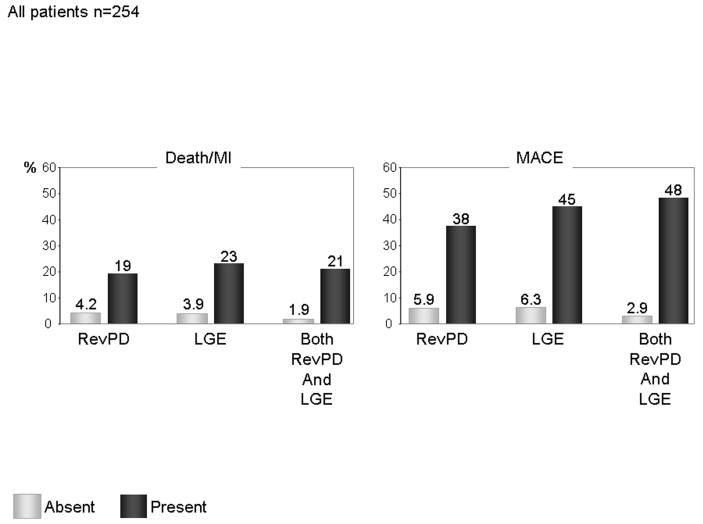
Figure 4 displayed the annual event rates in Death/MI and in MACE of the study cohort. Presence of RevPD and LGE both portended to elevated annual event rates of Death/MI (19 and 23%, respectively) or MACE (38 and 45%, respectively). Patients who demonstrated both RevPD and LGE had event rates of Death/MI and MACE at 21% and 48%, respectively. Patients with RevPD and LGE both absent had the lowest event rates in Death/MI and MACE.
Figure 4.
Annual event rates of Death/MI and MACE, respectively, in presence of RevPD, LGE, and both RevPD and LGE.
Subgroup Analysis in Patients Without a History of MI
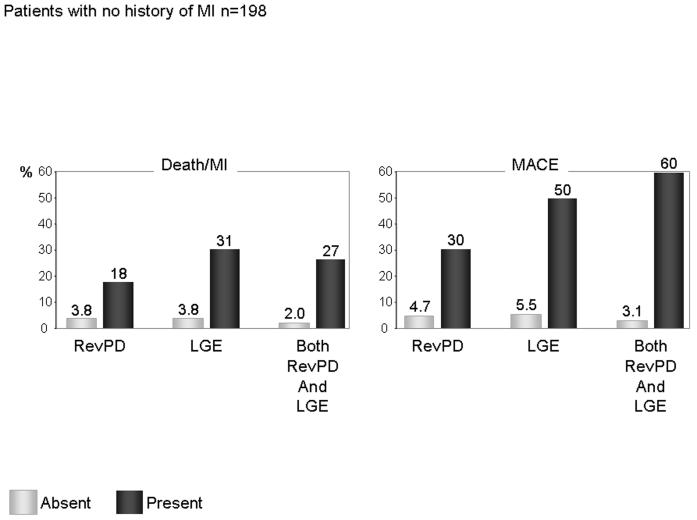
One hundred ninety-eight patients (78%) had no history of MI, among them 27 had LGE consistent with unrecognized MI. Twenty-seven patients (14%) experienced MACE including 9 cardiac deaths, 9 acute MI, 9 unstable angina hospitalizations, and no late revascularization. RevPD and LGE both demonstrated strong unadjusted association with Death/MI (HR 9.03, P<0.0001 and HR 6.06, P=0.0003, respectively) and MACE (HR 16.22 and 10.31, respectively, both P<0.0001). By stepwise forward selection, RevPD was selected to form the best final models for Death/MI and for MACE, respectively, and it was the strongest multivariable predictor of MACE. Figure 5 shows the Kaplan-Meier curves illustrating complementary prognostic associations of RevPD and LGE with clinical events in patients without a history of MI. Both RevPD and LGE were associated with reduced Death/MI-free survival (figure 5a and 5b). While RevPD also demonstrated strong association with reduced MACE-free survival (figure 5c), patients with both LGE and RevPD experienced the worst MACE-free survival distribution (figure 5d). In patients without a history of MI, both ReVPD and LGE demonstrated strong univariable association with Death/MI (HR 9.03 and 6.06, P<0.0001 and P=0.0003, respectively) and MACE (HR 16.22 and 10.31, respectively, both P<0.0001). By multivariable analyses, RevPD and LGE maintained strong association with Death/MI and MACE after adjustment to the effects of age, gender, and to each other. When age, gender, RevPD, and LGE were entered into a model for Death/MI, RevPD and LGE both demonstrated independent prognostic association (adjusted HRs of 4.52 and 3.72, respectively, both P=0.02). When age, gender, RevPD, and LGE were entered into a model for MACE, presence of RevPD demonstrated