Abstract
Arrhythmias figure prominently among the complications encountered in the varied and diverse population of patients with congenital heart disease, and are the leading cause of morbidity and mortality. The incidence generally increases as the patient ages, with multifactorial predisposing features that may include congenitally malformed or displaced conduction systems, altered hemodynamics, mechanical or hypoxic stress, and residual or postoperative sequelae. The safe and effective management of arrhythmias in congenital heart disease requires a thorough appreciation for conduction system variants, arrhythmia mechanisms, underlying anatomy, and associated physiology. We, therefore, begin this review by presenting the scope of the problem, outlining therapeutic options, and summarizing congenital heart disease-related conduction system anomalies associated with disorders of the sinus node and AV conduction system. Arrhythmias encountered in common forms of congenital heart disease are subsequently discussed. In so doing, we touch upon issues related to risk stratification for sudden death, implantable cardiac devices, catheter ablation, and adjuvant surgical therapy.
Keywords: Congenital heart disease, Atrial arrhythmias, Ventricular arrhythmias, Sudden cardiac death
Scope Of The Problem
Congenital heart disease is the most common form of birth defect, with an estimated 1-2% of live newborns afflicted by moderate or severe types [1]. Arrhythmias figure prominently among the healthcare issues encountered and pose unique and diverse challenges [2,3]. The incidence of arrhythmias generally increases as the patient with congenital heart disease ages. Indeed, by adulthood, arrhythmias are the leading cause of morbidity and hospital admissions [4,5], and sudden death of presumed arrhythmic etiology is the most common cause of mortality [6,7].
Arrhythmias may reflect congenitally malformed or displaced conduction systems, altered hemodynamics, mechanical and/or hypoxic stress, and/or residual or postoperative sequelae [5,8]. The entire gamut of arrhythmia subtypes may occur in patients with congenital heart disease, with several forms often coexisting. Bradyarrhythmias may involve disorders of the sinus node, atrioventricular (AV) node, His-Purkinje system, or intra-atrial propagation. Junctional tachyarrhythmias are common, particularly in young post-operative patients [9]. Atrial tachyarrhymias are highly prevalent and may be mediated by accessory pathways, dual AV nodal reentry, twin AV nodes, macroreentrant circuits, automatic rhythms, or non-automatic foci. Atrial fibrillation is increasingly prevalent in the growing and aging population of adults with congenital heart disease. Ventricular arrhythmias are thought to be the leading cause of sudden death in several subtypes of congenital heart disease.
Therapeutic options
Importantly, arrhythmias may herald a changing hemodynamic profile and should generally prompt a detailed work-up. The care of the patient with congenital heart disease and arrhythmias may involve pharmacological therapy, catheter ablation, implantable cardiac devices, and surgical interventions.
In the absence of specific evidence-based recommendations, pharmacological therapy is often guided by principles established in other forms of heart disease [10,11]. These include considerations regarding systemic ventricular dysfunction, sinus node disease, impaired AV node conduction, negative inotropic effects, and proarrhythmia. The comparative efficacy of antiarrhythmic agents remains poorly studied, with little data regarding dosing and toxicity for the various age groups with congenital heart disease. Amiodarone-associated thyroid dysfunction is common in adults with congenital heart disease, especially in women and those with complex cyanotic heart disease or univentricular hearts with Fontan palliation [12]. There is much interest in the new generation of class III antiarrhythmic agents that purport fewer multisystemic side-effects without increased mortality in the setting of left ventricular dysfunction. In a multicenter case series, dofetilide appeared to be a viable adjunct to catheter-based ablation and alternative pharmacological approaches for atrial arrhythmias in adult patients with congenital heart disease [10].
Anatomical complexities and vascular access issues may complicate catheter-based interventions and implantation of pacemakers or implantable cardioverter-defibrillators (ICD) [2,13,14]. Challenges in device therapy include circumventing obstructed vessels, conduits, or baffles; minimizing thromboembolic risk in the presence of intracardiac shunts; identifying appropriate candidates for cardiac resynchronization therapy and primary prevention ICDs; configuring adequate vectors for defibrillation despite small sizes and/or limited vascular access; and a high rate of inappropriate shocks and lead complications [2,13,15,16]. Although the success rate is modest, some patients may benefit from pacemakers with automated overdrive pacing algorithms to terminate atrial tachyarrhythmias [17].
With the advent of three-dimensional electroanatomic mapping and advances in catheter technology permitting larger and deeper lesions, transcatheter ablation has emerged as a promising alternative for many patients with tachyarrhythmias. While acute success rates in dedicated centers are high, recurrences and the onset of new arrhythmias remain problematic, particularly in patients with Fontan palliation. In certain circumstances, arrhythmia surgery, usually performed in conjunction with cardiac surgery for other indications, may complement less invasive options.
Conduction System Considerations
Sinus node
Most patients with congenital heart disease have a normally positioned sinus node. Exceptions include left juxtaposition of the atrial appendages, situs inversus, and heterotaxy syndromes. In left juxtaposition of the atrial appendages, both appendages are on the left side of the arterial pedicle [18]. The sinus node is displaced anteriorly and inferiorly, below the crista terminalis [19]. In atrial situs inversus, the atria are positioned in a mirror-image fashion, with a left-sided sinus node. Heterotaxy syndromes may generally be categorized as either right (asplenia syndrome) or left (polysplenia syndrome) atrial isomerism. Patients with right atrial isomerism often have bilateral sinus nodes. The governing node may shift from one to the other [20]. In left atrial isomerism, sinus nodes are either absent or hypoplastic and displaced posteroinferiorly [21]. Congenital sinus node dysfunction is common [21].
AV node and His-Purkinje system
The AV conduction system may be displaced if atrial and ventricular septae are malaligned, AV arrangements are discordant, or if the heart is univentricular. As a general rule of thumb, if the AV conduction system is displaced, it also tends to be more fragile and susceptible to degeneration, placing patients at greater risk for AV block.
In atrioventricular canal defects (AVCD), the AV node is displaced inferiorly and posteriorly [22]. The His-bundle extends along the lower rim of the ventricular septum. This inferior course and hypoplastic left anterior hemifascicle gives rise to the characteristic superior QRS axis. In congenitally corrected transposition of the great arteries, or L-TGA, the AV node is displaced anteriorly and laterally [23]. An elongated and fragile His-bundle courses across the anterior rim of the pulmonary valve. If a ventricular septal defect is present, it continues along its superior border [23]. In patients with tricuspid atresia, the AV node is typically found on the floor of the right atrium near a small dimple lined with endocardium [24]. The course of the His-Purkinje system may vary but is typically further leftward and away from more anterior ventricular septal defects [24]. For other forms of univentricular hearts, key determinants of the course of the AV conduction system include the direction of ventricular looping and morphology of the dominant ventricle [25]. The AV conduction system is often displaced with AV discordance and AVCD. In L-looped single left ventricles, two AV nodes may be present [26,27]. The elongated His-bundle may be susceptible to damage, with complete AV block [26]. With ventricular D-looping and a dominant right ventricle, the AV node remains within Koch's triangle [27].
