Abstract
Perchlorate is a commonly occurring environmental toxicant that may be transported across the placental barrier by the sodium-iodide symporter (NIS), possibly resulting in both increased perchlorate exposure and decreased iodide uptake by the fetus. Therefore, we measured levels of three physiologically relevant NIS-inhibitors (perchlorate, nitrate and thiocyanate) and iodide in maternal and fetal fluids collected during cesarean-section surgeries on 150 U.S. women. Geometric means of perchlorate, thiocyanate and nitrate levels in maternal urine (2.90, 947 and 47900 µg/L, respectively) were similar to previously published results, while urinary iodide levels (1420 µg/L) were significantly higher (p<0.0001), likely because of prevalent prenatal vitamin use in the study population (74%). Thiocyanate levels were higher in the maternal serum, cord serum, and amniotic fluid of smokers compared to women with environmental tobacco smoke exposure and non-smokers (p-values of 0.0006, p=0.0011, and 0.0026, respectively). Perchlorate was detected in most samples: urine (100%), maternal serum (94%), cord serum (67%), and amniotic fluid (97%). Maternal urinary perchlorate levels were positively correlated with perchlorate levels in amniotic fluid (r=0.57), indicating that maternal urine perchlorate is an effective biomarker of fetal perchlorate exposure. Maternal serum perchlorate was generally higher than cord serum perchlorate (median ratio 2.4:1 for paired samples), and maternal urine perchlorate was always higher than fetal amniotic fluid perchlorate levels (mean ratio 22:1); conversely, iodide levels were typically higher in fetal fluids compared to maternal fluids. We found no evidence of either disproportionate perchlorate accumulation or lack of iodide in the fetal compartment. In this panel of healthy infants, we found no association between cord blood levels of these anions and newborn weight, length and head circumference.
Introduction
Perchlorate (ClO4 −) is an inorganic anion used as an oxidant in solid rocket propellant, road flares, explosives, and pyrotechnics (1). Perchlorate also can form naturally in the atmosphere (2), leading to perchlorate accumulation in arid climates (3). This combination of human activities and natural processes results in the widespread presence of perchlorate in the environment (4). Perchlorate has been detected in drinking water from 35 different states and 2 territories (5). Perchlorate in irrigation water, soil, or some natural fertilizers results in perchlorate accumulation in food and forage crops (4,6). Perchlorate can also form in aging bleach solutions (7), although dilute bleach solutions sprayed on produce are unlikely to be a major source of human exposure (8). Thus, the prevalence of trace levels of perchlorate in food and water leads to widespread human exposure (9–10).
Environmental perchlorate exposure is of potential health concern because large doses of perchlorate competitively inhibit iodide uptake (11–12). Nitrate (NO3 −) and thiocyanate (SCN−) are two other iodide uptake inhibitors to which people are commonly exposed (13). Although nitrate and thiocyanate have lower sodium-iodide symporter (NIS) binding affinities than perchlorate, serum levels of thiocyanate and nitrate are likely to be higher than levels of perchlorate (14). In vitro studies of human NIS indicate that perchlorate, nitrate, and thiocyanate act additively to competitively inhibit iodide uptake (14). Therefore, quantification of iodide, thiocyanate, nitrate, and perchlorate levels provides information about the current likelihood of inhibition of iodide uptake. Sustained inhibition of iodide uptake could potentially impair thyroid hormone production. Indeed, a large cross-sectional study found increased urinary perchlorate levels were associated with decreased thyroxine and increased thyroid stimulating hormone in women with urinary iodine <100 µg/L (15), with the strongest associations in a subgroup with elevated thiocyanate levels (16).
NIS is expressed in human placenta (17), likely as a mechanism for active transport of iodide to the fetus (18). Recent studies found that perchlorate is actively transported across membrane barriers by NIS (19–20), raising the possibility of active transport of perchlorate, thiocyanate and nitrate into the fetal compartment, and of potentially impaired iodide transport across the placenta. By this mechanism, ubiquitous maternal exposure to perchlorate, nitrate and thiocyanate (9) could results in fetal exposure and impaired iodide transport. Reduced fetal iodide levels could impair fetal thyroid hormone production and possibly to reduced thyroid hormone reserves at birth. Direct measurement of these toxicologically related anions in maternal and fetal matrices is required for improved understanding of in utero exposure.
Fetal development is the life stage most sensitive to thyroid disruption (21–22). During pregnancy the thyroid needs to produce more thyroxine, and increased thyroxine production requires increased iodine intake (21). Therefore, the American Thyroid Association recommends iodine supplementation for all pregnant women (23). Direct measurement of perchlorate, nitrate, thiocyanate and iodide in maternal serum provide a snapshot of the relative levels of these anions competing for transport by NIS at the thyroid, lactating breast and placenta (14). Similar measurements in cord serum and amniotic fluid may provide information about transport of NIS-inhibitors and iodide across the placenta (24–25). One study of iodine-sufficient pregnant women exposed to perchlorate found no association between perchlorate exposure and infant health (26), although exposure assessment was based only on maternal measures. Improved in utero exposure assessment is needed because the National Academy of Sciences defines fetal development and infancy as life stages potentially more sensitive to perchlorate and other thyroid inhibitors (22), because of the crucial role of thyroid hormones in the development of various organs, especially the brain. Maternal hypothyroidism may affect fetal growth and neonatal thyroid function (21, 27). However, no previous studies have evaluated the relation between maternal and fetal exposures to iodine uptake inhibitors and impaired fetal growth. Therefore, we examined the relationship between levels of perchlorate, nitrate, thiocyanate and iodide in maternal and fetal fluids in a panel of healthy mothers/pregnancies. We also examined the association between cord blood levels of these anions and newborn weight, length and head circumference.
