Abstract
Therapeutically valuable proteins are often rare and/or unstable in their natural context, calling for production solutions in heterologous systems. A relevant example is that of the stress-induced, normally rare, and naturally unstable “read-through” human acetylcholinesterase variant, AChE-R. AChE-R shares its active site with the synaptic AChE-S variant, which is the target of poisonous organophosphate anticholinesterase insecticides such as the parathion metabolite paraoxon. Inherent AChE-R overproduction under organophosphate intoxication confers both short-term protection (as a bioscavenger) and long-term neuromuscular damages (as a regulator). Here we report the purification, characterization, and testing of human, endoplasmic reticulum-retained AChE-RER produced from plant-optimized cDNA in Nicotiana benthamiana plants. AChE-RER purified to homogeneity showed indistinguishable biochemical properties, with IC50 = 10−7 M for the organophosphate paraoxon, similar to mammalian cell culture-derived AChE. In vivo titration showed dose-dependent protection by intravenously injected AChE-RER of FVB/N male mice challenged with a lethal dose of paraoxon, with complete elimination of short-term clinical symptoms at near molar equivalence. By 10 days postexposure, AChE-R prophylaxis markedly limited postexposure increases in plasma murine AChE-R levels while minimizing the organophosphate-induced neuromuscular junction dismorphology. Our findings present plant-produced AChE-RER as a bi-modal agent, conferring both short- and long-term protection from organophosphate intoxication.
Keywords: paraoxon intoxication, neuromuscular junction, transgenic plants, long-term protection, bioscavenger
Accidental (environmental or occupational) and self-inflicted (suicide) exposure to organophosphate (OP) pesticides is encountered frequently in the emergency room, especially in the developing world (1, 2). These perennial public health issues are compounded by a growing concern over the potential use of OP nerve agents such as sarin as a means of terror and nonconventional warfare (3, 4). OPs disrupt neuro-transmission by inhibiting synaptic acetylcholinesterase (AChE-S), leading to accumulation of acetylcholine in the synapse and neural overstimulation (5). The severity of the ensuing nicotinic and muscarinic symptoms is dose dependent and can result in death due to cardiovascular and respiratory collapse (3, 4). Those surviving the initial insult often suffer long-term sequelae, including OP-induced delayed neuropathy, muscle weakness, permanent brain dismorphology, and social/behavioral deficits (3, 4, 6).
The mechanisms underlying OP-delayed toxicity and other stressful insults (e.g., psychological stress or head trauma) involve rapid elevation of c-fos, followed by up-regulation of the ACHE gene (7) and rapid but long-lasting shifted alternative splicing from AChE-S to the otherwise rare “read-through” variant (AChE-R, 8–10). Up-regulation and isoform switching are associated with short-term neuroprotection, but prolonged overexpression of AChE-R exerts long-lasting damage (5). Neuromuscular junctions (NMJ) show degenerated synaptic folds, enlarged motor endplates, disorganized muscle fibers, and branded terminal nerves (11) accompanied by physiological malfunctioning (12). Thus, the goal of any successful therapy should be prevention of the immediate life-threatening effects of OP intoxication and its long-term debilitating consequences.
Existing medical practices address the short-term, but not the delayed, consequences of OP intoxication (7, 13, 14). As an alternative, it was suggested to use human cholinesterases (ChEs) as OP bioscavengers (14), but the practicality of this approach depends on the availability of large amounts of these enzymes, which are required in stoichiometric, rather than catalytic, quantities. The considerable success with plasma-purified butyrylcholinesterase (BChE) as an OP bio-scavenger (15, 16) called for comparative studies with the physiologically relevant target of OPs, human AChE. AChE shows considerably faster OP binding kinetics than BChE (17), predicting that smaller doses may be effective. Of the various AChE isoforms, use of the soluble OP-induced serum protein AChE-R (18), which shows efficient OP binding capacities (8), appeared most promising. We hypothesize that AChE-R treatment would provide effective immediate protection and suppress host enzyme overproduction, thus alleviating at least part of the long-term sequelae of OP intoxication in blood and muscle alike.
Several strategies for production of BChE have been explored, including purification from outdated blood-banked human plasma (16, 19, 20) and milk of transgenic goats (21). Nevertheless, mammalian-based production systems seem less promising for large-scale production of AChE-R because of the low levels and relative instability of the protein and its cognate mRNA in such systems (22, 23). To solve these difficulties, we turned to plant production and endoplasmic reticulum (ER) retention of recombinant human AChE-R. We report here the efficient production and purification of this novel therapeutic protein, a single administration of which provides prophylactic protection from otherwise lethal OP challenges and attenuates the long-term serum AChE-R excess and NMJ damages caused by OP poisoning.
MATERIALS AND METHODS
Engineering of N. benthamiana plants expressing human AChE-R
A plant expression-optimized DNA construct encoding human AChE-R (GenBank #DQ140347) fused to the C-terminal ER retention signal SEKDEL was synthesized de novo, cloned into a plant expression vector, and used for Agrobacterium tumefaciens-mediated transformation of N. benthamiana (24). The plant line with the highest AChE activity was selected for propagation, seed production, and further analysis (25).
In vivo experiments
Circulatory t½ of plant-derived AChE-RER was determined in male 6- to 8-wk-old FVB/N mice that were lightly anesthetized with isoflurane (Rhodia, Bristol, UK) and placed in a restrainer prior to treatment. Mice were i.v. injected (tail vein) with 80 μl saline containing 400 or 1000 U (U) of purified l) AChE-RER. At the indicated times, blood samples (10–30 μl) from the tail vein were collected into 4 μl of 0.25M EDTA in PBS and kept on ice until separation of plasma by centrifugation (500 rpm for 30 min, 4°C). Paraoxon LD50 values for FVB/N mice were determined by bolus i.v. (tail vein) injections of aqueous solutions of paraoxon (Sigma, St. Louis, MO, USA) at different concentrations.