Arrhythmias In Specific Congenital Heart Disease Lesions
Atrial septal defect (ASD)
Macroreentrant atrial circuits are the most frequent arrhythmias encountered in patients with secundum and sinus venosus ASDs. In the absence of surgical repair, typical cavo-tricupsid isthmus-dependent atrial flutter is the most common form. In the presence of atriotomy incisions, sutures, and/or patches, non-isthmus dependent macroreentrant circuits may occur or coexist with typical flutter. Common substrates include macroreentry along the lateral right atrial wall and double-loop or figure-of-eight circuits [28,29].
The incidence of atrial arrhythmias increases with age and has been reported in 20% of adults [30,31]. Although surgical closure may decrease atrial arrhythmias, it is less effective in older patients [30-32]. In 218 adults with isolated ASDs, sustained atrial arrhythmias occurred in 19% prior to surgery: atrial flutter alone in 5%, atrial flutter and fibrillation in 2.8%, and atrial fibrillation alone in 11% [30]. Over a post-surgical follow-up of 3.8 years, atrial arrhythmias persisted or recurred in 60% of patients diagnosed preoperatively, and 2.3% developed new-onset arrhythmias. All patients with post-surgical atrial arrhythmias were over 40 years of age at time of repair. In a subsequent study that randomized 521 adults over 40 years of age with a secundum or sinus venosus ASD to surgical closure versus medical therapy, no difference in atrial tachyarrhythmias were noted at a median of 7 years post-operatively [31]. The impact of transcatheter ASD closure on atrial arrhythmias is less clear. In one series, all patients with persistent arrhythmias remained in atrial fibrillation or flutter after closure [32].
Ventricular septal defect (VSD)
In patients with unoperated VSDs, isolated premature ventricular contractions (PVC), couplets, and multiform PVCs are prevalent [33]. Non-sustained or sustained ventricular tachycardia has been observed in 6% [33]. A higher mean pulmonary artery pressure is associated with high-grade ectopy [33]. Nevertheless, in the absence of Eisenmenger syndrome, sudden cardiac death is uncommon [34,35]. Late sudden death has been reported in 4% of patients following surgical repair [36,37]. Of 296 patients with surgical VSD closure between 1954 and 1960, 20% of patients had transpired by 30 years of follow-up [37]. Risk factors for mortality included surgical repair after 5 years of age, pulmonary vascular resistance greater than 7 Woods units, and complete heart block. In patients who undergo transcatheter VSD closure, AV block is a major concern, with an estimated incidence of 3-4% [38].
Atrioventricular canal defect (AVCD)
In patients with AV canal defects, frequent ventricular ectopy has been described in 30%, while complex ventricular arrhythmias are predominantly confined to those with left ventricular dysfunction [39]. Persistent complete AV block occurs in 1% to 7% in the immediate post-operative period and approximately 2% thereafter [39,40]. Prolonged infra-Hisian conduction time may be a marker for increased risk of late AV block, even if the PR interval is normal [41]. In 18 patients with AVCDs, preoperative electrophysiologic studies revealed sinus node dysfunction in 1, supra-Hisian first degree AV block in 5, and intraatrial conduction delay in the majority [36]. Atrial fibrillation or flutter has been noted in 5% of patients after surgical repair [39,42].
Importantly, since the AV node is displaced just anterior to the mouth of the coronary sinus, interventional electrophysiologists should be cautious when ablating in the right inferior paraseptal region. For patients with cavo-tricuspid isthmus-dependent macroreentry, lateral ablation lines are generally preferred to prevent AV block. With dual AV node physiology, the slow pathway has been located superior to the His bundle, with the fast pathway inferior to the displaced AV node, as shown in Figure 1 [43].
Figure 1.
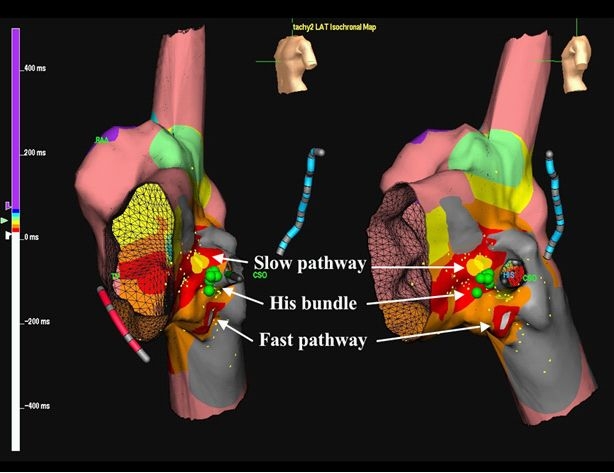
Cryomapping and cryoablation combined with 3D electroanatomic mapping in a patient with a partial AVCD. Three-dimensional electroanatomic maps of retrograde atrial activation during AV nodal reentrant tachycardia in left anterior oblique (Panel A) and left lateral (Panel B) views. The blue decapolar catheter is positioned in the coronary sinus and red quadripolar catheter in the right ventricle. Green circles indicate sites where His-bundle electrograms were recorded. Local activation times are color-coded, with the site of earliest atrial activation in white, inferior to the infero-posteriorly displaced His-bundle. The yellow circles represent the site of successful cryomapping and cryoablation of the slow pathway, superior to the His bundle. RAA denotes right atrial appendage; CSO, coronary sinus ostium. Reproduced from Khairy P. et al. Partial atrioventricular canal defect with inverted atrioventricular nodal input into an inferiorly displaced atrioventricular node. Heart Rhythm 2007;4(3):355-8.43. Copyright (2007), with permission from Elsevier.
Left ventricular outflow tract obstruction
In patients with left ventricular outflow tract obstruction, ventricular arrhythmias have been related to severity of obstruction, increased wall stress, and left ventricular hypertrophy [5,44-46]. In a series of adults with unoperated aortic stenosis, 34% had high grade ventricular ectopy on Holter monitoring, compared to 6% of controls [44]. Correlations with lower left ventricular ejection fraction and higher wall stress were later demonstrated [45,46]. Unfortunately, the risk of sudden death appears to persist despite surgical repair, warranting lifelong monitoring. Indeed, in a population-based study of sudden death after surgery for congenital heart disease, patients with aortic stenosis constituted the highest risk subgroup, with an incidence of 3% at 10 years and 20% at 30 years [6]. Aortic coarctation was also among the high risk lesions [6].
Congenitally corrected transposition of the great arteries (L-TGA)
Patients with L-TGA have notoriously fragile AV conduction systems. In 107 patients with L-TGA and mean age of 22 years, complete AV block occurred in 22% [47]. Risk of AV block was estimated to be 2% per year, irrespective of associated anomalies. Electrophysiological studies suggest that AV block occurs above or within the His bundle [48,49]. These studies are consistent with clinical and pathological observations. A stable narrow QRS escape rhythm often accompanies complete AV block [47] and fibrosis of the His bundle is observed histologically [49,50]. Since the AV node and His bundle are highly sensitive to catheter or surgical trauma, manipulation near these areas should be exercised with caution. Altered hemodynamics, such as the volume loading conditions of pregnancy, may place patients at higher risk of developing AV block. Complete AV block follows surgical repair of an associated VSD in over 25% [47,51].
Ebstein's anomaly
In Ebstein's anomaly, the atrialized portion of the right ventricle is morphologically and electrically right ventricle but functionally right atrium [52]. Mechanical stimulation of the atrialized right ventricle may induce ventricular arrhythmias. Otherwise, spontaneous ventricular tachycardia is uncommon in the absence of associated malformations, such as aortic coarctation or left ventricular outflow tract obstruction [53].