Material and Methods
Study Participants
This prospective study reports the analysis of perchlorate, thiocyanate, nitrate and iodide as part of a larger study examining a spectrum of environmental toxicants in maternal and fetal compartments. The study was approved by the Institutional Review Boards of The University of Medicine and Dentistry of New Jersey and Rutgers University under a cooperative agreement with the Centers for Disease Control and Prevention (CDC). Subjects eligible for recruitment included non-anemic women (hemoglobin ≥8 mg/dl) with singleton pregnancies scheduled for an elective cesarean at term (≥37 weeks). We chose cesarean deliveries to facilitate collection of amniotic fluid as an additional measure of fetal exposure. Subjects were identified before admission, and the potential subjects’ physicians were asked for permission to offer participation. Women were excluded if there was evidence of labor or rupture of membranes at delivery. Additional exclusion criteria included maternal conditions such as diabetes, hypertension and known thyroid dysfunction. All subjects were asked to fast starting at bedtime the night before the surgery. Thus, the 150 study participants had not eaten for 8–14 hours before sample collection and surgery. After enrollment and before placement of intravenous and bladder catheters, subjects completed a brief questionnaire to provide data on occupations, hobbies, and self-described tobacco smoke exposure.
Sample Collection
Maternal urine (MU) and blood (MB) samples were collected in the preoperative holding area within an hour of surgery and before intravenous drip began. Amniotic fluid (AF) was collected using an 18-gauge intravenous catheter sheath without the needle that was directly inserted through the membranes. If the membranes ruptured prior to AF collection, the fluid gush was collected into a sterile cup. Following fetal delivery the umbilical cord was clamped and 30–60 ml of cord blood (CB) was aspirated directly from the umbilical vein, transferred into serum separator tubes and processed immediately. All biological samples were stored at −70°C after collection and processing (see Supporting Information for further details).
Quantification of Perchlorate, Thiocyanate, Nitrate and Iodide
The levels of perchlorate, nitrate, thiocyanate and iodide were measured in all matrices using highly selective and sensitive methods coupling ion chromatography and tandem mass spectrometry (23). Maternal urine was diluted with aqueous internal standards and analyzed as previously described (28). Amniotic fluid was diluted with internal standards, filtered and analyzed as described earlier (24). Maternal and cord blood serum was analyzed following protein precipitation and solid phase extraction as previously described (29–30). All analytes were quantified using stable isotope dilution and daily calibration curves. Each batch of unknown samples also contained at least two pools of characterized quality control materials and blank samples to assess method accuracy, precision and contamination. Urine specific gravity was measured using a hand-held refractometer (National Instrument Company, Inc., Baltimore, MD), and this data was used to adjust for variable hydration of participants.
Statistical Analysis of Exposure Variables
Univariate analysis indicated that all analytes in all matrices were log-normally distributed. Therefore, subsequent bivariate analysis compared log10-transformed data. The proportion of samples from each compartment with detectable levels of each analyte was assessed, as were the median levels of each analyte. The levels of the four analytes in the four different matrices were compared based on Pearson correlation coefficient (r). For the few samples that did not contain measurable levels of perchlorate or thiocyanate, we imputed a value equal to the detection limit divided by the square root of two. Comparison of NIS-inhibitors in maternal blood with fetal iodide levels included the following potency factors based on an in vitro study of NIS-mediated competitive transport: perchlorate, 1; thiocyanate, 1/15; nitrate, 1/240 (14); these potency factors are based on a single in vitro study and thus include a degree of uncertainty. We calculated a perchlorate equivalence concentration variable (PEC) by summing the product of molar concentrations of each iodide uptake inhibitor and its corresponding potency factor (14). The t-test was used to determine if potential exposure modifiers affected measured levels of perchlorate and iodide, respectively. The Kruskal-Wallis test was used to evaluate differences in thiocyanate levels in amniotic fluid, cord blood serum and maternal blood serum collected from women with differing tobacco smoke exposure. All analyses were conducted using SAS/STAT software (version 9.1.3).
Statistical Analysis of Neonatal Outcome Data
Because perchlorate can inhibit iodine uptake and thus might impact thyroid function and fetal growth, we tested the hypothesis that increasing levels of perchlorate in cord blood (CB) would be associated with decreased head circumference, birth weight, and length. Therefore, we generated indicator variables based on quartiles of perchlorate concentration in CB. Using a linear regression, we regressed birth weight against these CB perchlorate variables. We also included maternal age (<30 years, 30–34, ≥35), body mass index at delivery (<25, 25–29, 30–34, ≥35), and gravida (≤2 pregnancies, 3, ≥4) for adjustment. To examine whether a dose-response relationship existed, we then performed a test for trend. These analyses were repeated for head circumference and birth length associated with CB iodide, CB nitrate, CB thiocyanate, and CB PEC.
Sensitivity Analysis
Thiocyanate is a marker of tobacco smoke exposure, and smoking is associated with decreased fetal growth parameters (31–32). Therefore, we evaluated whether our effect estimates for CB perchlorate, CB iodide, CB nitrate, and CB PEC were sensitive to adjustment for CB thiocyanate levels, by including CB thiocyanate indicator variables in the same models described previously. Since fetal growth is also associated with genetic factors (33), we tested this by including race (whites and non-whites) as an effect modifier of these associations.
Results
Table S1 of the online Supporting Information lists the characteristics of the maternal and neonatal study population. Nearly half (47%) of the mothers did not provide data on race; of those who did, 78% were white. BMI >30 was seen in 57% of subjects (n=143). Eleven percent of subjects were primiparous. Most study participants reported no current exposure to environmental tobacco smoke (81%). Distributions of newborn birth weight, head circumference and birth length are shown in Table S2.
Table 1 lists the distributions for perchlorate, thiocyanate, nitrate and iodide by matrix (also see Supporting Information Figures S1–S4). Perchlorate was detected in most samples: urine (100%), maternal serum (94%), cord serum (67%), and amniotic fluid (97%). Nitrate, thiocyanate and iodide were detected in almost all samples, consistent with the adequate sensitivity of the analytical methods.