Protection assays were conducted as follows. Mice were i.v. injected with 0–10,000 U of AChE-RER in 100 μl of 0.9% saline. ChE levels were determined from blood samples drawn 10 min before and 5 min after AChE injection. Mice were challenged by i.v. injection with paraoxon (2.73 μmol/kg) at 10 min post-AChE-RER injection and symptoms were observed. No supportive measures were given, and mice that did not show improvement within 15 min of challenge were euthanized. Experiments were approved by the institutional animal care and use committees of Arizona State University and The Hebrew University of Jerusalem.
Biochemical and molecular analyses
Total genomic leaf DNA blot analysis was done by a DIG-labeled probe (PCR amplified with the primers 5′ gtctgctacc-aatatgtggacaccc3′ and 5′ cctggaggacagcccacaaggtggg3′) as described previously (24). Purification of plant-derived AChE-RER from leaf tissue was as described (25). Purified plant-derived AChE-RER was kept in water at 4°C for up to 6 months. Typical preparations of pure enzyme had specific activities of ~3000 U/mg protein, and only preparations with specific activities larger than 2000 U/mg protein were used in vivo.
Protein preparations were resolved by SDS-PAGE and stained with either GelCode SilverSNAP Stain Kit II (Pierce, Rockford, IL, USA) or Coomassie brilliant blue or transferred to a PVDF membrane and immunodecorated with polyclonal Abs raised against either the common domain unique to human or mouse AChE (N-19 and E-19 respectively; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or the C-terminal domain of human AChE-R (26). HRP-conjugated secondary Abs (Sigma) and the ECL-plus kit (Amersham, Piscataway, NJ, USA) were used for detection. Total soluble protein (TSP) was determined as described (24).
AChE activity assays, enzyme kinetics, inhibition curves, and AChE activity staining of nondenaturing PAGE were as described previously (24). Activity assays and staining were performed in the presence of 0.5 × 10−5 M of the specific BChE inhibitor tetraisopropylpyrophosphoramide (iso-OMPA, Sigma).
For histochemical AChE activity staining of diaphragms, sacrificed mice were dissected to expose the diaphragm muscles, which were fixed in situ with 4% paraformaldehyde for 5 min, dissected out, and kept overnight in the fixative at room temperature. After two washes with PBS, the muscles were incubated in Karnovsky’s staining solution at 4°C until brown precipitants appeared (27). NMJ analyses involved the Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA).
RESULTS
Production of human ER-retained AChE-R in plants
Humans and other mammals express several molecular variants of AChE (28). All share a common core domain and enzymatic properties but possess distinct C-terminal amino acid residues, expression patterns, subcellular localization, and protein partner interactions (Fig. 1A, ref. 29). Previous reports from our group focused on plant expression of the common core domain (24, 30), which accumulated to low levels (~0.002% TSP) due to the inefficient translation of the human sequence in plant cells. De novo construction of plant expression-optimized genes encoding all three physiological C-terminal variants resulted in an ~50-fold higher accumulation levels of the proteins in N. benthamiana (~0.05% TSP) compared with the native human sequence (data not shown).
Figure 1.
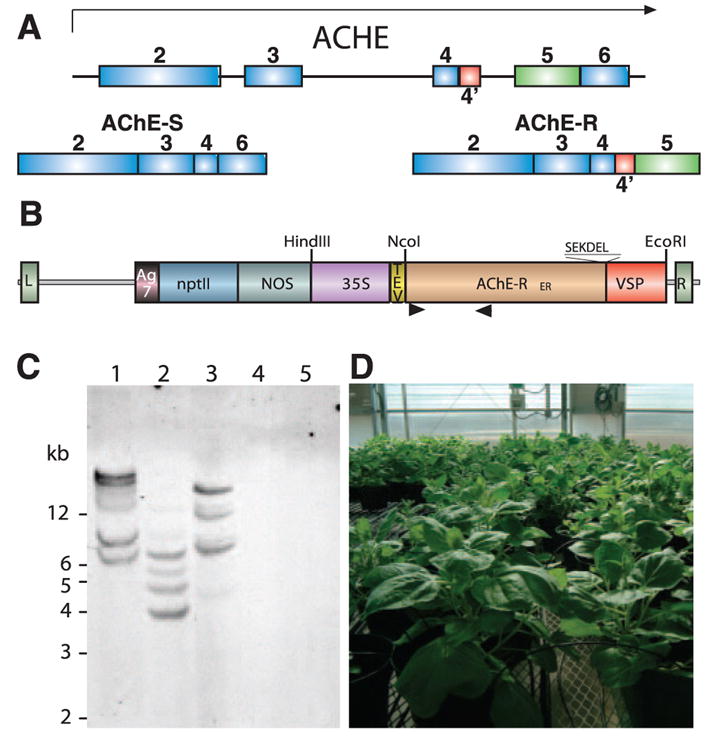
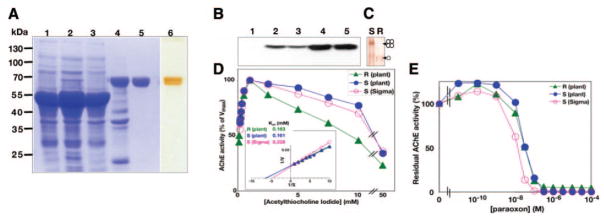
Expression of AChE-RER in plants. A) AChE-S and AChE-R are alternative splicing variants with distinct C termini. B) The AChE-RER expression vector. L, left border; R, right border; Ag7, the nopaline synthase polyadenylation signal; nptII, the kanamycin resistance marker; NOS, the nopaline synthase promoter; 35S, cauliflower mosaic virus 35S promoter; TEV, the translation enhancer region of the tobacco etch virus; AChE-RER, codon-optimized coding region for human AChE-R containing the C-terminal SEKDEL endoplasmic reticulum retention signal; VSP, the 3′ UTR of the soybean vegetative storage protein; arrowheads, locations of primers used to generate probe for DNA blot analysis. C) DNA blot analysis. Genomic DNA was extracted from the highest expressing N. benthamiana line (2D) (lanes 1–3) and WT plants (lanes 4, 5) and digested with EcoRI (lanes 1, 4), NcoI (lanes 2, 5), and HindIII (lane 3). D) Large-scale cultivation of 2D N. benthamiana in controlled growth facility at Arizona State University.