Right-sided accessory pathways, classically associated with Ebstein's anomaly, have been reported in 25% and may be multiple [52,54,55]. In addition to AV reciprocating tachycardia, ectopic atrial tachycardia and atrial fibrillation or flutter can occur. Tachyarrhythmias may be poorly tolerated in patients with severe Ebstein's malformation and/or an associated ASDs that shunt right-to-left during tachycardia [5]. High risk or multiple pathways may support rapid conduction during atrial fibrillation or flutter [55]. Mapping and ablation can be challenging as signals in the atrialized portion of the right ventricle may be complex and the true AV groove, along which accessory pathways are targeted, may not be readily apparent. To identify the AV groove, coronary angiography may be performed or a thin multielectrode catheter may be inserted in the right coronary artery [56]. Of 65 patients with Ebstein's anomaly and accessory pathways, short-term ablation success rates ranged from 75% to 89%, depending on pathway location, with late recurrences in up to 32% [57].
Univentricular hearts with Fontan palliation
The Fontan procedure has undergone multiple modifications to become the treatment of choice for various forms of single ventricle physiology [25]. Sudden cardiac death of presumed arrhythmic etiology is a major cause of late mortality [58]. Atrial arrhythmias are a challenge to manage, often incur substantial morbidity, and may be poorly tolerated hemodynamically. Although some respond favorably to pharmacological therapy [10] results are often disappointing.
In general, the incidence of atrial tachyarrhythmias appears lower in patients with total cavo-pulmonary connections in comparison to classic right atrium to pulmonary artery connections. Studies comparing the relative incidence of atrial tachyarrhythmias in patients with lateral tunnel versus extracardiac conduits are ongoing. Overall, the most common arrhythmia is atrial macroreentry [59]. Although atrial fibrillation may occur, it is surprisingly less common than one would anticipate, especially considering the often extremely dilated right atrium. Tachycardia circuits may be complex and/or multiple [28,60,61]. Single circuits that are quite amenable to catheter ablation may occasionally be encountered (Figure 2). Overall acute success rates exceed 80% [62-64], although recurrences or new onset arrhythmias remain problematic, in the order of 30% to 45% within the first year [62,65].
Figure 2.
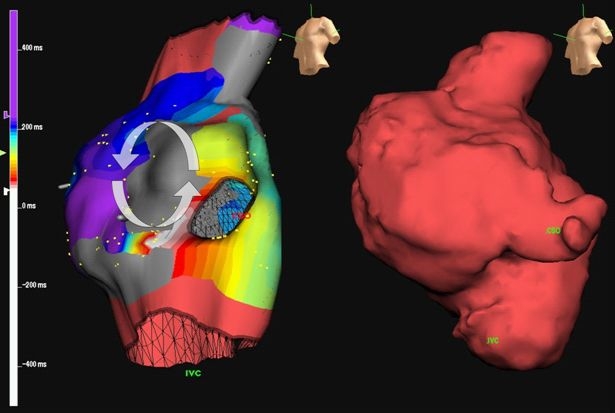
Electroanatomic mapping in a right atrium to pulmonary artery Fontan. An electroanatomic map (left) and imported CMR image (right) are shown in a patient with a classic modified Fontan and recalcitrant atrial tachyarrhythmias. The grey regions denote areas of dense scar. Local activation times are color-coded, from white to red, orange, yellow, green, light blue, dark blue, and purple. Note the narrow channel of tissue between two dense scars. The arrhythmia circuit propagated counterclockwise around the upper scar and was successfully interrupted by ablating this narrow isthmus. Reproduced from Khairy P. EP challenges in adult congenital heart disease Heart Rhythm 2008;5(10):1464-72. Copyright (2008), with permission from Elsevier.
Patients with failing Fontans and refractory atrial arrhythmias should be considered for surgical conversion to a total cavopulmonary connection with concomitant arrhythmia surgery. This typically includes debulking the right atrium, removing thrombus, excising right atrial scar tissue, epicardial pacemaker implantation, a modified right atrial Maze procedure and, in patients with prior documented atrial fibrillation, a left-sided Maze procedure as well [66]. Case series with short-term follow-up report promising results, with arrhythmia recurrence rates of 13% to 30% [66-68]. Although the incidence of atrial tachyarrhythmias may be lower, extracardiac Fontans substantially complicate transvenous access to arrhythmia circuits, as exemplified by Figure 3 [69].
Figure 3.
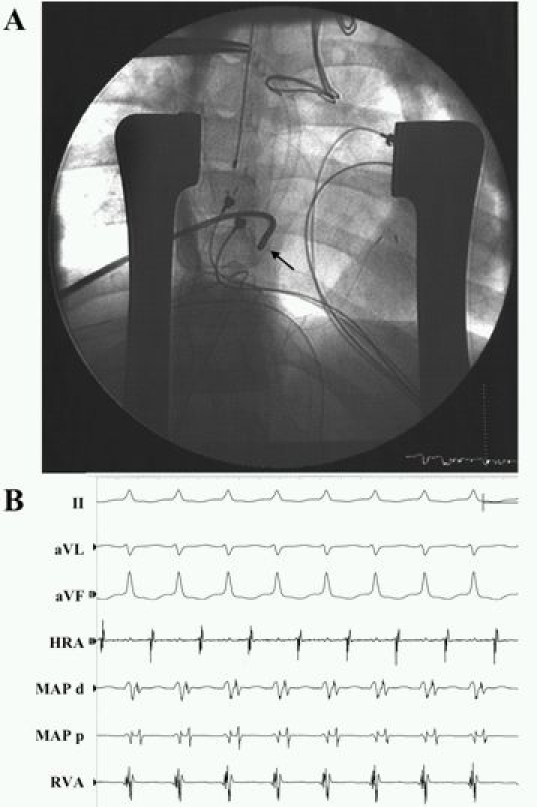
Catheter ablation in an extracardiac Fontan via a direct atriotomy approach. A patient with hypoplastic left heart syndrome and Ebstein's malformation of the right-sided AV valve had poorly tolerated incessant orthodromic AV reciprocating tachycardia postoperatively after an extracardiac Fontan with surgical accessory pathway ligation. In Panel A, an anteroposterior view is shown at the site of successful ablation, portraying the position of the radiofrequency ablation catheter (black arrow). The sternum is splayed open by means of thoracic retractors. Epicardial bipolar atrial and ventricular pacing leads are seen. Shown in Panel B are recordings from surface ECG leads II, aVL, and aVF; epicardial high right atrium (HRA); distal (MAP d) and proximal (MAP p) electrode pairs of the radiofrequency ablation catheter; and epicardial ventricle (RVA). Orthodromic AV reciprocating tachycardia is seen with the mapping catheter positioned at the site of successful ablation. Reproduced from Khairy P et al. Transcatheter ablation via a sternotomy approach as a hybrid procedure in a univentricular heart. PACE 2008;31(5):639-40, with permission from Wiley-Blackwell.