Table 1.
Distribution of perchlorate, thiocyanate, nitrate and iodide (µg/ L) in maternal and fetal matrices
| Analyte | Matrix | na | % detects |
5th %tile |
25th %tile |
50th %tile |
75th %tile |
95th %tile |
|---|---|---|---|---|---|---|---|---|
| perchlorate | AFb | 130 | 97% | 0.056 | 0.101 | 0.145 | 0.197 | 0.380 |
| perchlorate | CBc | 126 | 67% | <0.050 | 0.095 | 0.139 | 0.223 | 0.480 |
| perchlorate | MBd | 132 | 94% | <0.050 | 0.160 | 0.223 | 0.350 | 0.893 |
| perchlorate | MUe | 34 | 100% | 0.900 | 1.72 | 2.76 | 4.35 | 7.71 |
| thiocyanate | AF | 130 | 98% | 18.4 | 63.5 | 171 | 470 | 1050 |
| thiocyanate | CB | 126 | 100% | 304 | 524 | 840 | 1220 | 3030 |
| thiocyanate | MB | 132 | 100% | 278 | 603 | 936 | 1300 | 2780 |
| thiocyanate | MU | 34 | 100% | 287 | 571 | 839 | 1720 | 3780 |
| nitrate | AF | 130 | 100% | 862 | 1465 | 2090 | 3025 | 5480 |
| nitrate | CB | 126 | 100% | 1400 | 1900 | 2480 | 3310 | 4530 |
| nitrate | MB | 132 | 100% | 1220 | 1710 | 2410 | 3220 | 5120 |
| nitrate | MU | 34 | 100% | 24800 | 38100 | 48500 | 61300 | 94500 |
| iodide | AF | 130 | 100% | 4.66 | 10.8 | 21.4 | 59.0 | 377 |
| iodide | CB | 126 | 100% | 3.57 | 5.64 | 8.00 | 11.9 | 19.2 |
| iodide | MB | 105 | 100% | 1.19 | 1.81 | 2.58 | 5.24 | 26.4 |
| iodide | MU | 34 | 100% | 309 | 643 | 1680 | 3000 | 5420 |
number of specimens
fetal amniotic fluid
fetal cord blood serum
maternal blood serum
maternal urine, adjusted by specific gravity
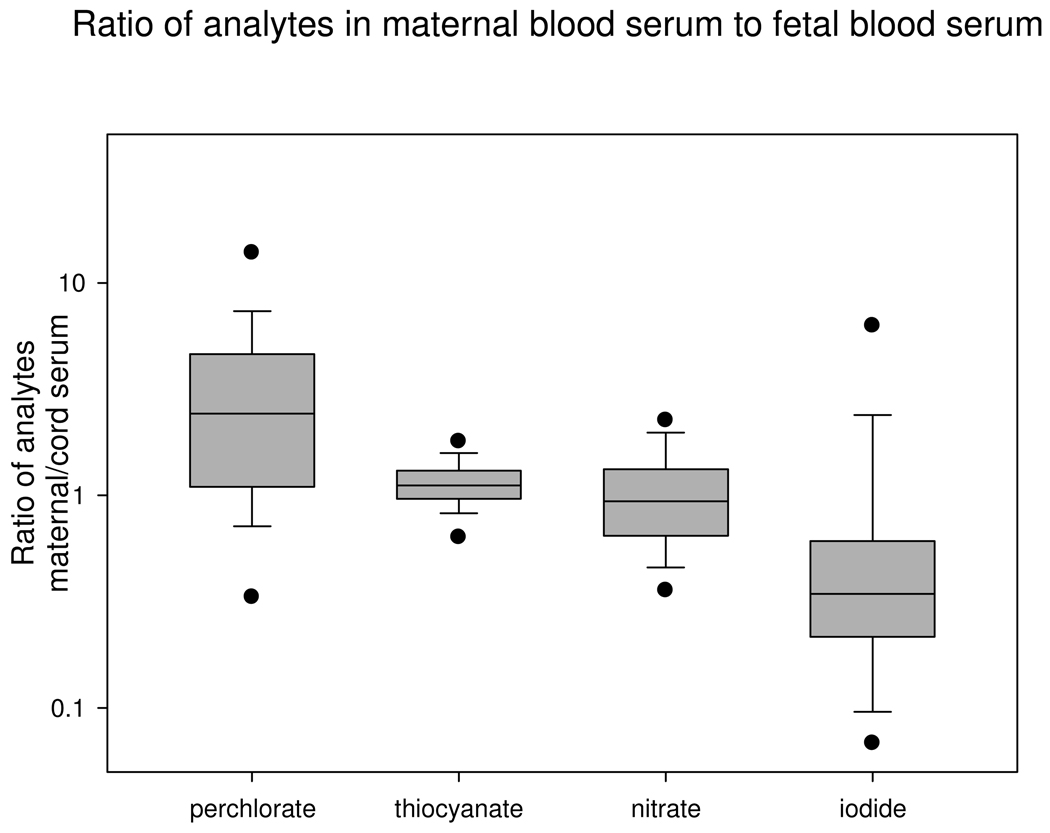
Perchlorate, nitrate and iodide levels were higher in maternal urine compared to other matrices. Maternal serum perchlorate levels exceeded cord serum perchlorate levels for most of the mother/child pairs (median ratio 2.4, inter-quartile range 1.1–4.6; Fig 1). Maternal serum perchlorate levels were two times higher (mean 0.417 vs. 0.220 µg/L, p=0.037) than cord serum perchlorate, with a higher detection rate as well (97% vs. 67%). Only iodide was consistently lower in maternal blood serum compared to cord blood serum (median ratio 0.34, inter-quartile range 0.22–0.61), although the highly skewed distribution had a mean maternal to child iodide ratio of 0.97±2.0.