Plants, like other heterologous expression systems, may produce recombinant human proteins that are decorated with nonhuman complex glycans, predicting their fast circulatory clearance (31, 32). In addition, plant glycans may contain unique glyco-epitopes that may be allergenic in some cases (31, 32). We therefore engineered AChE-R to contain the C-terminal peptide SEKDEL (Fig. 1B), allowing its retrogade transport from the cis-Golgi back to the ER (33). These modifications enabled us to select a plant line harboring at least four copies of the transgene (Fig. 1C), which accumulated AChE-RER to 0.5%–1.0% TSP (equivalent to a specific activity in the crude extract of ~19 U/mg protein, or ~30 mg/kg fresh weight). The selected line was propagated for seed and biomass production in a USDA-approved containment greenhouse (Fig. 1D). The plants appeared phenotypically indistinguishable from WT plants under normal growth conditions.
The AChE-RER protein could be detected in crude plant extracts by SDS-PAGE, revealing a 67 kDa band absent from the WT’s extract (Fig. 2A). AChE-RER was purified to homogeneity from plant extracts using procainamide affinity chromatography followed by anion exchange chromatography (25), with final specific activity of the purified protein >3000 U/mg. When resolved by SDS-PAGE and visualized by Coomassie or silver staining, the purified protein appeared as a doublet that strongly reacts with AChE-specific Abs (Fig. 2A, B). As predicted by its retention in the ER, the protein contains high-mannose glycans (data not shown), and the two bands apparent in the gel possibly represent two differently glycosylated species. Protein sequencing revealed that the accumulating enzyme was correctly processed, with the mature protein’s N-terminal residue being R35, as previously demonstrated for AChE expressed in a human cell line (34). Nondenaturing PAGE (Fig. 2C) demonstrated that, unlike recombinant AChE-S, AChE-RER is monomeric, compatible with previous observations (18, 27). Pure preparations of plant-produced AChE-RER were found to be stable at 4°C for at least 12 months.
Figure 2.
Characterization of plant-produced AChE-RER. A) Fractions from successive purification steps were subjected to SDS-PAGE and visualized by Coomassie staining (lanes 1–5) or silver staining (lane 6) as follows: crude WT extract (lane 1), crude 2D extract (lane 2), procainamide affinity chromatography flow-through (lane 3), eluate after extensive dialysis (lane 4), final product after anion-exchange chromatography and concentration (lanes 5, 6). B) The above fractions were subjected to immunoblot analysis (lanes 1–5) with AChE-specific Abs. C) Nondenaturing PAGE, followed by staining for ChE activity analysis of plant-derived AChE-S (S) and AChE-RER. D) Plant-produced AChE-RER (R) displays equivalent Km and substrate inhibition compared with mammalian cell culture (S, Sigma) or plant produced AChE-S (S, plant). Insert: Lineweaver-Burke analysis (insert). E) Residual activities of plant-derived AChE-RER and AChE-S and mammalian cell culture-derived AChE-S were assayed in the presence of the indicated concentration of the OP paraoxon.
Kinetic characterization of plant-derived AChE-RER
Activities of plant-derived human AChE-RER and human AChE-S (T. S. Mor, unpublished results) were compared with cell culture-derived human AChE-S (from Sigma; ref. 35). Km values for all three variants were similar to each other and to those reported in the literature (~0.2 mM, Fig. 2D, refs. 34, 36). The enzymes exhibited characteristic inhibition by substrate concentration of >2 mM, with no significant differences between the plant- and cell-derived AChE-S. Substrate inhibition was more pronounced in the case of the R variant, compatible with C terminus active site intramolecular signaling (37). All AChE variants tested here had a similar ability to bind and to be inhibited by paraoxon, the active OP metabolite of the pesticide parathion (Fig. 2E).
Circulatory clearance of plant-derived AChE-RER
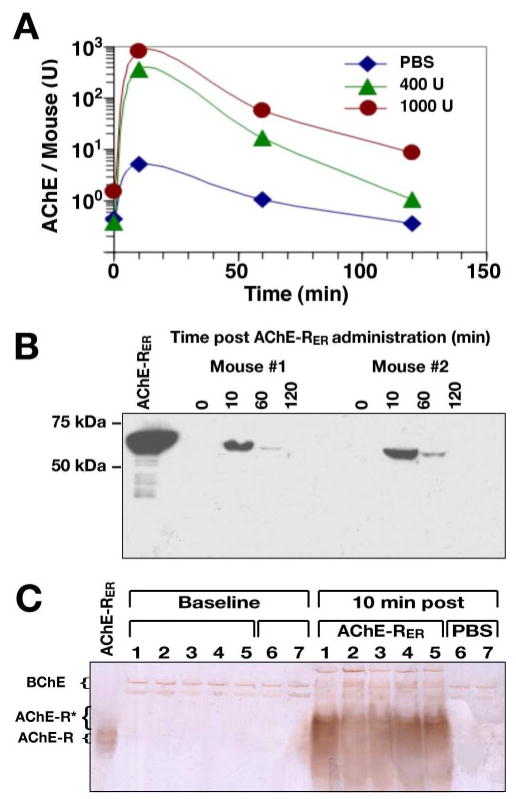
The clearance of plant-derived AChE-RER in mice was determined by i.v. injection of enzyme (either 400 U or 1000 U) and measuring its residual activity in plasma samples in the presence of the BChE-specific inhibitor iso-OMPA (Fig. 3A). The plant-derived human enzyme was cleared rapidly with circulatory t½ = 24 min, similar to observations with AChE from other sources (38).
Figure 3.