Tetralogy of Fallot
The frequency and nature of arrhythmias encountered in tetralogy of Fallot are related, in part, to the type of surgical correction. Repairs were initially performed by means of a ventricular incision. This approach was largely abandoned in favor of transatrial/transpulmonary access to reduce risk for potentially fatal ventricular tachyarrhythmias [70]. The most common atrial circuit is typical clockwise or counterclockwise atrial flutter utilizing the sub-Eustachian isthmus between the tricuspid valve annulus and inferior vena cava, even if the P-wave morphology is not characteristic for these arrhythmias [71]. Other circuits often involve the lateral wall and may be multiple, often with a double-loop type of reentry. Non-automatic focal atrial tachycarrhythmias most commonly arise adjacent to suture points, with radial spread of activation. A practical approach to catheter ablation in tetralogy of Fallot has been previously described [71].
Sudden cardiac death is the most common cause of mortality late after repair [72,73]. Monomorphic ventricular tachycardia occurs in approximately 10% of patients by 20 years of follow-up, an example of which is depicted in Figure 4 [71]. Nearly all forms are critically dependent on at least one of four discrete narrow channels, or isthmuses, portrayed in Figure 5 [71,74]. Considerable efforts have been directed towards identifying risk factors for ventricular tachycardia and sudden death. In the largest cohort study with a mean follow-up > 20 years, non-invasive risk factors were a QRS interval ≥ 180 ms, an annual increase in QRS duration, older age at repair, and presence of a right ventricular outflow tract patch [75]. Patients with ventricular tachycardia or sudden cardiac death were more likely to have increased cardiothoracic ratios, at least moderate pulmonary and tricuspid regurgitation, and peripheral pulmonary stenosis. A higher QT dispersion was also noted, believed to reflect increased heterogeneity in myocardial repolarization. Other reported risk factors include frequent ectopic beats [76] increased right ventricular systolic pressures [77-79] complete heart block [77,80] and increased JT dispersion [81,82]. In patients deemed at moderate risk, further risk stratification by means of programmed ventricular stimulation may be helpful [83-85]. Event-free survival rates in non-inducible and inducible patients are shown in Figure 6 [83].
Figure 4.
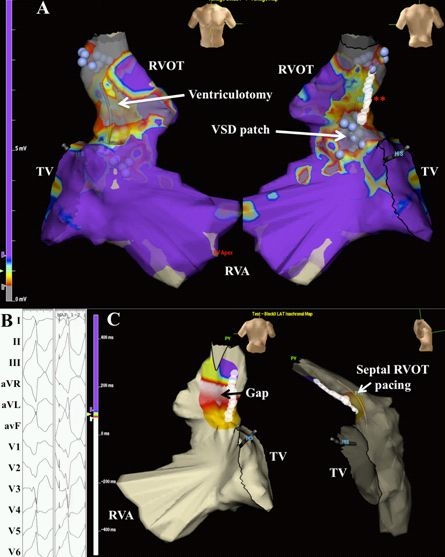
Voltage and pace mapping of ventricular tachycardia in tetralogy of Fallot. In Panel A, a color-coded voltage map of the right ventricle in sinus rhythm is shown in a patient with tetralogy of Fallot and ventricular tachycardia. Values below 0.5 mV are depicted in grey and above 1.5 mV in purple. The presumed ventriculotomy incision and ventricular septal defect patch are indicated by the white arrows. In areas of low voltage, unipolar pacing at 10 mAmp/2 ms was performed. Unexcitable tissue was marked by blue spheres. Linear ablation (white circles at the double red asterisk) was performed connecting two unexcitable areas, i.e. pulmonary annulus and ventricular septal defect patch. TV denotes tricuspid valve; RVA, right ventricular apex; RVOT, right ventricular outflow tract. This site was selected for ablation following pace mapping of the septal RVOT, shown in Panel B. Bouts of non-sustained ventricular tachycardia were inducible, corresponding to documented sustained events. Left and right hand panels capture non-sustained ventricular tachycardia and successful 12-lead pace mapping, respectively. In Panel C, block was verified by pacing from a decapolar catheter septal to the ablation line and mapping local activation. Local activation times are color-coded from white to red, orange, yellow, green, light blue, dark blue, and purple. Early activation along the mid-portion of the RVOT adjacent to the ablation line suggests a conduction gap. Additional ablation was performed along the mid-portion of the line, until bidirectional block was achieved. Reproduced from Khairy P, Stevenson WG. Catheter ablation in tetralogy of Fallot. Heart Rhythm 2009;6(7):1069-74.71. Copyright (2009), with permission from Elsevier.
Figure 5.
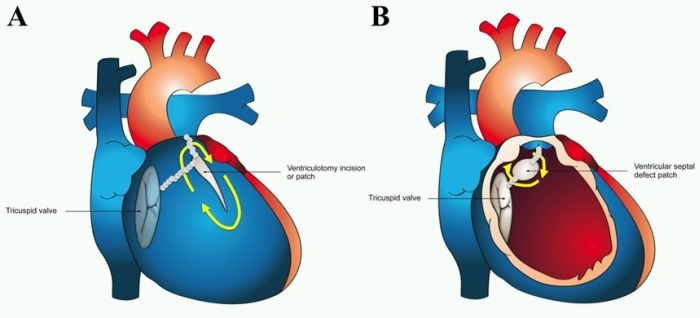
Potential critical isthmuses for ventricular tachycardia in surgically repaired tetralogy of Fallot. Potential arrhythmia circuits along the right ventricular free wall (Panel A) and septum (Panel B) are indicated by yellow arrows. Small grey circles schematically represent four critical isthmuses for ventricular tachycardia that may be transected by catheter ablation. Reproduced from Khairy P, Stevenson WG. Catheter ablation in tetralogy of Fallot. Heart Rhythm 2009;6(7):1069-74.71. Copyright (2009), with permission from Elsevier.
Figure 6.
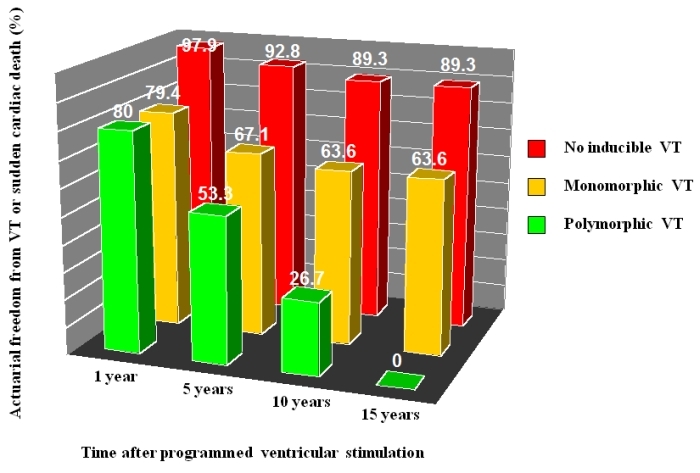
Value of programmed ventricular stimulation in tetralogy of Fallot. Actuarial freedom from ventricular tachycardia (VT) and sudden cardiac death is depicted in patients with no inducible VT, inducible monomorphic VT, and inducible polymorphic VT at 1, 5, 10, and 15 years following programmed ventricular stimulation. Adapted with permission from Khairy P et al. Value of programmed ventricular stimulation after tetralogy of fallot repair: a multicenter study. Circulation 2004;109(16):1994-2000.
