Abstract
Reduced telomere length has recently been reported in T lymphocytes of individuals with trisomy 21 Down syndrome (DS) and dementia. Shorter telomeres also have been documented in dyskeratosis congenita, cell senescence, Alzheimer disease, and neoplastic transformation. These observations suggest that similar shortening may occur in people with fragile X-associated tremor/ataxia syndrome (FXTAS), which frequently is accompanied by dementia. To test this hypothesis, telomere length has been quantified in T lymphocytes from older male carriers of premutation FMR1 alleles, with or without FXTAS, and FXTAS with dementia. Shorter telomeres (relative to age-matched controls) were observed in 5/5 individuals with FXTAS and dementia, in 2/2 individuals with FXTAS without dementia, and in 3/3 individuals with the fragile X premutation only (p values ranged from <.001 to <.05; Student’s t test), indicating that telomere shortening is associated with the premutation expansion of the FMR1 gene. The current study design allowed simultaneous comparisons among control, premutation, FXTAS, and FXTAS with dementia samples, and showed nearly equal degrees of shortening relative to controls among the three premutation sample groups. Thus, telomere shortening may serve as a biomarker for cellular dysregulation that may precede the development of the symptoms of FXTAS.
Keywords: telomere shortening, metaphase, FMR1, Parkinson, dementia, FXTAS
INTRODUCTION
There is general agreement that telomere shortening occurs with increasing age [Hastie et al., 1990; Lindsey et al., 1991]. Moreover, there is growing evidence that telomere shortening may play a causative role in certain age-related human disorders, including heart disease [Samani et al., 2001; Benetos et al., 2004], osteoporosis [Valdes et al., 2007], obesity [Valdes et al., 2005], dyskeratosis congenita [Vulliamy and Dokal, 2007], Alzheimer disease [Panossian et al., 2003], and Down syndrome [Jenkins et al., 2006a]. The recent discovery of UUAGGG-repeat telomeric RNAs may help to better understand the role of the telomere in the above conditions including aging and cancer [Schoeftner and Blasco, 2007].
We have recently reported shorter telomeres in T lymphocytes of people with trisomy 21 (Down syndrome; DS) with dementia relative to DS without dementia [Jenkins et al., 2006a]. As an extension of these studies, we have investigated whether the same association between telomere shortening and dementia occurs in some cases of the late-onset neurodegenerative disorder, fragile X-associated tremor/ataxia syndrome (FXTAS) [Hagerman and Hagerman, 2007; Jacquemont et al., 2007; Bourgeois et al., 2007; Berry-Kravis et al., 2007]. FXTAS, which affects carriers (mainly males) of premutation alleles (55-200 CGG repeats) of the fragile X mental retardation 1 gene (FMR1), is often, but not invariably, accompanied by dementia [Grigsby et al., 2006]. Therefore, the principal objective of the current study was to test the hypothesis that T lymphocytes from males with FXTAS and dementia will exhibit shorter telomeres than either FXTAS cases without dementia, or carriers without FXTAS or age-matched controls.
MATERIALS AND METHODS
Subjects
Subjects who were carriers of premutation alleles, documented by FMR1 genotyping, were recruited through the Fragile X Research and Treatment Center at the University of California at Davis MIND Institute. Subjects signed informed consent for this research, which was reviewed and approved by an Institutional Review Board (IRB). Each subject underwent detailed neurological studies to confirm the presence of FXTAS, and neuropsychological testing to evaluate the presence of cognitive deficits. Using the diagnostic criteria for FXTAS reported by Jacquemont et al. [2003] and the diagnostic criteria for dementia of DSM-IV [Ghaemi et al., 2008], patients were separated into 3 groups: 5 with FXTAS and dementia; 2 with FXTAS but without dementia and 3 with the premutation and without FXTAS (see Table I).
Table I.