Figure 1.
Distribution of ratios of analytes in maternal blood serum to cord blood serum. Box- and-whisker plot indicates the 5th and 95th percentiles (●) 10th and 90th percentiles (whisker, |), the 25th, 50th, and 75th percentiles (box, —). Y-axis is logarithmic scale.
We assessed the effect of current tobacco smoke exposure on measured levels of perchlorate, thiocyanate, nitrate and iodide. As shown in Table 2, smokers had higher thiocyanate levels compared to non-smokers.
Table 2.
Distribution of thiocyanate in different matrices based on questionnaire- assessed tobacco smoke exposure
| Non-smokera (median, range) |
ETSb (median, range) |
Smokerc (median, range) |
Kruskal-Wallis p-value |
|
|---|---|---|---|---|
| AFd | 147 (<0.05 – 2400) |
255 (<0.05 – 4230) |
3510 (469 – 6900) |
0.0026 |
| CBe | 752 (88.3 – 3180) |
755 (212 – 3220) |
5240 (727 – 10300) |
0.0011 |
| MBf | 862 (114 – 1880) |
883 (231 – 2780) |
4615 (1300 – 9110) |
0.0006 |
Non-smoker (n=100, 103, and 93 for AF, CB, and MB, respectively)
ETS=non-smoker with environmental tobacco smoke exposure (n=20, 18, and 17 for AF, CB, and MB, respectively)
Smoker (n=5, 7, and 6 for AF, CB, and MB, respectively)
amniotic fluid
cord blood serum
maternal blood serum
We evaluated the correlation of analyte levels across the four maternal and fetal matrices (Table 3). Maternal urinary perchlorate levels were positively correlated with matched newborn perchlorate levels in amniotic fluid (r=0.57). When one outlying point was removed, the correlation coefficient for perchlorate in urine and amniotic fluid increased to 0.92 (see Supporting Information Figure S5). Similar correlation analysis for thiocyanate found that thiocyanate levels in all matrices were positively correlated (r=0.60 to 0.94). Amniotic fluid nitrate levels were positively correlated with nitrate levels in cord blood, maternal blood and maternal urine (r=0.62, 0.41 and 0.58, respectively). Maternal urinary iodide levels were positively correlated only with iodide levels in maternal serum (r=0.35).
Table 3.
Pearson correlation coefficients between log-transformed analytes and matrices.
| Perchlorate | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Matrix | AFa | CBb | MBc | MUd | ||||||||||||
| Perchlorate | AF | --- | 0.53 | 0.18 | 0.57 | ||||||||||||
| Perchlorate | CB | 0.53 | --- | −0.03 | 0.20 | ||||||||||||
| Perchlorate | MB | 0.18 | −0.03 | --- | −0.10 | Thiocyanate | |||||||||||
| Perchlorate | MU | 0.57 | 0.20 | −0.10 | --- | AF | CB | MB | MU | ||||||||
| Thiocyanate | AF | −0.07 | −0.04 | 0.07 | −0.12 | --- | 0.60 | 0.61 | 0.63 | ||||||||
| Thiocyanate | CB | −0.14 | 0.02 | 0.21 | 0.06 | 0.60 | --- | 0.94 | 0.94 | ||||||||
| Thiocyanate | MB | −0.17 | −0.06 | 0.22 | −0.15 | 0.61 | 0.94 | --- | 0.88 | Nitrate | |||||||
| Thiocyanate | MU | −0.04 | −0.14 | 0.08 | 0.08 | 0.63 | 0.94 | 0.88 | --- | AF | CB | MB | MU | ||||
| Nitrate | AF | 0.25 | 0.15 | −0.02 | −0.01 | 0.11 | 0.04 | −0.03 | 0.31 | --- | 0.62 | 0.41 | 0.58 | ||||
| Nitrate | CB | 0.23 | 0.26 | 0.02 | 0.03 | 0.05 | 0.03 | 0.01 | 0.03 | 0.62 | --- | 0.22 | 0.31 | ||||
| Nitrate | MB | 0.09 | 0.11 | 0.34 | −0.05 | −0.04 | −0.01 | 0.11 | 0.19 | 0.41 | 0.22 | --- | 0.25 | Iodide | |||
| Nitrate | MU | 0.08 | −0.10 | −0.13 | 0.35 | 0.23 | 0.47 | 0.14 | 0.51 | 0.58 | 0.31 | 0.25 | --- | AF | CB | MB | MU |
| Iodide | AF | −0.03 | −0.09 | 0.02 | −0.34 | 0.41 | 0.06 | 0.01 | 0.20 | 0.11 | −0.09 | 0.07 | −0.09 | --- | 0.01 | −0.12 | 0.02 |
| Iodide | CB | −0.12 | −0.26 | 0.14 | −0.30 | −0.04 | −0.01 | 0.04 | −0.27 | −0.03 | 0.05 | −0.02 | −0.20 | 0.01 | --- | 0.06 | −0.03 |
| Iodide | MB | 0.04 | 0.13 | 0.07 | 0.01 | 0.09 | 0.28 | 0.13 | 0.00 | 0.02 | 0.21 | −0.11 | −0.17 | −0.12 | 0.06 | --- | 0.35 |
| Iodide | MU | −0.22 | −0.14 | −0.34 | −0.16 | 0.09 | 0.38 | 0.23 | 0.42 | −0.02 | −0.06 | −0.22 | 0.17 | 0.02 | −0.03 | 0.35 | --- |
fetal amniotic fluid, n=130
fetal cord blood serum, n=126
maternal blood serum, n=132 for perchlorate, thiocyanate and nitrate, n=105 for iodide
maternal urine, adjusted by specific gravity, n=34
We examined the relationship of maternal serum levels of NIS-inhibitors and cord blood levels of iodide, and found no correlation between maternal serum concentrations of any of the NIS-inhibitors and cord serum concentrations of iodide. Furthermore, we evaluated whether increasing levels of CB perchlorate, thiocyanate, nitrate, iodide, and PEC were associated with decreasing or increasing head circumference, birth weight, and birth length. CB levels of perchlorate, thiocyanate, nitrate, and iodide were essentially uncorrelated. We did not find any quartile of CB perchlorate to be associated with head circumference, decreased weight, or birth length (Table 4). Further, there were no clear patterns of increasing/decreasing effect estimates for increasing perchlorate quartile for any outcome. Increasing quartile of CB nitrate concentration was associated with increasing head circumference, although the trend was not statistically significant for any of the anions or PEC (Table 4).