Pharmacokinetics of plant-produced AChE-RER. A) AChE-RER clearance profile. Groups of 5 mice were injected with either 400 U, 1000 U of plant-produced AChE-RER or an equivalent volume of saline. Plasma samples were collected and assayed for AChE activity in the presence of Iso-OMPA, a selective BChE inhibitor. B) Immunoblot detection of intact circulating AChE-RER. Plasma protein samples from 2 mice injected with 400 U AChE-RER were resolved by SDS-PAGE and subjected to immunoblotting. A 1.3 μg sample of pure plant-derived AChE-RER was resolved alongside for comparison. C) Circulating AChE-RER displays decreased migration in nondenaturing PAGE. Plasma samples from 5 mice injected with 400 U AChE-RER and 2 mice injected with PBS were prepared and subjected to nondenaturing PAGE, followed by staining of catalytically active AChE. Fast migrating AChE-R monomers appeared only after injection of the recombinant enzyme. In panels B, C, purified plant-produced AChE-RER was resolved alongside the serum samples for comparison.
Human AChE was detected by immunoblotting with Abs specific to the common domain of human AChE in plasma samples from mice treated with 400 U AChE-RER, but not from control animals (Fig. 3B and data not shown). AChE protein levels decreased after their initial peak at 10 min and were no longer detectable at 2 h, compatible with the enzymatic assay (Fig. 3A).
Nondenaturing PAGE, followed by ChE activity staining, revealed slow-migrating ChE bands, likely representing BChE oligomers inaccessible to iso-OMPA inhibition, in the plasma of all mice, including controls. Catalytically active monomeric enzyme was, however, observed in plasma of AChE-RER-injected but not PBS-injected controls (Fig. 3C). Surprisingly, compared with the purified plant-derived AChE-RER (Fig. 3C, left lane), the injected enzyme in the mouse plasma samples migrated more slowly as several broad bands (Fig. 3C). This unexpected mobility shift of the enzyme, compatible with findings in the cerebrospinal fluid of Alzheimer’s disease patients (39), is suggestive of protein-protein interactions with as-yet-unidentified plasma protein (or proteins).
Protection from OP challenge by plant-produced AChE-RER
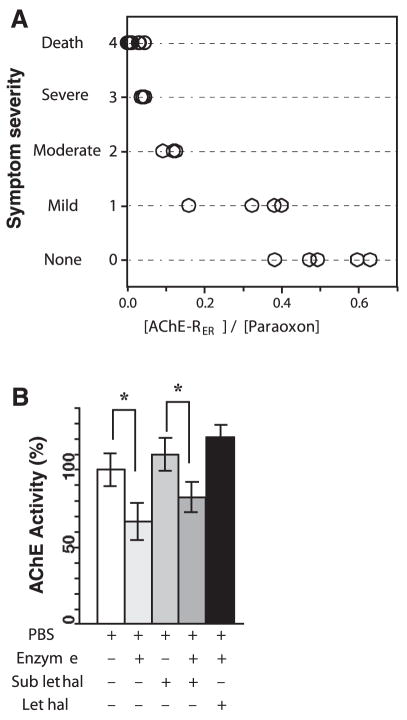
To test whether exogenously applied AChE-RER could protect animals from an OP challenge, we determined the experimental single bolus LD50 of paraoxon. In 6-to 8-wk-old male FVB/N mice, the LD50 was 2.4 μmol/kg. Pretreatment with increasing amounts of plant-produced human AChE-RER (dose adjusted to body weight) was then followed by challenge with 1.14 × LD50 paraoxon, and the symptoms were observed and recorded (Fig. 4). This dose of paraoxon is 100% fatal for otherwise untreated mice, with death, preceded by severe cholinergic symptoms (convulsions and strained respiratory ability), occurring within 5–10 min. Pre-treating 17 mice with plant-derived AChE-RER reduced mortality even when the molar ratio of AChE-RER/OP was as low as 0.04, far lower than the comparable stoichiometric protection by BChE (40). At this low molar ratio, the surviving animals initially displayed severe symptoms, followed by more moderate symptoms (less drastic convulsions and tremor). Symptoms gradually diminished, and within 4 h mice exhibited only mild indications (decreased motor activity and general malaise). All mice that survived the first 15 min after paraoxon injection showed an apparent complete recovery within 24 h.
Figure 4.
A) Plant-derived AChE-RER can protect mice against lethal challenge of paraoxon. Mice were injected i.v. with plant-derived AChE-RER followed, after 10 min, by another i.v. injection of paraoxon (750 mg/kg) to yield the indicated enzyme/OP ratio. Each circle represents an individual mouse. Symptoms were scored as described in the text. B) Plant-derived AChE-RER and paraoxon can reciprocally modulate accumulation of murine AChE-R in vivo. Plasma samples were harvested 10 days after treatments and analyzed for their content of AChE activity. Asterisks indicate statistically significant changes (P<0.05, Student’s t test).
Larger prophylactic doses of plant-derived AChE-RER provided even better protection. At enzyme/agent molar ratios of >0.09, initial symptoms were moderate; at >0.2, initial symptoms were only mild. Mice treated with an enzyme/agent ratio of >0.5 showed no sign of postexposure symptoms. Six-month monitoring of all surviving mice revealed no obvious neuropathy or other symptoms. Exceedingly low titers of IgGs specific to the administered human enzyme were observed; however, neither AChE-specific IgEs nor auto-Abs directed against murine AChE (tested against pure preparations of the latter; ref. 41) could be detected even in serum samples from mice treated with the largest dose of plant-derived AChE-RER (~10,000 U, data not shown).