CGG repeat number, age, diagnostic and dementia status of 15 older menwith and without the fragile X premutation
| Log Number | Category | CGG Repeats | Age | FXTAS | Dementia |
|---|---|---|---|---|---|
| 19-04 | normal | 32 | 69 | no | no |
| 151-04 | normal | 33 | 66 | no | no |
| 160-04 | normal | 33 | 59 | no | no |
| 285-04 | normal | 30 | 73 | no | no |
| 1313-06 | normal | 30 | 64 | no | no |
| 384-04 | premutation | 73 | 73 | no | no |
| 501-05 | premutation | 60 | 56 | no | no |
| 293-06 | premutation | 81 | 69 | no | no |
| 176-05 | premutation | 90 | 70 | yes | no |
| 464-05 | premutation | 103 | 65 | yes | no |
| 4-04 | premutation | 87 | 68 | yes | yes |
| 5-04 | premutation | 88 | 66 | yes | yes |
| 112-04 | premutation | 90 | 69 | yes | yes |
| 192-04 | premutation | 115 | 64 | yes | yes |
| 383-05 | premutation | 142 | 64 | yes | Yes |
Molecular Analysis
Genomic DNA was isolated from peripheral blood leucocytes (5 ml of whole blood) using standard methods (Puregene Kit; Gentra Inc.). Southern blot analysis and PCR analysis were performed as described by Tassone et al. [2008]. Analysis and calculation of the repeat size were carried out using an Alpha Innotech FluorChem 8800 Image Detection System.
Telomere length analysis
Anonymous frozen buffy coat cells were obtained from older (53-73) age-matched, male individuals who: (1) did not carry the fragile X premutation; (2) carried the premutation and were unaffected by FXTAS; (3) had FXTAS, but without dementia; (4) had FXTAS with dementia. The buffy coat samples were cultured at 37°C for four days at an initial concentration of 200,000 – 400,000 viable mononuclear cells per ml of PHA-containing medium.
Telomere length differences were determined by detecting changes in fluorescence intensity using an FITC-labeled peptide nucleic acid (PNA) probe and DAPI counterstaining, (Applied Biosystems; DAKO) and Applied Imaging software similar to that previously reported [Jenkins et al., 2006a] including studies that validated the use of fluorescence intensity measurements for both whole metaphase analyses and individual chromosome comparisons. Nothing similar is possible with Southern analysis [Lansdorp et al., 1996; Londoño-Vallejo et al., 2001; Perner et al., 2003; Mayer et al., 2006]. Analyses of 20 metaphase and 20 interphase preparations from short-term T lymphocyte cultures from each of 15 individuals were analyzed using quantitative PNA FISH technology blind to dementia, FXTAS, and premutation status. Pairwise comparisons of light intensity values, e.g., 20 light intensity values from a fragile X premutation versus 20 light intensity values from an age-matched control, were made using the Student’s t test.
RESULTS
An example of telomere labeling is depicted in Figure 1, which shows telomeres at the ends of the short and long arms of metaphase chromosomes. Interphase labeling of telomeres is also shown following short-term lymphocyte culture of buffy coat samples from a person with FXTAS and dementia. Table II provides quantitative measures of the mean light intensities detected from metaphases of 15 male individuals with and without the fragile X premutation, FXTAS, and FXTAS with dementia in 7 studies where an age-matched control sample was cultured and analyzed in parallel with a premutation and/or FXTAS and/or FXTAS with dementia sample. Interphase results, uninformative for Study 1, were otherwise similar to those from metaphase (data not shown but is available upon request). As shown in Table II, metaphase analyses showed shorter telomeres in 3 of 3 cases of premutation only, compared to age-matched controls. Similarly, shorter telomeres were observed in 2 of 2 cases of FXTAS compared to age-matched controls, and 5 of 5 cases of FXTAS with dementia exhibited shorter telomeres than age-matched controls. When values for premutation without FXTAS, FXTAS, and FXTAS with dementia were compared in Study 1, there were no statistically significant pairwise differences of light intensities, similar to results for FXTAS, and FXTAS with dementia in Study 2.
Fig. 1.
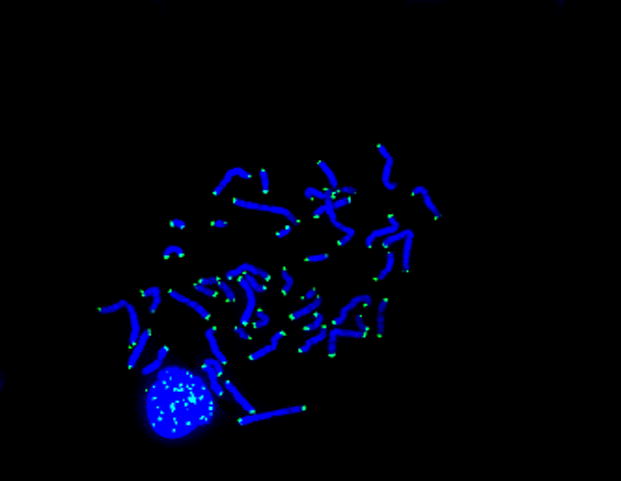
Telomeres in metaphase and interphase nuclei shown by an FITC-labeled PNA probe with DAPI counterstaining, from a person with FXTAS and dementia
Table II.