Table 4.
Change (and 95% confidence interval) in head circumference, birth weight, and length associated with each quartile of perchlorate, thiocyanate, iodide, nitrate, and perchlorate equivalence concentration in the cord blood serum (ng/mL). Analyses adjusted for maternal age, maternal body mass index, and gravida
| ANION BY OUTCOME |
na | QUARTILE 1 |
QUARTILE 2 |
QUARTILE 3 |
QUARTILE 4 |
Test for trend p-value |
|---|---|---|---|---|---|---|
| Head circumference (cm) | ||||||
| Perchlorate | 115 | 0.00 | −0.05 (−0.80, 0.70) |
−0.63 (−1.31, 0.05) |
−0.14 (−0.81, 0.53) |
0.36 |
| Thiocyanate | 115 | 0.00 | −0.07 (−0.82, 0.69) |
−0.08 (−0.82, 0.65) |
0.36 (−0.39, 1.11) |
0.37 |
| Iodide | 115 | 0.00 | −0.16 (−0.88, 0.56 |
0.21 (−0.53, 0.95) |
−0.23 (−0.95, 0.49) |
0.75 |
| Nitrate | 115 | 0.00 | 0.06 (−0.65, 0.78) |
0.12 (−0.60, 0.84) |
0.76 (0.03, 1.48) |
0.06 |
| PECb | 115 | 0.00 | 0.78 (0.05, 1.52) |
0.25 (−0.46, 0.96) |
0.32 (−0.39, 1.03) |
0.72 |
| Birth weight (g) | ||||||
| Perchlorate | 120 | 0.00 | −99 (−332, 135) |
−100 (−306, 106) |
19 (−189, 227) |
0.98 |
| Thiocyanate | 120 | 0.00 | −113 (−343, 118) |
−112 (−340, 116) |
−107 (−337, 124) |
0.40 |
| Iodide | 120 | 0.00 | 116 (−336, 105) |
−110 (−339, 105) |
−91 (−316, 133) |
0.47 |
| Nitrate | 120 | 0.00 | 14 (−211, 240) |
61 (−165, 287) |
109 (−119, 337) |
0.31 |
| PEC | 120 | 0.00 | 157 (−73, 386) |
86 (−133, 306) |
14 (−208, 236) |
0.93 |
| Length (cm) | ||||||
| Perchlorate | 115 | 0.00 | −0.94 (−2.81, 0.94) |
−0.82 (−2.50, 0.86) |
−1.01 (−2.67, 0.65) |
0.23 |
| Thiocyanate | 115 | 0.00 | 0.00 (−1.84, 1.85) |
1.23 (−0.57, 3.04) |
0.01 (−1.83, 1.85) |
0.66 |
| Iodide | 115 | 0.00 | −0.54 (−2.32, 1.24) |
0.47 (−1.37, 2.30) |
−0.03 (−1.80, 1.75) |
0.76 |
| Nitrate | 115 | 0.00 | −1.32 (−3.10, 0.45) |
−1.67 (−3.46, 0.12) |
−0.75 (−2.54, 1.05) |
0.33 |
| PEC | 115 | 0.00 | −0.24 (−2.08, 1.60) |
−1.16 (−2.93, 0.61) |
−1.07 (−2.83, 0.70) |
0.15 |
number of specimens
perchlorate equivalence concentration
We evaluated whether adjustment for CB thiocyanate concentration, a marker of tobacco smoke exposure, would change the effect estimates reported above. Mothers who reported smoking during pregnancy had a higher mean CB thiocyanate level (Mean ± SD = 4038 ± 3302 µg/L; n=9) than mothers who did not report smoking (899 ± 574 µg/L; n=117). However, there was no change in the quartile-specific effect estimates (data not shown). We evaluated effect modification of these associations by maternal race, and did not detect any. Compared to CB perchlorate quartile 1, having a CB perchlorate in the 2nd quartile was associated with a decrease in birth weight; however, higher quartiles of perchlorate were not associated with changes in birth weight (Table 5). There was no clear trend of increasing or decreasing birth weight, length, or head circumference associated with increasing quartile of CB anion level, and none of the tests for trend was statistically significant (Table 5).
Table 5.