Implantable cardioverter-defibrillators (ICD) are increasingly utilized in the primary and secondary prevention of sudden death in patients with tetralogy of Fallot, with patients experiencing relatively high rates of appropriate shocks [86]. For patients with primary prevention indications, a risk score was derived from surgical, hemodynamic, electrocardiographic, and electrophysiological factors (Table 1) [86]. As depicted in Figure 7, patients with < 3 points (low risk) experienced no appropriate shocks. In patients with 3-5 points (intermediate risk) and > 5 points (high risk), appropriate shocks were received by 3.8% and 17.5% of patients per year, respectively [86].
Table 1.
Risk score for appropriate ICD shocks in patients with tetralogy of Fallot and primary prevention indications
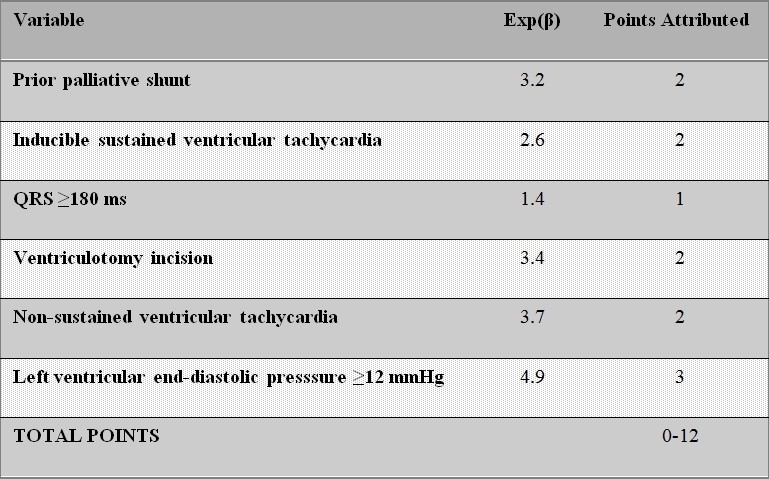
Figure 7.
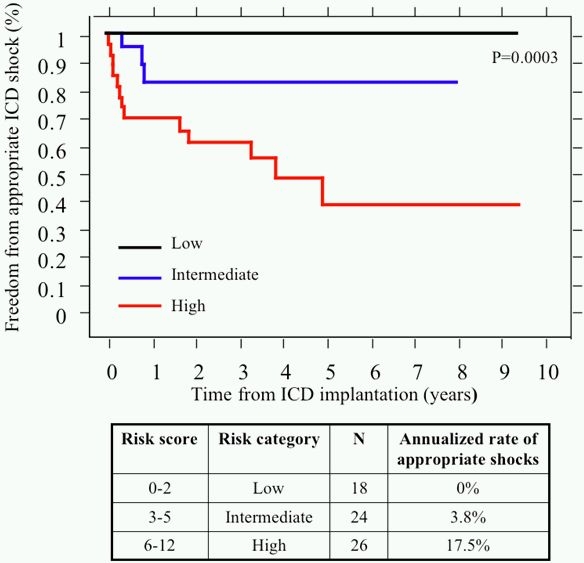
Freedom from appropriate ICD shocks in primary prevention patients with tetralogy of Fallot according to their risk category. In patients with primary prevention indications, Kaplan-Meier survival curves for freedom from first appropriate ICD shock are plotted and compared according to risk score classification. Risk score, corresponding risk category, number of patients, and annualized rate of appropriate shocks are summarized below. Reproduced with permission from Khairy P et al. Circulation 2008;117(3):363-70.
D-transposition of the great arteries (D-TGA)
Although arterial switch surgery has supplanted atrial redirection as the procedure of choice for D-TGA, most adults with D-TGA have had intraatrial baffle repairs of the Mustard or Senning variety. Sinus node dysfunction is highly prevalent with increasing age, with loss of sinus rhythm in 60% at 20 years [87]. In a meta-analysis comparing outcomes in 885 patients from 7 studies, sinus node dysfunction was more common in patients with Mustard procedures [88]. Atrial tachyarrhythmias have been reported in 24% of patients at 20 years [87] with similar rates in patients with Mustard and Senning baflles [88]. Sudden death is the leading cause of late mortality after intraatrial baffle surgery [6,87,89,90]. Recent reports suggest that atrial tachyarrhythmias are an important contributor to sudden death [91]. Contributing factors may include longer cycle lengths than typical atrial flutter (favoring 1:1 conduction), impaired AV transport with failure to augment right ventricular filling rates during tachycardia [92], systemic right ventricular dysfunction [93] and subendocarial ischemia resulting from a right coronary circulation irrigating a systemic ventricle [94]. As exemplified by Figure 8, catheter ablation of atrial arrhythmias frequently requires access to the pulmonary venous atrium and is often successful [95].
Figure 8.
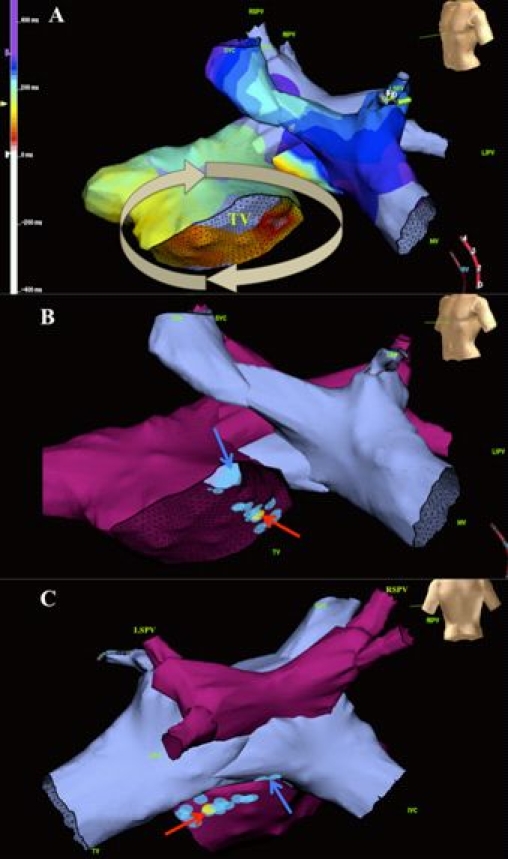
Ablation of the cavotricuspid isthmus in D-TGA with Mustard baffle. Panel A depicts an electroanatomic map of systemic and pulmonary venous atria. Local activation times are color-coded, from white to red, orange, yellow, green, light blue, dark blue, and purple. A circuit rotates clockwise around the tricuspid valve (TV). The anatomical relationship between systemic (blue) and pulmonary (purple) venous atria is demonstrated in left anterior oblique (Panel B) and posterior (Panel C) views. Blue circles mark ablation sites and the yellow circle the site of successful arrhythmia termination. Blue and red arrows indicate ablation lesions within systemic and pulmonary venous portions of the cavotricuspid isthmus, respectively. Reproduced from Khairy P, Van Hare G. Catheter ablation in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm 2009;6(2):283-9. Copyright (2009), with permission from Elsevier.
Studies are beginning to address the role of ICDs [91] and cardiac resynchronization therapy for failing systemic right ventricles [16,96]. Unfortunately, risk stratification for sudden death remains ineffective. In a retrospective multicenter case-control study, risk factors were limited to the presence of arrhythmia or heart failure symptoms and history of documented atrial fibrillation or flutter [97]. The electrocardiogram, chest x-ray, and Holter findings were not predictive of sudden death, and medical therapy and pacemakers were not found to be protective. Moreover, in a multicenter cohort study, very few appropriate ICD shocks were received in patients with primary prevention indications [91]. The presence or degree of right ventricular dysfunction was not associated with appropriate shocks. Encouragingly, beta-blocker therapy appeared protective.