AChE-RER treatment limits the OP-induced up-regulation of host AChE in plasma
OP exposure induces two seemingly inverse effects. First, it decreases ChE activities by blockade; and second, it induces long-lasting feedback overproduction of AChE-R (8, 10). In another set of experiments, we examined the ability of AChE-RER to protect from these delayed effects of OP poisoning. To this end, mice were pretreated by i.v. administration of PBS ± AChE-RER (400 U), followed by a sublethal (0.8 LD50) or lethal (1.13 LD50) paraoxon challenge. Unprotected, sublethally challenged mice or AChE-protected, lethally challenged mice all exhibited severe cholinergic symptoms followed by recovery (survival rates were 100% and 70% for sublethally and lethally challenged mice, respectively). Ten days after treatment, mice were euthanized and plasma and skeletal muscle samples were harvested. At this time, all mice predictably experienced a small increase in their plasma AChE levels induced by the OP challenge (Fig. 4B, Supplemental Fig. 1A). Plasma AChE activity at this delayed time point was substantially lower in enzyme-treated animals than in unprotected sublethally challenged mice, suggesting an effective yet incomplete offset of the long-lasting AChE-R overproduction by administration of plant-derived AChE-RER (Fig. 4B). Administration of plant-derived AChE-RER alone significantly limited levels of plasma AChE-R activity 10 days postinjection (Fig. 4B, Supplemental Fig. 1A), supporting the notion that this offset was a feedback response to the high levels of AChE molecules in the circulatory system. Furthermore, the enzyme pretreatment modulation of the OP-induced increase in plasma AChE activity is mirrored by corresponding changes in AChE monomers, probably murine AChE-R, observed by nondenaturing gels of samples obtained 10 days postchallenge (Supplemental Fig. 1A). We therefore conclude that the postpoisoning increase in the plasma levels of endogenous murine AChE, specifically murine AChE-R, can be mitigated at least partially by exogenously applied plant-derived enzyme.
AChE-RER treatment ameliorates NMJ dismorphology induced by OP poisoning
Enzyme pretreatment exerted considerable attenuation effects on the postexposure accumulation of the murine AChE-R in skeletal (leg) muscles after both lethal and sublethal OP insults and prevented its inhibition (Supplemental Fig. 1C). At the same time, muscle AChE activities in AChE-RER-pretreated mice were similar to those of unchallenged controls (Supplemental Fig. 1D, E) and compatible with the hypothesis that the administered AChE-RER served as a “decoy” protecting muscle AChE-R from inhibition. It therefore appears that administration of plant-derived AChE-RER reciprocally modulated the OP-induced changes in AChE gene expression, preventing its inhibition in muscles while decreasing OP-induced AChE-R production in the mouse circulation.
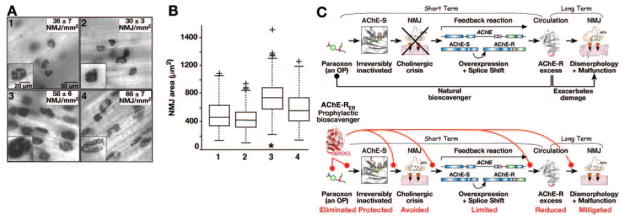
To study the effect of AChE-RER on the long-term dismorphology of NMJs induced by OPs, intact diaphragm muscles were dissected 10 days post-treatment, stained for catalytically active AChE, and analyzed with respect to NMJ density and mean individual area (Fig. 5). Paraoxon exposure increased both the density and the fraction of large NMJs (P<0.01, median test, Fig. 5A, B) compared with the PBS control. Pretreatment with 400 U AChE-RER prevented at least part of the exposure-induced enlargement of NMJ area, whereas treatment with 400 U AChE-RER alone did not alter NMJ density or area within the nonexposed diaphragm (P>0.05, median test, Fig. 5A, B). These findings attribute the attenuated NMJ dismorphology effects to an ameliorated feedback response in host muscle tissues.
Figure 5.
AChE-RER pretreatment ameliorates morphological NMJ damage induced by OP poisoning. A) Mice were either treated with PBS (1), 400 U AChE-RER (2), an 0.8 × LD50 of paraoxon (3), or 400 U AChE-RER and an 0.8 × LD50 of paraoxon (4). 10 days post-treatment, diaphragm muscle was dissected and stained for catalytically active AChE to visualize the number and size of NMJ. NMJ density (NMJs/mm2) was measured and indicated by an average ± SE within each section, A1–A4. B) Analysis of NMJ density and morphology. NMJ area (μm2) from the analysis above was box plotted using the MATLAB 7.0.1 software. The box is built from lines at the lower quartile, median, and upper quartile values. The whiskers show the rest of the data and outliers indicated by +, present data beyond the whiskers. *P < 0.01 (median test). Both analyses suggest that paraoxon exposure increased both the density of NMJs and the fraction of large synapses (see also insets in A3 compared with A1). Pretreatment with plant-derived AChE-RER prevented at least part of the effect on NMJ area, while plant-derived AChE-RER alone did not alter NMJ density or area within the nonexposed diaphragm. C) Model for mechanism of protection from acute and chronic consequences of OP intoxication.
DISCUSSION
The search for a useful prophylactic and/or therapeutic agent to counteract the effects of OP poisoning must consider many factors, including production costs, safety, and of course the ability to ensure both short-and long-term protection. Here we have demonstrated that plant production addresses the first two concerns, and choosing the soluble stress-related read-through splice variant of AChE addresses the third and fourth.
We expressed human AChE-RER in transgenic plants, which have recently emerged as a promising system, still in its experimental infancy, for the production of protein pharmaceuticals (42). Plant systems offer low production costs (with comparable purification and regulatory costs), production scalability and flexibility (with low capital investment), and improved safety (no concern of human pathogens and prions). Similar to other heterologous expression systems, the N-linked glycosylation process in plants differs in details from that in humans (31, 32). In this context, two major concerns are raised: accelerated circulatory clearance because of the inability of plants to sialilate their glycoproteins, and risk of increased allergenicity because of the presence of unique plant glyco-epitopes on complex glycans (31). To minimize these risks, we have directed the accumulation of AChE-R to the ER, thus avoiding the trans-Golgi, where xylose and fucose residues, occasionally associated with allergic reactions in humans, are added (31). Indeed, our preliminary data show that plant-derived AChE-RER is decorated by high-mannose glycans, which are not different from those of humans and therefore lack the “allergenic” glyco-epitopes.