Change (and 95% confidence interval) in birth weight (g) associated with each quartile of perchlorate, thiocyanate, iodide, nitrate, and perchlorate equivalence concentration in cord blood serum, by race. Analyses adjusted for maternal age, maternal body mass index, and gravida (N=120 newborns)
| ANION BY OUTCOME |
na | QUARTILE 1 |
QUARTILE 2 |
QUARTILE 3 |
QUARTILE 4 |
Test for trend p-value |
|---|---|---|---|---|---|---|
| Whites | ||||||
| Perchlorate | 87 | 0.00 | 67 (−199, 333) |
1 (−226, 228) |
93 (−140, 326) |
0.54 |
| Thiocyanate | 87 | 0.00 | −59 (−327, 209) |
−131 (−397, 136) |
−33 (−297, 231) |
0.76 |
| Iodide | 87 | 0.00 | −156 (−387, 74) |
−22 (−262, 218) |
20 (−238, 278) |
0.70 |
| Nitrate | 87 | 0.00 | 82 (−154, 318) |
201 (−42, 443) |
124 (−122, 370) |
0.19 |
| PECb | 87 | 0.00 | 91 (−172, 354) |
89 (−166, 344) |
−59 (−307, 189) |
0.58 |
| Non-Whites | ||||||
| Perchlorate | 33 | 0.00 | −512 (−960, −64) |
−540 (−965, −115) |
−343 (−780, 94) |
0.11 |
| Thiocyanate | 33 | 0.00 | −457 (−967, 54) |
−375 (−887, 136) |
−350 (−986, 287) |
0.26 |
| Iodide | 33 | 0.00 | 45 (−500, 591) |
−300 (−887, 287) |
−128 (−632, 375) |
0.46 |
| Nitrate | 33 | 0.00 | −292 (−910, 325) |
−191 (−823, 442) |
60 (−522, 641) |
0.63 |
| PEC | 33 | 0.00 | 152 (−332, 637) |
203 (−252, 658) |
−32 (−562, 498) |
0.85 |
number of specimens
perchlorate equivalence concentration
Discussion
This study provides novel data relevant to maternal and fetal perchlorate exposure in the perinatal period. By measuring perchlorate and toxicologically related anions (thiocyanate, nitrate and iodide) in maternal urine, maternal blood serum, cord blood serum and amniotic fluid, we relate maternal exposure and fetal exposure. Perchlorate levels were much lower than iodide levels in all four matrices studied (Table 1). For example, geometric means of perchlorate and iodide levels in maternal urine were 2.90 and 1420 µg/L, respectively. In vitro experiments indicate that human NIS has a 30-fold higher affinity for perchlorate compared to iodide (14). Therefore, based on the relative levels of perchlorate and iodide found in maternal blood and urine, we would not expect inhibition of iodide transport into the fetal compartment in these individuals at the time of the study.
No other published study has measured perchlorate, thiocyanate and nitrate in perinatal samples collected from both maternal and fetal compartments. However, several studies have examined these anions individually in either maternal or fetal matrices (9,26,29,34–36). For perspective, we compared the levels of perchlorate, thiocyanate and nitrate found in maternal and fetal compartments to levels found in other published studies. Geometric mean concentrations have been published for urinary levels of these analytes in women of reproductive age in the general U.S. population (9,34). Geometric means for urinary anion levels in our study are consistent with these reference values for perchlorate (2.90 µg/L [this study] vs. 2.14 µg/L [reference study]), thiocyanate (947 µg/L [this study] vs. 699 µg/L [reference study]), and nitrate (47,900 µg/L [this study] vs. 35,400 µg/L [reference study]). Geometric means for analytes in maternal blood serum in our study are also consistent with control population data from a study of Israeli adults (29): perchlorate (0.246 vs. 0.440 µg/L), thiocyanate (899 vs. 1067 µg/L), and nitrate (2425 vs. 4660 µg/L), for our study and the published study (29), respectively. Geometric mean concentrations for analytes in amniotic fluid in our study are consistent with data from 48 U.S. women undergoing second trimester amniocentesis (24): perchlorate (0.144 vs. 0.180 µg/L), thiocyanate (168 vs. 89 µg/L), and nitrate (2156 vs. 1620 µg/L). We extrapolated perchlorate dose based on existing methods (37) and maternal blood perchlorate levels; the resulting median perchlorate dose was 0.094 µg/kg/day, well below the EPA reference dose (RfD) of 0.7 µg/kg/day, and consistent with previous estimates of the median perchlorate dose (0.064 µg/kg/day) in U.S. adults (9). Thus the perchlorate levels we found in all matrices are consistent with previously reported background exposure levels for adults from developed countries.
Administration of intravenous fluids (~1 liter), immediately prior to surgery may dilute analytes in physiological fluids. In our study, maternal blood and urine samples were not affected because they were collected prior to infusion of fluids; however, the fetal blood and amniotic fluid were likely diluted by this effect. Infusion of 1 liter intravenous fluid has been associated with increased amniotic fluid volume (38), such that 29% of the final amniotic fluid volume was attributable to the intravenous fluid. However, this magnitude of amniotic fluid dilution is inadequate to explain the 22-fold higher perchlorate levels in maternal urine compared with fetal amniotic fluid. Thus, the relative levels of perchlorate in maternal urine and fetal amniotic fluid are consistent with no concentration of perchlorate in the fetal compartment.
Urinary iodide levels in this study population (geometric mean 1420 µg/L) were higher than for women of reproductive age in the general U.S. population (geometric mean 132.5 µg/L) (30). The higher levels of iodine found in our study population may result from widespread use of prenatal vitamins in our study population (74%). Study participants who said they used prenatal vitamins daily tended to have higher iodide levels compared to non-users; however, this difference was not statistically significant (p=0.69, 0.06, 0.78, and 0.45 for AF, CB, MB, and MU, respectively). The lack of significantly elevated iodine levels in self-identified vitamin users may be caused by the high degree of variability in prenatal vitamin formulations. A recent study indicates that only 51% of prenatal vitamins contain iodine, and 17% of those that claim to contain iodine actually contain less than half of the amount listed (39). The high degree of variability of iodine in supplements may lead to no significant correlation between self assessed vitamin use and increased urinary iodine. Based on the importance of adequate iodine intake during pregnancy, the American Thyroid Association recommends that all pregnant women take daily iodine supplement (23).
Higher iodine intake in our study population may explain the modest accumulation of iodide in fetal serum compared with maternal serum. The ratio of maternal serum iodide to cord serum iodide was less than 0.5 for 67% of paired maternal and child samples (Fig 1). By comparison, only 11% of mother/child pairs had plasma iodide ratios of less than 0.5 in a U.S. study of similar size (40). Iodide is actively transported across the placenta and studies of experimental animals indicate that iodide can accumulate in the fetal compartment (18,41). Additionally, the higher iodide levels in the study population may reduce the potential effects of exposure to NIS inhibitors. Thus, a follow-up study in a population with lower iodine intake is warranted.