Shorter telomeres shown by reduced light intensities (x103) in fragile Xpremutations with and without FXTAS and/or dementia in whole metaphases from 7 studies
| Metaphase | Control | Premutation | FXTAS | FXTAS/D | P |
|---|---|---|---|---|---|
| Study 1 | 216731 [285-04] 30cgg |
17473 [384-04] 73cgg |
18270 [176-05] 90cgg |
18269 [112-04] 90cgg |
<.0032 |
| 2 | 20369 [19-04] 32cgg |
NA | 15365 [464-05] 103cgg |
13766 [5-04] 88cgg |
<.000004 |
| 3 | 22864 [1313-06] 30cgg |
NA | NA | 16464 [383-05] 142cgg |
<.000002 |
| 4 | 18166 [151-04] 33cgg |
NA | NA | 15264 [192-04] 115cgg |
<.05 |
| 5 | 255693 [19-04] 32cgg |
NA | NA | 16968 [4-04] 87cgg |
<.000001 |
| 6 | 24359 [160-04] 33cgg |
14756 [501-05] 60cgg |
NA | NA | <.00007 |
| 7 | 172733 [285-04] 30cgg |
93.369 [293-06] 81cgg |
NA | NA | <.000001 |
21673: means light intensity value = 216 from a 73-year-old control subject whose cgg repeat size was 30 and whose log number is [285-04].
p values are the largest numerical values obtained among comparisons between control vs. premutation; control vs. premutation with FXTAS; control vs. premutation with FXTAS and dementia, within Studies 1–7.
different samples from the same control individuals were cultured at different times for Studies 1 and 7 and Studies 2 and 5 so that the total number of control individuals in this project was 5 with a total of 7 cultured control buffy coat samples.
The same result was obtained when individual chromosome 1 was analyzed for telomere shortening among the samples listed in Table I. That is, all samples with fragile X premutations with and without FXTAS and/or dementia exhibited shorter telomeres on chromosome 1 than age-matched controls, while this was not observed when individual chromosomes 21 and X were analyzed (data not shown but available upon request).
DISCUSSION
As listed in Table II, 7 studies have clearly shown that telomeres were shorter in 10 older male individuals with the Fragile X premutation with and without FXTAS and/or dementia, compared to age-matched controls. Also, it appears that either whole metaphase analysis or individual chromosome 1 analysis may be used to detect shorter telomeres in older males with the Fragile X premutation. Individual chromosomes were studied as well as whole metaphases and interphases because Zou et al. [2004] have studied whether a subset of short telomeres determines replicative senescence, and because a previous study showed that individual chromosomes 21 had shorter telomeres in people with Down syndrome and dementia versus those with DS only [Jenkins et al., 2006b]. Future studies will show whether other individual chromosomes may be utilized to detect increased telomere shortening in people with the fragile X premutation.
In Studies 1 and 2 (Table II), it was surprising to find virtually no difference in telomere length (based on light intensity differences) from premutation specimens with and without FXTAS and/or dementia. It is possible that increased telomere shortening had already begun in males with the premutation only, as shown here in three of three individuals studied. To confirm the above results, further studies are warranted. Specifically, analysis of the premutation only will be carried out to determine at what age significant telomere shortening begins compared to controls. As can be seen from Table II, premutation lengths did not appear to be inversely correlated with telomere length except for all controls versus all pre-mutations with or without FXTAS and/or dementia. It should also be mentioned that control sample 285-04 had a light intensity value of 216 in Study 1 and of 172 in Study 7, likely due to inter-study variability since all studies could not be done simultaneously.
Our observations of telomere shortening in individuals with the FMR1 premutation remain to be confirmed and elucidated. Additional studies, conducted on younger premutation carriers, will determine whether increased telomere shortening can serve as a biomarker that may result in earlier detection of individuals at increased risk for developing FXTAS and FXTAS/dementia. Early detection could lead to future intervention strategies to extend the quality of life of both them and their families and may even lead to interventions aimed at slowing down or reversing the disease progression.