independent prognostic association (adjusted HRs 8.92, P=0.0008) whereas LGE a trend toward independent association (adjusted HRs 3.00, P=0.07). In 156 patients without history of MI and did not demonstrate RevPD, LGE presence indicated a >11-fold elevated hazards to Death/MI (HR 11.48, P=0.001). Figure 6 displayed the annual event rates of Death/MI or MACE in patients without a history of MI. A presence of RevPD and LGE were both associated with high rates of Death/MI (18 and 31%, respectively, versus 3.8%) and MACE (30 and 50%, respectively, versus 4.7% and 5.5%, respectively). In patients without a history of MI, patients who had both RevPD and LGE have high rates of Death/MI (27%) and MACE (60%), a stark comparison to the low annual rates in Death/MI (2%) or MACE (3.1%) amongst patients with absent RevPD and LGE.
Figure 5.
Kaplan-Meier curves for Death/MI (top) and MACE (bottom) stratified by RevPD and LGE, respectively, in patients without a history of MI (n=198).
Figure 6.
Annual event rates of Death/MI and MACE, respectively, in presence of RevPD, LGE, and both RevPD and LGE, among patients without a history of MI.
Diagnosing QCAStenosis by RevPD with and without LGE
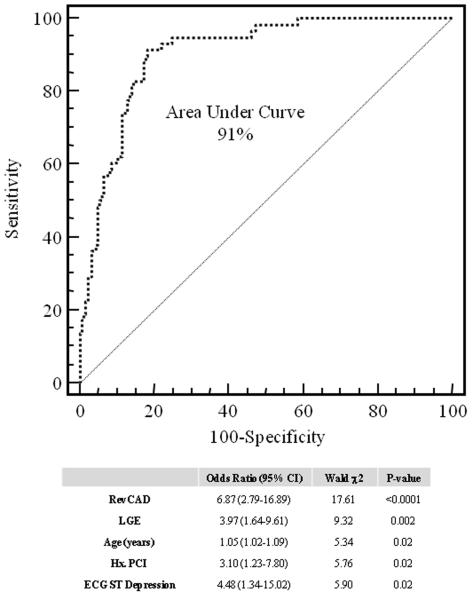
Per the discretion of the referring physician, 68 patients (27%) underwent X-ray coronary angiography within the first 12 months after CMR with 43 patients (63%) demonstrating QCAstenosis, among them 20 patients underwent percutaneous revascularization. RevPD was present in 40 of the 43 patients with QCAstenosis (sensitivity 93%) and was normal in 21 of the 25 patients without QCAstenosis (specificity 84%). Adding presence of LGE detected 1 additional case of QCAstenosis (sensitivity increased to 95%) but at the expense of 4 “false positive” cases without QCAstenosis (specificity dropped to 70%). RevPD detects single-vessel, 2-vessel, and 3-vessel QCAstenosis at sensitivities of 92%, 94%, and 100%. In the 186 patients who were not referred to undergo coronary angiography, 12 patients experienced MACE within the first 12 months after CMR (3 cardiac deaths, 5 acute MI, 3 hospitalizations for unstable angina, and 1 late revascularization). Figure 7 illustrates the best final multivariable model for QCAstenosis/MACE12months using stepwise forward selection. This model, which selected both RevPD and LGE and other variables as shown, yielded a 91% area under the curve in its association with QCAstenosis/MACE12months.