Thiocyanate levels were higher in all matrices from smokers compared to non-smokers, likely because of cyanide exposure from tobacco smoke (35). Thiocyanate levels were similar in paired mother and child samples, underscoring the ability of many smoke components to cross the placenta and lead to fetal exposure. Thiocyanate can potentially interact with perchlorate to inhibit iodide uptake (14) and possibly lead to changes in thyroid hormone levels (16). The potential interaction among thiocyanate from tobacco smoke, perchlorate from environmental exposure and low iodine intake merits further research (15–16).
Consistent with published reports describing widespread perchlorate exposure (9,24), perchlorate was detected in most samples: urine (100%), maternal serum (94%), cord serum (67%), and amniotic fluid (97%). None of the study participants reported occupations that could involve perchlorate exposure, and the study area has no known perchlorate contamination of tap water. Perchlorate is commonly found in food (6,10,28). Thus, the levels of perchlorate measured in the study participants may be lower than usual because of the presurgery fasting and the 8-hr physiological half-life of perchlorate (9). A similar pattern would be expected for nitrate and iodide because of no recent oral intake from food and supplements. Thiocyanate levels would be less likely impacted by fasting because of the relatively long half life (6 days) and potentially ongoing exposure for the active smokers.
Although there were only 34 maternal urine samples, these perchlorate levels were positively correlated with perchlorate levels in amniotic fluid (Figure S5). Thus, maternal urine perchlorate may be an effective biomarker of fetal perchlorate exposure. The use of maternal urine perchlorate as a surrogate measure of fetal exposure will allow studies comparing fetal exposure with subsequent developmental health endpoints (22).
Measurement of perchlorate levels in cord blood serum provides relevant information about in utero exposure. Perchlorate exposure patterns change significantly when the newborn begins to consume breast milk or formula, because both of these foods contain perchlorate (42–44). Because perchlorate is concentrated in milk during lactation (19,42), infants consuming milk-based formula or breast milk may have elevated perchlorate exposure compared with other life stages (42,44–46). The lower exposure levels we report do not conflict with this literature because our study assesses perchlorate levels immediately before and during birth, rather than once feeding has begun.
Our findings of no association between inhibitors of iodide uptake and newborn growth parameters are consistent with results from a longitudinal epidemiologic study of pregnant women in Northern Chile (26). The Chilean study classified perchlorate exposure of the healthy, iodine-replete women based on maternal and environmental measurements instead of infant cord blood measurements. Our analyses were done in a panel of healthy, iodine-replete mothers who had perchlorate exposure <RfD. Thus, it is unlikely that we would observe abnormalities in fetal growth. Since the subjects were healthy, we may have excluded those subjects in which we would see abnormal fetal growth associated with anion levels. Further, our measurement of anions at birth may not represent exposure at time(s) earlier in pregnancy that could affect fetal growth. Therefore, a follow-up study is warranted to examine exposure throughout pregnancy, and to include mothers/pregnancies at higher risk for adverse fetal growth. Additional analysis of this data with physiologically-based models will further clarify perchlorate distribution during pregnancy.
In summary, we found that maternal serum perchlorate was generally higher than cord serum perchlorate, and maternal urine perchlorate was always higher than fetal amniotic fluid perchlorate levels. Conversely, iodide levels were higher in fetal fluids compared to maternal fluids. Based on the levels of iodide and NIS-inhibitors found in the maternal and fetal compartments in the perinatal time frame, we found no evidence of inhibition of iodine transport across the placenta. Additionally, cord blood levels of NIS-inhibitors were not associated with changes in birth weight, length, and head circumference.
Supplementary Material
Acknowledgements
The authors thank Janice Menuel, John Morrow, and Marian Lake for technical support. This work was supported by NIEHS Grant T32ES007148 (MR), NIEHS Grant P30ES005022 (MR), and NJDEP Grant SR04-058 (MR).
Footnotes
Supporting Information Available
Supporting Information includes a brief description of population characteristics, sample collection, analytical methods and additional tables and figures cited in the paper (Tables S1–S2 and Figures S1–S5). This material is available free of charge at http://pubs.acs.org.
References
- 1.Mendiratta SK, Dotson RL, Brooker RT. Perchloric Acid and Perchlorates. New York, NY: John Wiley & Sons, Inc; 2005. [Google Scholar]
- 2.Dasgupta PK, Martinelango PK, Jackson WA, Anderson TA, Tian K, Tock RW, Rajagopalan S. The origin of naturally occurring perchlorate: the role of atmospheric processes. Environ.Sci.Technol. 2005;39:1569–1575. doi: 10.1021/es048612x. [DOI] [PubMed] [Google Scholar]
- 3.Rao B, Anderson TA, Orris GJ, Rainwater KA, Rajagopalan S, Sandvig RM, Scanlon BR, Stonestrom DA, Walvoord MA, Jackson WA. Widespread natural perchlorate in unsaturated zones of the southwest United States. Environ.Sci.Technol. 2007;41:4522–4528. doi: 10.1021/es062853i. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta PK, Dyke JV, Kirk AB, Jackson WA. Perchlorate in the United States. Analysis of Relative Source Contributions to the Food Chain. Environ.Sci.Technol. 2006;40:6608–6614. doi: 10.1021/es061321z. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Environmental Protection Agency. Unregulated Contaminant Monitoring Regulation (UCMR) data from public water systems. Available at http://www.epa.gov/safewater/ucmr/data.html.