ER retention of the recombinant AChE-R allowed its accumulation to high levels (~1% TSP). Similar to its native counterpart (23), the plant-derived protein was cleared rapidly from the circulation. The circulatory t½ of mammalian ChEs depends on amino acid domains on the protein’s surface, on glycan structures, and on the protein’s oligomerization state (43–45). The elimination rate is influenced by the species in which it is measured, by the genomic source of the tested protein, and by the production host. For example, clearance in primates of simian and human AChE-S (produced in a human cell line) is only marginally affected by the protein’s oligomerization state or its glycosylation (59–99 min) (44). Similarly, BChE purified from human blood showed characteristically long circulatory t½ in rodents (40, 45), whereas recombinant human BChE produced in a hamster cell line (45) or in the mammary glands of transgenic goats (C. N. Karatzas et al., poster in VIII International Meeting on Cholinesterases, Perugia, Italy, 2004) was eliminated much faster. The inferior pharmacokinetic parameters encountered by administration of recombinant ChEs can be improved by decorating the proteins with poly(ethylene glycol), which masks the surface features responsible for the rapid clearance. Accordingly, “PEGylated” recombinant human AChE-S (44) or recombinant BChE derived from milk of transgenic goats (C. N. Karatzas, et al., ibid) displayed significantly longer circulatory t½ than did the non-PEGylated proteins in either rodents, pigs, or monkeys. In preliminary experiments we observed a dramatic increase in t½ after PEGylation with different-sized PEGs (B. C. Geyer and T. S. Mor, unpublished results).
We demonstrate that recombinant human AChE-RER can accumulate in transgenic plant tissues to commercially viable levels, and that it can be readily purified to homogeneity and possesses kinetic hallmarks of the authentic human enzyme with respect to substrate hydrolysis and OP binding. We found plant production of ER-retained, soluble AChE-R to be advantageous at several levels. Recombinant human AChE-RER accumulates in plant tissues to commercially viable levels (Fig. 1). When purified to homogeneity, it reaches the kinetic hallmarks of the authentic human enzyme with respect to substrate hydrolysis and OP binding (Fig. 2). Its near stoichiometric administration to animals dramatically raised the level of circulating ChEs (Fig. 3), fully protecting them from the acute symptoms of OP poisoning and death (Fig. 4). Furthermore, AChE-R prophylaxis ameliorated the chronic effects of OP toxicity by mitigating long-term up-regulation of ACHE gene expression (Figs. 4, 5, Supplemental Fig. 1), adding a new dimension to the concept of ChE therapy. Over and above its function as a passive OP bioscavenger, this particular isoform of AChE can actively interfere with the vicious cycle associated with the long-lasting molecular feedback response to OP exposure. It is unclear whether AChE-S or BChE may also enable such long-term protection.
Exposure to OP ChE inhibitors rapidly increases the levels of synaptic ACh in NMJs and in peripheral tissues, initiating the so-called “cholinergic crisis” (Fig. 5C). AChE-R overproduction quickly restores the cholinergic balance by increasing ACh hydrolysis potential throughout the circulation; however, persistent overproduction of AChE-R associates with overproduction of proinflammatory cytokines (46), behavioral impairments (29), declarative memory loss (47), muscle malfunctioning (48), and excessive myelopoiesis (49). Pre-treatment with the enzyme would likely cause a transient elevation of inflammation markers (46); however, the built-in transience of the plant-derived AChE-RER would limit the duration of such effects. Moreover, mitigated overproduction of endogenous AChE-R in the circulation should further limit the duration of such symptoms, suggesting that pretreatment with this particular AChE isoform not only can prevent the acute cholinergic crisis after OP intoxication, but may also limit its deleterious chronic aftermath.
Supplementary Material
Acknowledgments
Supported in part by DARPA research contract N66001–01-C-8015 (to T.S.M. and H.S.), by U.S. National Institutes of Health Center of Excellence Grant U54N5058183-01 (to T.S.M.), and by Israel Science Foundation Grant 618/02–1 (to H.S.). The authors are indebted to Richard Malloy and the Biodesign greenhouse staff at the Polytechnic Campus of Arizona State University. We thank Palmer Taylor for the generous gift of mouse AChE.
References
- 1.Dharmani C, Jaga K. Epidemiology of acute organophosphate poisoning in hospital emergency room patients. Rev Environ Health. 2005;20:215–232. doi: 10.1515/reveh.2005.20.3.215. [DOI] [PubMed] [Google Scholar]
- 2.London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med. 2005;47:308–321. doi: 10.1002/ajim.20147. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield RA, Brown BR, Hutchins JB, Iandolo JJ, Jackson R, Slater LN, Bronze MS. Microbiological, biological, and chemical weapons of warfare and terrorism. Am J Med Sci. 2002;323:326–340. doi: 10.1097/00000441-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Lee EC. Clinical manifestations of sarin nerve gas exposure. J Am Med Assoc. 2003;290:659–662. doi: 10.1001/jama.290.5.659. [DOI] [PubMed] [Google Scholar]
- 5.Soreq H, Seidman S. Acetylcholinesterase—new roles for an old actor. Nat Rev Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 6.Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman A, Kaufer D, Shemer J, Hendler I, Soreq H, Tur-Kaspa I. Pyridostigmine brain penetration under stress enhances neuronal excitability and induces early immediate transcriptional response. Nat Med. 1996;2:1382–1385. doi: 10.1038/nm1296-1382. [DOI] [PubMed] [Google Scholar]