Figure 7.
Best final logistic regression model for association with QCAStenosis/MACE12months.
DISCUSSION
Consistent with recent reports, in this study the presence and the extent of RevPD by CMR stress perfusion imaging provide excellent prognostic stratification of patients with known or suspected CAD. However, our study provides new and important findings: RevPD and LGE provide complementary prognostication to Death/MI in patients referred to CMR for assessment of known or suspected CAD. This is evident by RevPD and LGE both maintained a > 3-fold association with Death/MI when adjusted to each other and to the effects of patient age and gender (adjusted HR 3.31, P=0.02 and 3.43, P=0.01, respectively). We also found that while RevPD was the strongest multivariable predictor selected for MACE, those with RevPD and LGE both absent had the lowest annual event rates of Death/MI and MACE (<2% and <3%, respectively).
Several studies have shown strong prognostic value of CMR perfusion imaging. Ingkanisorn, et al. studied chest pain patients in the emergency room and reported excellent cardiac event-free survival among patients with negative vasodilating stress CMRMPI.15, 16 Jahnke, et al.1 reported that in 513 patients with abnormal adenosine CMRMPI portended to a 12-fold increased risk for cardiac events, while a normal combined examination portended a three-year event-free survival of 99%. However, these studies did not assess the relative or incremental prognostic value of LGE available from the same imaging session. While RevPD is sensitive to alteration of regional blood flow secondary to coronary stenosis, LGE can detect and quantify a broad range of infarction with or without a clinical knowledge of prior MI. It is therefore conceivable that this observed complementary prognostic association by RevPD and LGE, is due to their characterization of different pathologic alteration of myocardial physiology consequent to CAD. In this regard, it is consistent with our observation that LGE as evidence of prior infarction was associated with hard events such as new MI or cardiac death; whereas RevPD as evidence of flow-limiting coronary stenosis was associated with less critical events such as unstable angina. LGE without a history of MI most likely indicates an untreated coronary event resulting in subclinical infarction, supported by the >11-fold adjusted hazards increase to Death/MI despite an absence of RevPD in the current study. While patients who had both RevPD and LGE positive represented the highest risk group who experienced a >20% annual rates of Death/MI, patients with CMR negative for both RevPD and LGE indicated a favorable negative event rate for Death/MI of >98%. On the other hand, LGE data did not significantly improve the detection of QCAStenosis evidenced on X-ray angiography by RevPD. Unrecognized MI as detected by LGE can occur as the result of a spontaneous thrombolysis and recannulation of an acute coronary lesion. Thus, while LGE provide a foot-print of myocardial damage as a result of a prior coronary event, may not be associated with a flow-limiting coronary lesion.
LIMITATIONS
Some selection bias exists from the pattern CMR was referred at our institution and thus the CAD prevalence in our cohort appeared high compared to other similar studies. While we also demonstrated that the current strong prognosticating potentials offered by CMR perfusion and LGE imaging was robust and consistent in the subgroup of patients without a history of MI, future studies will need to determine if the current results may extrapolate to the same degree in a population with substantially lower CAD prevalence. Another limitation relates to the small subset of patients with angiographic verification of coronary stenosis and therefore test specificity can only be estimated based on the limited data. The principal aim of our study was to evaluate the prognostic value of clinical and CMR data, not its ability to detect anatomic CAD. Finally, we did not collect analogous patient data from stress nuclear myocardial perfusion imaging or CT angiography and therefore cannot comment regarding the relative clinical value of these imaging modalities compared to CMR for the evaluation of patients presenting for evaluation of cardiac ischemic symptoms.
CONCLUSIONS
In summary, in patients with known or suspected CAD, the current study provides evidence in support of robust and complementary roles from perfusion and LGE imaging by CMR in risk-stratifying against cardiac death or MI.