Summary
Patients with congenital heart disease represent a heterogeneous population with varied arrhythmic diagnoses and issues. The last decade has witnessed major advances in our understanding of arrhythmia mechanisms and therapeutic options. Sudden cardiac death of presumed arrhythmic etiology is the leading cause of mortality, particularly in patients with left-sided obstructive lesions, D-TGA and intraatrial baffles, tetralogy of Fallot, and severe systemic ventricular dysfunction. Sinus node dysfunction is common in left atrial isomerism and with Mustard, Senning, Glenn, or Fontan surgery. Complete AV block frequently occurs in patients with L-looped ventricles, left atrial isomerism, and AVCD. Atriotomy incisions, sutures, baffles, and conduits may predispose to the development of scar-based macroreentrant atrial circuits, as commonly encountered in surgically repaired ASD, Mustard and Senning baffles, tetralogy of Fallot, and Fontan palliation.
ICDs and cardiac resynchronization therapy are increasingly utilized in patients with congenital heart disease. Unlike standard indications for patients without congenital heart disease, evidence supporting this technology is limited but growing. Guidelines have begun incorporating issues relevant to this patient population and targeted training programs are now offered. A thorough understanding of conduction system variants, arrhythmia mechanisms, underlying anatomy, and physiology is important to safely and effectively manage arrhythmias in this unique and diverse patient population.
References
- Hoffman JI, et al. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. EP challenges in adult congenital heart disease. Heart Rhythm. 2008;5:1464. doi: 10.1016/j.hrthm.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Walsh EP, et al. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115:534. doi: 10.1161/CIRCULATIONAHA.105.592410. [DOI] [PubMed] [Google Scholar]
- Engelfriet P, et al. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. Eur Heart J. 2005;26:2325. doi: 10.1093/eurheartj/ehi396. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Arrhythmias in adult congenital heart disease. Expert Rev Cardiovasc Ther. 2006;4:83. doi: 10.1586/14779072.4.1.83. [DOI] [PubMed] [Google Scholar]
- Silka MJ, et al. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32:245. doi: 10.1016/s0735-1097(98)00187-9. [DOI] [PubMed] [Google Scholar]
- Oechslin EN, et al. Mode of death in adults with congenital heart disease. Am.J.Cardiol. 2000;86:1111. doi: 10.1016/s0002-9149(00)01169-3. [DOI] [PubMed] [Google Scholar]
- Kanter RJ, et al. Atrial arrhythmias during chronic follow-up of surgery for complex congenital heart disease. Pacing Clin.Electrophysiol. 1997;20:502. doi: 10.1111/j.1540-8159.1997.tb06207.x. [DOI] [PubMed] [Google Scholar]
- Rekawek J, et al. Risk factors for cardiac arrhythmias in children with congenital heart disease after surgical intervention in the early postoperative period. J Thorac Cardiovasc Surg. 2007;133:900. doi: 10.1016/j.jtcvs.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Wells R, et al. Dofetilide for Atrial Arrhythmias in Congenital Heart Disease: A Multicenter Study. Pacing Clin Electrophysiol. 2009 doi: 10.1111/j.1540-8159.2009.02479.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hoyer AW. The safety and efficacy of ibutilide in children and in patients with congenital heart disease. Pacing Clin Electrophysiol. 2007;30:1003. doi: 10.1111/j.1540-8159.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- Thorne SA, et al. Amiodarone-associated thyroid dysfunction: risk factors in adults with congenital heart disease. Circulation. 1999;100:149. doi: 10.1161/01.cir.100.2.149. [DOI] [PubMed] [Google Scholar]
- Stephenson EA, et al. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol. 2006;17:41. doi: 10.1111/j.1540-8167.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Batra AS, et al. Pacing in adults with congenital heart disease. Expert Rev Cardiovasc Ther. 2006;4:663. doi: 10.1586/14779072.4.5.663. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: a multicenter study. Circulation. 2006;113:2391. doi: 10.1161/CIRCULATIONAHA.106.622076. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Defibrillators and cardiac resynchronisation therapy in congenital heart disease. Exp Rev Med Devices. 2008;5:267. doi: 10.1586/17434440.5.3.267. [DOI] [PubMed] [Google Scholar]
- Stephenson EA, et al. Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol. 2003;92:871. doi: 10.1016/s0002-9149(03)00905-6. [DOI] [PubMed] [Google Scholar]
- Anjos RT, et al. Surgical implications of juxtaposition of the atrial appendages. A review of forty-nine autopsied hearts. J Thorac Cardiovasc Surg. 1990;99:897. [PubMed] [Google Scholar]
- Ho SY, et al. Disposition of the sinus node in left-sided juxtaposition of the atrial appendages. Br Heart J. 1979;41:129. doi: 10.1136/hrt.41.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairy P, et al. Clinical use of electrocardiography in adults with congenital heart disease. Circulation. 2007;116:2734. doi: 10.1161/CIRCULATIONAHA.107.691568. [DOI] [PubMed] [Google Scholar]
- Momma K, et al. Characteristics and natural history of abnormal atrial rhythms in left isomerism. Am J Cardiol. 2009;65:231. doi: 10.1016/0002-9149(90)90090-n. [DOI] [PubMed] [Google Scholar]
- Anderson RH, et al. The morphology of the specialized atrioventricular junctional area: the evolution of understanding. Pacing Clin Electrophysiol. 2002;25:957. doi: 10.1046/j.1460-9592.2002.00957.x. [DOI] [PubMed] [Google Scholar]
- Anderson RH, et al. The conducting tissues in congenitally corrected transposition. Circulation. 1974;50:911. doi: 10.1161/01.cir.50.5.911. [DOI] [PubMed] [Google Scholar]
- Bharati S, et al. The conduction system in tricuspid atresia with and without regular (d-) transposition. Circulation. 1977;56:423. doi: 10.1161/01.cir.56.3.423. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Univentricular heart. Circulation. 2007;115:800. doi: 10.1161/CIRCULATIONAHA.105.592378. [DOI] [PubMed] [Google Scholar]
- Bharati S, et al. The course of the conduction system in single ventricle with inverted (L-) loop and inverted (L-) transposition. Circulation. 1975;51:723. doi: 10.1161/01.cir.51.4.723. [DOI] [PubMed] [Google Scholar]
- Wilkinson JL, et al. Conducting tissues in univentricular heart of right ventricular type with double or common inlet. J Thorac Cardiovasc Surg. 1979;77:691. [PubMed] [Google Scholar]
- Delacretaz E, et al. Multi atrial maco-re-entry circuits in adults with repaired congenital heart disease: entrainment mapping combined with three-dimensional electroanatomic mapping. J Am Coll Cardiol. 2001;37:1665. doi: 10.1016/s0735-1097(01)01192-5. [DOI] [PubMed] [Google Scholar]
- Shah D, et al. Dual-loop intra-atrial reentry in humans. Circulation. 2000;101:631. doi: 10.1161/01.cir.101.6.631. [DOI] [PubMed] [Google Scholar]