Acknowledgments
This work was supported in part by the New York State Office of Mental Retardation and Developmental Disabilities, NICHD grants HD 036071, HD02274 and NINDS NS044299. This research was also supported in part through the NIH Roadmap initiative (UL1 RR024922, NCRR: RL1 AG032119, NIA). We also thank Ezzat El-Akkad of our Institute’s Graphics Resources and Multimedia Services (GRAMS) and Lawrence Black, Institute Librarian.
References
- Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Bauer M, Cassidy F, Malhi GS, Mitchell P, Phelps J, Vieta E, Youngstrom E ISBD Diagnostic Guidelines Task Force. Diagnostic guidelines for bipolar disorder: a summary of the International Society for Bipolar Disorders Diagnostic Guidelines Task Force Report. Bipolar Disord. 2008;10:117–128. doi: 10.1111/j.1399-5618.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Brega AG, Jacquemont S, Loesch DZ, Leehey MA, Goodrich GK, Hagerman RJ, Epstein J, Wilson R, Cogswell JB, Jardini T, Tassone F, Hagerman PJ. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248:227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Leehey M, Grigsby J, Zhang L, Grunberg J, Greco C, Des Portes V, Jardini T, Berry-Kravis E, Brown WT, Schaeffer S, Kissle J, Tassone F, Hagerman PJ. Fragile X tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins EC, Velinov MT, Ye L, Gu H, Li S, Jenkins EC, Jr, Brooks SS, Pang D, Devenny DA, Zigman WB, Schupf N, Silverman WP. Telomere shortening in T lymphocytes of older individuals with Down syndrome and dementia. Neurobiology of Aging. 2006a;27:941–945. doi: 10.1016/j.neurobiolaging.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Jenkins EC, Velinov MT, Ye L, Gu H, Pang D, Devenny DA, Zigman WB, Schupf N, Silverman WP. Telomere shortening in T lymphocyte metaphases and interphases and individual chromosomes 21 and 1, from older individuals with Down syndrome and dementia. Presented at the annual meeting of The American Society of Human Genetics; October 11th, 2006; New Orleans, LA. 2006b. Available from http://www.ashg.org/cgi-bin/ashg06s/ashg06. [Google Scholar]
- Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little M-T, Dirks RW, Raap AK, Tanke JH. Heterogeneity in telomere length of human chromosomes. Human Molecular Genetics. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- Londoño-Vallejo JA, DerSarkissian H, Cazes L, Thomas G. Differences in telomere length between homologous chromosomes in humans. Nucleic Acids Res. 2001;29(15):3164–71. doi: 10.1093/nar/29.15.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S, Brüderlein S, Perner S, Waibel I, Holdenried A, Ciloglu N, Hasel C, Mattfeldt T, Nielsen KV, Möller P. Sex-specific telomere length profiles and age-dependent erosion dynamics of individual chromosome arms in humans. Cytogenet Genome Res. 2006;112:194–201. doi: 10.1159/000089870. [DOI] [PubMed] [Google Scholar]
- Panossian LA, Porter VR, Valenzuela HG, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer’s diseases status. Neurobiol Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Perner S, Brüderlein, Hasel C, Walbel I, Holdenried A, Ciloglu N, Chopurian H, Nielsen KV, Plesch A, Högel J, Möller P. Quantifying telomere lengths of human individual chromosome arms by centromere-calibrated fluorescence in situ hybridization and digital imaging. Am J Pathol. 2003;163:1751–56. doi: 10.1016/S0002-9440(10)63534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening and atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Published online 23 December 2007. 2007 doi: 10.1038/incb1685. [DOI] [PubMed] [Google Scholar]
- Tassone F, Pan R, Amiri K, Taylor AK, Hagerman PJ. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10:43–49. doi: 10.2353/jmoldx.2008.070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Valdes AM, Richards JB, Gardner JP, Swaminathan R, Kimura M, Xiaobin L, Aviv A, Spector TD. Telomere length in leukocytes correlates with bone mineral density and is shorter with osteoporosis. Osteoporos Int. 2007;18:1203–1210. doi: 10.1007/s00198-007-0357-5. [DOI] [PubMed] [Google Scholar]
- Vulliamy TJ, Dokal I. Dyskeratosis congenita: The diverse clinical presentation of mutations in the telomerase complex. Biochimie. 2007 Jul 31; doi: 10.1016/j.biochi.2007.07.017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]