CMR reversible myocardial perfusion defect (RevPD) has demonstrated not only high accuracy in detection of flow-limiting coronary stenosis, but strong prognostic value in risk stratifying patients presented with suspected ischemia. With high tissue contrast and spatial resolution, CMR late gadolinium enhancement (LGE) is the most sensitive current imaging technique in detecting small subendocardial infarction that elevates a patient’s risk of cardiac events. In a clinical cohort of 254 patients referred for stress CMR imaging, we tested the hypothesis that RevPD and LGE imaging in a single CMR study can provide complementary prognostic values towards major adverse events including cardiac death or non-fatal MI (Death/MI). While RevPD and LGE both demonstrated strong unadjusted association with Death/MI (HR of 6.88 and 5.32, respectively, both P<0.0001), robust association with Death/MI by RevPD and LGE was maintained when the effects of these variables were adjusted to each other and to patient age and gender. In patients without a history of MI who were found to have no RevPD, presence of LGE portended to more than 11-fold hazards increase to Death/MI. We found that patients with both RevPD and LGE absent had the most favorable annual negative event rate for Death/MI at > 98%. We therefore conclude that CMR stress myocardial perfusion and LGE imaging performed in a CMR study provide complementary prognostic implication to cardiac death or acute non-fatal MI.
Acknowledgments
Funding Sources This study was supported by the Brigham and Women’s Hospital Cardiovascular Imaging Funds. Dr. Kwong is supported in part by a research grant from the National Institutes of Health (NIH RO1 HL091157).
Footnotes
Conflict of Interest Disclosures None
References
- 1.Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, Fleck E, Paetsch I. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 2.Diamond GA, Staniloff HM, Forrester JS, Pollock BH, Swan HJ. Computer-assisted diagnosis in the noninvasive evaluation of patients with suspected coronary artery disease. J Am Coll Cardiol. 1983;1:444–455. doi: 10.1016/s0735-1097(83)80072-2. [DOI] [PubMed] [Google Scholar]
- 3.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 4.Chaitman BR, Bourassa MG, Davis K, Rogers WJ, Tyras DH, Berger R, Kennedy JW, Fisher L, Judkins MP, Mock MB, Killip T. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS) Circulation. 1981;64:360–367. doi: 10.1161/01.cir.64.2.360. [DOI] [PubMed] [Google Scholar]
- 5.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr., Fihn SD, Fraker TD, Jr., Gardin JM, O’Rourke RA, Pasternak RC, Williams SV. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 7.Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP, Judd RM. An improved MR imaging technique for the visualization of myocardial infarction. Radiology. 2001;218:215–223. doi: 10.1148/radiology.218.1.r01ja50215. [DOI] [PubMed] [Google Scholar]
- 8.Salton CJ, Chuang ML, O’Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J.Am.Coll.Cardiol. 2002;39:1055–1060. doi: 10.1016/s0735-1097(02)01712-6. [DOI] [PubMed] [Google Scholar]
- 9.Alfakih K, Reid S, Jones T, Sivananthan M. Assessment of ventricular function and mass by cardiac magnetic resonance imaging. Eur.Radiol. 2004;14:1813–1822. doi: 10.1007/s00330-004-2387-0. [DOI] [PubMed] [Google Scholar]
- 10.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 11.Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 12.Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083–1089. doi: 10.1161/01.cir.0000027818.15792.1e. [DOI] [PubMed] [Google Scholar]
- 13.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N.Engl.J.Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Cliniical Cardiology of the American Heart Association. Int.J.Cardiovasc.Imaging. 2002;18:539–542. [PubMed] [Google Scholar]
- 15.Ingkanisorn WP, Kwong RY, Bohme NS, Geller NL, Rhoads KL, Dyke CK, Paterson DI, Syed MA, Aletras AH, Arai AE. Prognosis of negative adenosine stress magnetic resonance in patients presenting to an emergency department with chest pain. J Am Coll Cardiol. 2006;47:1427–1432. doi: 10.1016/j.jacc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 16.Aletras AH, Ingkanisorn WP, Mancini C, Arai AE. DENSE with SENSE. J Magn Reson. 2005;176:99–106. doi: 10.1016/j.jmr.2005.05.010. [DOI] [PubMed] [Google Scholar]