- Gatzoulis MA, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340:839. doi: 10.1056/NEJM199903183401103. [DOI] [PubMed] [Google Scholar]
- Attie F, et al. Surgical treatment for secundum atrial septal defects in patients >40 years old. A randomized clinical trial. J Am Coll Cardiol. 2001;38:2035. doi: 10.1016/s0735-1097(01)01635-7. [DOI] [PubMed] [Google Scholar]
- Silversides CK, et al. Symptomatic atrial arrhythmias and transcatheter closure of atrial septal defects in adult patients. Heart. 2004;90:1194. doi: 10.1136/hrt.2003.022475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman L. Noninvasive prediction of pulmonary artery pressure in patients with isolated ventricular septal defect. Pediatr Cardiol. 2000;21:197. doi: 10.1007/s002460010039. [DOI] [PubMed] [Google Scholar]
- Cohle SD, et al. Sudden death due to ventricular septal defect. Pediatr Dev Pathol. 1999;2:327. doi: 10.1007/s100249900130. [DOI] [PubMed] [Google Scholar]
- Sarubbi B, et al. Sudden death in an adult with a small ventricular septal defect and an aneurysmal membranous septum. Cardiol Young. 1999;9:99. doi: 10.1017/s1047951100007514. [DOI] [PubMed] [Google Scholar]
- Fournier A, et al. Electrophysiologic cardiac function before and after surgery in children with atrioventricular canal. Am J Cardiol. 1986;57:1137. doi: 10.1016/0002-9149(86)90688-0. [DOI] [PubMed] [Google Scholar]
- Moller JH, et al. Late results (30 to 35 years) after operative closure of isolated ventricular septal defect from 1954 to 1960. Am J Cardiol. 1991;68:1491. doi: 10.1016/0002-9149(91)90284-r. [DOI] [PubMed] [Google Scholar]
- Zhou T, et al. Complications associated with transcatheter closure of perimembranous ventricular septal defects. Catheter Cardiovasc Interv. 2008;71:559. doi: 10.1002/ccd.21412. [DOI] [PubMed] [Google Scholar]
- Daliento L, et al. Electrical instability in patients undergoing surgery for atrioventricular septal defect. Int J Cardiol. 1991;30:15. doi: 10.1016/0167-5273(91)90119-a. [DOI] [PubMed] [Google Scholar]
- McGrath LB, et al. Actuarial survival, freedom from reoperation, and other events after repair of atrioventricular septal defects. J Thorac Cardiovasc Surg. 1987;94:582. [PubMed] [Google Scholar]
- Jacobsen JR, et al. Intracardiac electrography in endocardial cushion defects. Circulation. 1976;54:599. doi: 10.1161/01.cir.54.4.599. [DOI] [PubMed] [Google Scholar]
- Vetter VL, et al. Electrophysiologic residua and sequelae of surgery for congenital heart defects. Am J Cardiol. 1982;50:588. doi: 10.1016/0002-9149(82)90328-9. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Partial atrioventricular canal defect with inverted atrioventricular nodal input into an inferiorly displaced atrioventricular node. Heart Rhythm. 2007;4:355. doi: 10.1016/j.hrthm.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Klein RC. Ventricular arrhythmias in aortic valve disease: analysis of 102 patients. Am J Cardiol. 1984;53:1079. doi: 10.1016/0002-9149(84)90641-6. [DOI] [PubMed] [Google Scholar]
- von Olshausen K, et al. Determinants of the incidence and severity of ventricular arrhythmias in aortic valve disease. Am J Cardiol. 1983;51:1103. doi: 10.1016/0002-9149(83)90353-3. [DOI] [PubMed] [Google Scholar]
- Santinga JT, et al. Left ventricular function in patients with ventricular arrhythmias and aortic valve disease. Ann Thorac Surg. 1983;35:152. doi: 10.1016/s0003-4975(10)61452-x. [DOI] [PubMed] [Google Scholar]
- Huhta JC, et al. Complete atrioventricular block in patients with atrioventricular discordance. Circulation. 1983;67:1374. doi: 10.1161/01.cir.67.6.1374. [DOI] [PubMed] [Google Scholar]
- Daliento L, et al. Rhythm and conduction disturbances in isolated, congenitally corrected transposition of the great arteries. Am J Cardiol. 1986;58:314. doi: 10.1016/0002-9149(86)90069-x. [DOI] [PubMed] [Google Scholar]
- Gillette PC, et al. Electrophysiologic studies in patients with ventricular inversion and "corrected transposition". Circulation. 1979;60:939. doi: 10.1161/01.cir.60.4.939. [DOI] [PubMed] [Google Scholar]
- Foster JR, et al. Congenitally corrected transposition of the great vessels: localization of the site of complete atrioventricular block using his bundle electrograms. Am J Cardiol. 1976;38:383. doi: 10.1016/0002-9149(76)90181-8. [DOI] [PubMed] [Google Scholar]
- McGrath LB, et al. Death and other events after cardiac repair in discordant atrioventricular connection. J Thorac Cardiovasc Surg. 1985;90:711. [PubMed] [Google Scholar]
- Ho SY, et al. The atrioventricular junctions in Ebstein malformation. Heart. 2000;83:444. doi: 10.1136/heart.83.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WM, et al. The electrophysiologic basis and management of symptomatic recurrent tachycardia in patients with Ebstein's anomaly of the tricuspid valve. Am J Cardiol. 1982;49:1223. doi: 10.1016/0002-9149(82)90048-0. [DOI] [PubMed] [Google Scholar]
- Hebe J, et al. Ebstein's anomaly in adults. Arrhythmias: diagnosis and therapeutic approach. Thorac.Cardiovasc.Surg. 2000;48:214. doi: 10.1055/s-2000-6897. [DOI] [PubMed] [Google Scholar]
- Attie F. The adult patient with Ebstein anomaly. Outcome in 72 unoperated patients. Medicine (Baltimore) 2000;79:27. doi: 10.1097/00005792-200001000-00003. [DOI] [PubMed] [Google Scholar]
- Shah MJ, et al. Improved localization of right-sided accessory pathways with microcatheter-assisted right coronary artery mapping in children. J Cardiovasc Electrophysiol. 2004;15:1238. doi: 10.1046/j.1540-8167.2004.04100.x. [DOI] [PubMed] [Google Scholar]
- Reich JD, et al. The Pediatric Radiofrequency Ablation Registry's experience with Ebstein's anomaly. Pediatric Electrophysiology Society. J.Cardiovasc.Electrophysiol. 1998;9:1370. doi: 10.1111/j.1540-8167.1998.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- Gatzoulis MA, et al. Definitive palliation with cavopulmonary or aortopulmonary shunts for adults with single ventricle physiology. Heart. 2000;83:51. doi: 10.1136/heart.83.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triedman JK, et al. Intra-atrial reentrant tachycardia after palliation of congenital heart disease: characterization of multiple macroreentrant circuits using fluoroscopically based three-dimensional endocardial mapping. J Cardiovasc Electrophysiol. 1997;8:259. doi: 10.1111/j.1540-8167.1997.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Triedman JK, et al. Electroanatomic mapping of entrained and exit zones in patients with repaired congenital heart disease and intra-atrial reentrant tachycardia. Circulation. 2001;103:2060. doi: 10.1161/01.cir.103.16.2060. [DOI] [PubMed] [Google Scholar]
- Kannankeril PJ, et al. Frequency of late recurrence of intra-atrial reentry tachycardia after radiofrequency catheter ablation in patients with congenital heart disease. Am.J.Cardiol. 2003;92:879. doi: 10.1016/s0002-9149(03)00908-1. [DOI] [PubMed] [Google Scholar]