- 8.Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393:373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 9.Shohami E, Kaufer D, Chen Y, Seidman S, Cohen O, Ginzberg D, Melamed-Book N, Yirmiya R, Soreq H. Antisense prevention of neuronal damages following head injury in mice. J Mol Med. 2000;78:228–236. doi: 10.1007/s001090000104. [DOI] [PubMed] [Google Scholar]
- 10.Meshorer E, Erb C, Gazit R, Pavlovsky L, Kaufer D, Friedman A, Glick D, Ben-Arie N, Soreq H. Alternative splicing and neuritic mRNA translocation under long-term neuronal hypersensitivity. Science. 2002;295:508–512. doi: 10.1126/science.1066752. [DOI] [PubMed] [Google Scholar]
- 11.Lev-Lehman E, Evron T, Broide RS, Meshorer E, Ariel I, Seidman S, Soreq H. Synaptogenesis and myopathy under acetylcholinesterase overexpression. J Mol Neurosci. 2000;14:93–105. doi: 10.1385/JMN:14:1-2:093. [DOI] [PubMed] [Google Scholar]
- 12.Farchi N, Soreq H, Hochner B. Chronic acetyl-cholinesterase overexpression induces multilevelled aberrations in mouse neuromuscular physiology. J Physiol. 2003;546:165–173. doi: 10.1113/jphysiol.2002.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor P. Anticholinesterase agents. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 1996. pp. 161–176. [Google Scholar]
- 14.Maxwell DM, Brecht KM, Doctor BP, Wolfe AD. Comparison of antidote protection against soman by pyridostigmine, HI-6 and acetylcholinesterase. J Pharmacol Exp Ther. 1993;264:1085–1089. [PubMed] [Google Scholar]
- 15.Ashani Y. Prospective of human butyrylcholinesterase as a detoxifying antidote and potential regulator of controlled-release drugs. Drug Dev Res. 2000;50:298–308. [Google Scholar]
- 16.Doctor BP, Saxena A. Bioscavengers for the protection of humans against organophosphate toxicity. Chem Biol Interact. 2005;157–158:167–171. doi: 10.1016/j.cbi.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan D, Ordentlich A, Barak D, Ariel N, Kronman C, Velan B, Shafferman A. Does “butyrylization” of acetylcholinesterase through substitution of the six divergent aromatic amino acids in the active center gorge generate an enzyme mimic of butyrylcholinesterase? Biochemistry. 2001;40:7433–7445. doi: 10.1021/bi010181x. [DOI] [PubMed] [Google Scholar]
- 18.Sklan EH, Lowenthal A, Korner M, Ritov Y, Landers DM, Rankinen T, Bouchard C, Leon AS, Rice T, Rao DC, et al. Acetylcholinesterase/paraoxonase genotype and expression predict anxiety scores in health, risk factors, exercise training, and genetics study. Proc Natl Acad Sci U S A. 2004;101:5512–5517. doi: 10.1073/pnas.0307659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunwald J, Marcus D, Papier Y, Raveh L, Pittel Z, Ashani Y. Large-scale purification and long-term stability of human butyrylcholinesterase: a potential bioscavenger drug. J Biochem Biophys Methods. 1997;34:123–135. doi: 10.1016/s0165-022x(97)01208-6. [DOI] [PubMed] [Google Scholar]
- 20.Lenz DE, Maxwell DM, Koplovitz I, Clark CR, Capacio BR, Cerasoli DM, Federko JM, Luo C, Saxena A, Doctor BP, Olson C. Protection against soman or VX poisoning by human butyrylcholinesterase in guinea pigs and cynomolgus monkeys. Chem Biol Interact. 2005;157–158:205–210. doi: 10.1016/j.cbi.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Cerasoli DM, Griffiths EM, Doctor BP, Saxena A, Fedorko JM, Greig NH, Yu QS, Huang Y, Wilgus H, Karatzas CN. In vitro and in vivo characterization of recombinant human butyrylcholinesterase (Protexia™) as a potential nerve agent bioscavenger. Chem Biol Interact. 2005;157–158:363–365. doi: 10.1016/j.cbi.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 22.Chan RY, Adatia FA, Krupa AM, Jasmin BJ. Increased expression of acetylcholinesterase T and R transcripts during hematopoietic differentiation is accompanied by parallel elevations in the levels of their respective molecular forms. J Biol Chem. 1998;273:9727–9733. doi: 10.1074/jbc.273.16.9727. [DOI] [PubMed] [Google Scholar]
- 23.Cohen O, Reichenberg A, Perry C, Ginzberg D, Pollmacher T, Soreq H, Yirmiya R. Endotoxin-induced changes in human working and declarative memory associate with cleavage of plasma “readthrough” acetylcholinesterase. J Mol Neurosci. 2003;21:199–212. doi: 10.1385/jmn:21:3:199. [DOI] [PubMed] [Google Scholar]
- 24.Mor TS, Sternfeld M, Soreq H, Arntzen CJ, Mason HS. Expression of recombinant human acetylcholinesterase in transgenic tomato plants. Biotechnol Bioeng. 2001;75:259–266. doi: 10.1002/bit.10012. [DOI] [PubMed] [Google Scholar]
- 25.Geyer BC, Muralidharan M, Cherni I, Doran J, Fletcher SP, Evron T, Soreq H, Mor TS. Purification of transgenic plant-derived recombinant human acetylcholinesterase-R. Chem Biol Interact. 2005;157–158:331–334. doi: 10.1016/j.cbi.2005.10.097. [DOI] [PubMed] [Google Scholar]
- 26.Sternfeld M, Shoham S, Klein O, Flores-Flores C, Evron T, Idelson GH, Kitsberg D, Patrick JW, Soreq H. Excess “read-through” acetylcholinesterase attenuates but the “synaptic” variant intensifies neurodeterioration correlates. Proc Natl Acad Sci U S A. 2000;97:8647–8652. doi: 10.1073/pnas.140004597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidman S, Sternfeld M, Ben Aziz-Aloya R, Timberg R, Kaufer-Nachum D, Soreq H. Synaptic and epidermal accumulations of human acetylcholinesterase are encoded by alternative 3′-terminal exons. Mol Cell Biol. 1995;15:2993–3002. doi: 10.1128/mcb.15.6.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meshorer E, Toiber D, Zurel D, Sahly I, Dori A, Cagnano E, Schreiber L, Grisaru D, Tronche F, Soreq H. Combinatorial complexity of 5′ alternative ACHE transcripts and protein products. J Biol Chem. 2004;279:29740–29751. doi: 10.1074/jbc.M402752200. [DOI] [PubMed] [Google Scholar]