- Triedman JK, et al. Arrhythmias in adults with congenital heart disease. Heart. 2002;87:383. doi: 10.1136/heart.87.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triedman JK, et al. Prospective trial of electroanatomically guided, irrigated catheter ablation of atrial tachycardia in patients with congenital heart disease. Heart Rhythm. 2005;2:700. doi: 10.1016/j.hrthm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Triedman JK, et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39:1827. doi: 10.1016/s0735-1097(02)01858-2. [DOI] [PubMed] [Google Scholar]
- Mavroudis C. The beneficial effects of total cavopulmonary conversion and arrhythmia surgery for the failed Fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002;5:12. doi: 10.1053/pcsu.2002.31489. [DOI] [PubMed] [Google Scholar]
- Setty SP, et al. Extracardiac conduit with a limited maze procedure for the failing Fontan with atrial tachycardias. Ann Thorac Surg. 2002;74:1992. doi: 10.1016/s0003-4975(02)04123-1. [DOI] [PubMed] [Google Scholar]
- Petko M, et al. Surgical reinterventions following the Fontan procedure. Eur.J.Cardiothorac.Surg. 2003;24:255. doi: 10.1016/s1010-7940(03)00257-4. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Transcatheter ablation via a sternotomy approach as a hybrid procedure in a univentricular heart. Pacing Clin Electrophysiol. 2008;31:639. doi: 10.1111/j.1540-8159.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- Karamlou T, et al. Surgery insight: late complications following repair of tetralogy of Fallot and related surgical strategies for management. Nat Clin Pract Cardiovasc Med. 2006;3:611. doi: 10.1038/ncpcardio0682. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Catheter ablation in tetralogy of Fallot. Heart Rhythm. 2009;6:1069. doi: 10.1016/j.hrthm.2009.02.042. [DOI] [PubMed] [Google Scholar]
- Gillette PC, et al. Sudden cardiac death in the pediatric population. Circulation. 1992;85(1 Suppl):I64. [PubMed] [Google Scholar]
- Nollert G, et al. Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol. 1997;30:1374. doi: 10.1016/s0735-1097(97)00318-5. [DOI] [PubMed] [Google Scholar]
- Zeppenfeld K, et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116:2241. doi: 10.1161/CIRCULATIONAHA.107.723551. [DOI] [PubMed] [Google Scholar]
- Gatzoulis MA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- Harrison DA, et al. Sustained ventricular tachycardia in adult patients late after repair of tetralogy of Fallot. J Am Coll Cardiol. 1997;30:1368. doi: 10.1016/s0735-1097(97)00316-1. [DOI] [PubMed] [Google Scholar]
- Jonsson H, et al. Late sudden deaths after repair of tetralogy of Fallot. Electrocardiographic findings associated with survival. Scand J Thorac Cardiovasc Surg. 1995;29:131. doi: 10.3109/14017439509107219. [DOI] [PubMed] [Google Scholar]
- Garson A, et al. Status of the adult and adolescent after repair of tetralogy of Fallot. Circulation. 1979;59:1232. doi: 10.1161/01.cir.59.6.1232. [DOI] [PubMed] [Google Scholar]
- Katz NM, et al. Late survival and symptoms after repair of tetralogy of Fallot. Circulation. 1982;65:403. doi: 10.1161/01.cir.65.2.403. [DOI] [PubMed] [Google Scholar]
- Hokanson JS, et al. Significance of early transient complete heart block as a predictor of sudden death late after operative correction of tetralogy of Fallot. Am J Cardiol. 2001;87:1271. doi: 10.1016/s0002-9149(01)01518-1. [DOI] [PubMed] [Google Scholar]
- Berul CI, et al. Electrocardiographic markers of late sudden death risk in postoperative tetralogy of Fallot children. J Cardiovasc Electrophysiol. 1997;8:1349. doi: 10.1111/j.1540-8167.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Vogel M, et al. Regional wall motion and abnormalities of electrical depolarization and repolarization in patients after surgical repair of tetralogy of Fallot. Circulation. 2001;103:1669. doi: 10.1161/01.cir.103.12.1669. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Value of programmed ventricular stimulation after tetralogy of fallot repair: a multicenter study. Circulation. 2004;109:1994. doi: 10.1161/01.CIR.0000126495.11040.BD. [DOI] [PubMed] [Google Scholar]
- Khairy P. Programmed ventricular stimulation for risk stratification in patients with tetralogy of Fallot: a Bayesian perspective. Nat Clin Pract Cardiovasc Med. 2007;4:292. doi: 10.1038/ncpcardio0882. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Risk stratification in surgically repaired tetralogy of Fallot. Expert Rev Cardiovasc Ther. 2009;7:755. doi: 10.1586/erc.09.38. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363. doi: 10.1161/CIRCULATIONAHA.107.726372. [DOI] [PubMed] [Google Scholar]
- Gelatt M, et al. Arrhythmia and mortality after the Mustard procedure: a 30-year single-center experience. J Am Coll Cardiol. 1997;29:194. doi: 10.1016/s0735-1097(96)00424-x. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Long-term outcomes after the atrial switch for surgical correction of transposition: a meta-analysis comparing the Mustard and Senning procedures. Cardiol Young. 2004;14:284. doi: 10.1017/S1047951104003063. [DOI] [PubMed] [Google Scholar]
- Gatzoulis MA, et al. Late arrhythmia in adults with the mustard procedure for transposition of great arteries: a surrogate marker for right ventricular dysfunction? Heart. 2000;84:409. doi: 10.1136/heart.84.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen M. Late results of Senning operation. J.Thorac.Cardiovasc.Surg. 1999;117:488. doi: 10.1016/s0022-5223(99)70329-6. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baflles: a multicenter study. Circulation: Arrhythmia Electrophysiol. 2008;1:250. doi: 10.1161/CIRCEP.108.776120. [DOI] [PubMed] [Google Scholar]
- Derrick GP, et al. Failure of stroke volume augmentation during exercise and dobutamine stress is unrelated to load-independent indexes of right ventricular performance after the Mustard operation. Circulation. 2000;102(19 Suppl 3):III154. doi: 10.1161/01.cir.102.suppl_3.iii-154. [DOI] [PubMed] [Google Scholar]
- Pettersen E, et al. Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol. 2007;49:2450. doi: 10.1016/j.jacc.2007.02.062. [DOI] [PubMed] [Google Scholar]
- Millane T, et al. Role of ischemia and infarction in late right ventricular dysfunction after atrial repair of transposition of the great arteries. J Am Coll Cardiol. 2000;35:1661. doi: 10.1016/s0735-1097(00)00585-4. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Catheter ablation in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm. 2009;6:283. doi: 10.1016/j.hrthm.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Khairy P, et al. Cardiac resynchronization therapy in congenital heart disease. Int J Cardiol. 2006;109:160. doi: 10.1016/j.ijcard.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kammeraad JA, et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004;44:1095. doi: 10.1016/j.jacc.2004.05.073. [DOI] [PubMed] [Google Scholar]