- 29.Birikh KR, Sklan EH, Shoham S, Soreq H. Interaction of “readthrough ” acetylcholinesterase with RACK1 and PKCbeta II correlates with intensified fear-induced conflict behavior. Proc Natl Acad Sci U S A. 2003;100:283–288. doi: 10.1073/pnas.0135647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher SP, Geyer BC, Smith A, Evron T, Joshi L, Soreq H, Mor TS. Tissue distribution of cholinesterases and anticholinesterases in native and transgenic tomato plants. Plant Mol Biol. 2004;55:33–43. doi: 10.1007/s11103-004-0394-9. [DOI] [PubMed] [Google Scholar]
- 31.Gomord V, Faye L. Posttranslational modification of therapeutic proteins in plants. Curr Opin Plant Biol. 2004;7:171–181. doi: 10.1016/j.pbi.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–686. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 33.Jurgens G. Membrane trafficking in plants. Annu Rev Cell Dev Biol. 2004;20:481–504. doi: 10.1146/annurev.cellbio.20.082503.103057. [DOI] [PubMed] [Google Scholar]
- 34.Kronman C, Velan B, Gozes Y, Leitner M, Flashner Y, Lazar A, Marcus D, Sery T, Papier Y, Grosfeld H, Cohen S, Shafferman A. Production and secretion of high levels of recombinant human acetylcholinesterase in cultured cell lines: microheterogeneity of the catalytic subunit. Gene. 1992;121:295–304. doi: 10.1016/0378-1119(92)90134-b. [DOI] [PubMed] [Google Scholar]
- 35.Soreq H, Ben-Aziz R, Prody CA, Seidman S, Gnatt A, Neville L, Lieman-Hurwitz J, Lev-Lehman E, Ginzberg D, Lipidot-Lifson Y, Zakut H. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc Natl Acad Sci U S A. 1990;87:9688–9692. doi: 10.1073/pnas.87.24.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sternfeld M, Ming G, Song H, Sela K, Timberg R, Poo M, Soreq H. Acetylcholinesterase enhances neurite growth and synapse development through alternative contributions of its hydrolytic capacity, core protein, and variable C termini. J Neurosci. 1998;18:1240–1249. doi: 10.1523/JNEUROSCI.18-04-01240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafferman A, Velan B, Ordentlich A, Kronman C, Grosfeld H, Leitner M, Flashner Y, Cohen S, Barak D, Ariel N. Substrate inhibition of acetylcholinesterase: residues affecting signal transduction from the surface to the catalytic center. EMBO J. 1992;11:3561–3568. doi: 10.1002/j.1460-2075.1992.tb05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kronman C, Cohen O, Velan B, Shafferman A. Host-regulated disposition of mammalian AChEs. Chem Biol Interact. 2005;157–158:51–55. doi: 10.1016/j.cbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Darreh-Shori T, Hellstrom-Lindahl E, Flores-Flores C, Guan ZZ, Soreq H, Nordberg A. Long-lasting acetyl-cholinesterase splice variations in anticholinesterase-treated Alzheimer’s disease patients. J Neurochem. 2004;88:1102–1113. doi: 10.1046/j.1471-4159.2003.02230.x. [DOI] [PubMed] [Google Scholar]
- 40.Raveh L, Grunwald J, Marcus D, Papier Y, Cohen E, Ashani Y. Human butyrylcholinesterase as a general prophylactic antidote for nerve agent toxicity. In vitro and in vivo quantitative characterization. Biochem Pharmacol. 1993;45:2465–2474. doi: 10.1016/0006-2952(93)90228-o. [DOI] [PubMed] [Google Scholar]
- 41.Marchot P, Ravelli RB, Raves ML, Bourne Y, Vellom DC, Kanter J, Camp S, Sussman JL, Taylor P. Soluble monomeric acetylcholinesterase from mouse: expression, purification, and crystallization in complex with fasciculin. Protein Sci. 1996;5:672–679. doi: 10.1002/pro.5560050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giddings G, Allison G, Brooks D, Carter A. Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol. 2000;18:1151–1155. doi: 10.1038/81132. [DOI] [PubMed] [Google Scholar]
- 43.Kronman C, Chitlaru T, Elhanany E, Velan B, Shafferman A. Hierarchy of post-translational modifications involved in the circulatory longevity of glycoproteins. Demonstration of concerted contributions of glycan sialylation and subunit assembly to the pharmacokinetic behavior of bovine acetylcholinesterase. J Biol Chem. 2000;275:29488–29502. doi: 10.1074/jbc.M004298200. [DOI] [PubMed] [Google Scholar]
- 44.Cohen O, Kronman C, Velan B, Shafferman A. Amino acid domains control the circulatory residence time of primate acetylcholinesterases in rhesus macaques (Macaca mulatta) Biochem J. 2004;378:117–128. doi: 10.1042/BJ20031305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saxena A, Ashani Y, Raveh L, Stevenson D, Patel T, Doctor BP. Role of oligosaccharides in the pharmacokinetics of tissue-derived and genetically engineered cholinesterases. Mol Pharmacol. 1998;53:112–122. doi: 10.1124/mol.53.1.112. [DOI] [PubMed] [Google Scholar]
- 46.Grisaru D, Pick M, Perry C, Sklan EH, Almog R, Goldberg I, Naparstek E, Lessing JB, Soreq H, Deutsch V. Hydrolytic and nonenzymatic functions of acetylcholinesterase comodulate hemopoietic stress responses. J Immunol. 2006;176:27–35. doi: 10.4049/jimmunol.176.1.27. [DOI] [PubMed] [Google Scholar]
- 47.Nijholt I, Farchi N, Kye M, Sklan EH, Shoham S, Verbeure B, Owen D, Hochner B, Spiess J, Soreq H, Blank T. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol Psychiatry. 2004;9:174–183. doi: 10.1038/sj.mp.4001446. [DOI] [PubMed] [Google Scholar]
- 48.Brenner T, Hamra-Amitay Y, Evron T, Boneva N, Seidman S, Soreq H. The role of read through acetylcholinesterase in the pathophysiology of myasthenia gravis. FASEB J. 2003;17:214–222. doi: 10.1096/fj.02-0609com. [DOI] [PubMed] [Google Scholar]
- 49.Pick M, Perry C, Lapidot T, Guimaraes-Sternberg C, Naparstek E, Deutsch V, Soreq H. Stress-induced cholinergic signaling promotes inflammation-associated thrombopoiesis. Blood. 2005;107:3397–3406. doi: 10.1182/blood-2005-08-3240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.