- 6.Sanchez CA, Barraj L, Blount BC, Scrafford C, Valentin-Blasini L, Smith KM, Kreiger RI. Perchlorate Exposure from Food Crops Produced in the Lower Colorado River Region. Journal of Exposure Science and Environmental Epidemiology. 2009;19:359–368. doi: 10.1038/jes.2008.26. [DOI] [PubMed] [Google Scholar]
- 7.Greiner P, McLellan C, Bennett D, Ewing A. Occurrence of perchlorate in sodium hypochlorite. J.AWWA. 2008;100:68–74. [Google Scholar]
- 8.Sanchez CA, Fonseca JM, Blount BC, Krieger RI. Hypochlorite Treatments are not Significant Source of Perchlorate Exposure in Lettuce. J. Agric. Food Chem. doi: 10.1021/jf8033013. Article ASAP. doi:10.1021/jf8033013. [DOI] [PubMed] [Google Scholar]
- 9.Blount BC, Valentin-Blasini L, Mauldin JP, Pirkle JL, Osterloh JD. Perchlorate Exposure of the U.S. Population, 2001–2002. J Expo.Sci.Environ. Epidemiol. 2007;17:400–407. doi: 10.1038/sj.jes.7500535. [DOI] [PubMed] [Google Scholar]
- 10.Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration's Total Diet Study: Dietary intake of perchlorate and iodine. J Expo.Sci.Environ.Epidemiol. 2008 doi: 10.1038/sj.jes.7500648. [DOI] [PubMed] [Google Scholar]
- 11.Greer MA, Goodman G, Pleus RC, Greer SE. Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ.Health Perspect. 2002;110:927–937. doi: 10.1289/ehp.02110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff J. Perchlorate and the thyroid gland. Pharmacol.Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- 13.Wyngaarden JB, Stanbury JB, Rapp B. The effects of iodide, perchlorate, thiocyanate and nitrate administration upon the iodide concentrating mechanism of the rat thyroid. Endocrinology. 1953;52:568–574. doi: 10.1210/endo-52-5-568. [DOI] [PubMed] [Google Scholar]
- 14.Tonacchera M, Pinchera A, Dimida A, Ferrarini E, Agretti P, Vitti P, Santini F, Crump K, Gibbs J. Relative potencies and additivity of perchlorate, thiocyanate, nitrate, and iodide on the inhibition of radioactive iodide uptake by the human sodium iodide symporter. Thyroid. 2004;14:1012–1019. doi: 10.1089/thy.2004.14.1012. [DOI] [PubMed] [Google Scholar]
- 15.Blount BC, Pirkle JL, Osterloh J, Valentin-Blasini L, Caldwell KL. Urinary Perchlorate and Thyroid Hormone Levels in Adolescent and Adult Men and Women Living in the United States. Environ.Health Perspect. 2006;114:1867–1871. doi: 10.1289/ehp.9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinmaus C, Miller MD, Howd R. Impact of smoking and thiocyanate on perchlorate and thyroid hormone associations in the 2001–2002 national health and nutrition examination survey. Environ.Health Perspect. 2007;115:1333–1338. doi: 10.1289/ehp.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dohan O, Carrasco N. Advances in Na(+)/I(−) symporter (NIS) research in the thyroid and beyond. Mol.Cell Endocrinol. 2003;213:59–70. doi: 10.1016/j.mce.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 18.Logothetopoulus J, Scott RF. Active iodide transport across the placenta of the guinea-pig, rabbit and rat. J Physiol. 1956;132:365–371. doi: 10.1113/jphysiol.1956.sp005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dohan O, Portulano C, Basquin C, Reyna-Neyra A, Amzel LM, Carrasco N. The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc.Natl.Acad.Sci.U.S.A. 2007;104:20250–20255. doi: 10.1073/pnas.0707207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran N, Valentin-Blasini L, Blount BC, McCuistion CG, Fenton MS, Gin E, Salem A, Hershman JM. Thyroid-stimulating hormone increases active transport of perchlorate into thyroid cells. Am.J Physiol Endocrinol Metab. 2008;294:E802–E806. doi: 10.1152/ajpendo.00013.2008. [DOI] [PubMed] [Google Scholar]
- 21.Haddow J, Palomake G, Allan W, Williams J, Knight G, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. NewEngl.J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council. Washington, DC: National Academy Press; Health Implications of Perchlorate Ingestion. 2005
- 23.Becker DV, Braverman LE, Delange F, Dunn JT, Franklyn JA, Hollowell JG, Lamm SH, Mitchell ML, Pearce E, Robbins J, Rovet JF. Iodine supplementation for pregnancy and lactation-United States and Canada: recommendations of the American Thyroid Association. Thyroid. 2006;16:949–951. doi: 10.1089/thy.2006.16.949. [DOI] [PubMed] [Google Scholar]
- 24.Blount BC, Valentin-Blasini L. Analysis of perchlorate, thiocyanate, nitrate and iodide in human amniotic fluid using ion chromatography and electrospray tandem mass spectrometry. Analytica Chimica Acta. 2006;567:87–93. doi: 10.1016/j.aca.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Clewell RA, Merrill EA, Yu KO, Mahle DA, Sterner TR, Mattie DR, Robinson PJ, Fisher JW, Gearhart JM. Predicting fetal perchlorate dose and inhibition of iodide kinetics during gestation: a physiologically-based pharmacokinetic analysis of perchlorate and iodide kinetics in the rat. Toxicol.Sci. 2003;73:235–255. doi: 10.1093/toxsci/kfg081. [DOI] [PubMed] [Google Scholar]
- 26.Tellez RT, Chacon PM, Abarca CR, Blount BC, Landingham CB, Crump KS, Gibbs JP. Long-term environmental exposure to perchlorate through drinking water and thyroid function during pregnancy and the neonatal period. Thyroid. 2005;15:963–975. doi: 10.1089/thy.2005.15.963. [DOI] [PubMed] [Google Scholar]
- 27.Blazer S, Moreh-Waterman Y, Miller-Lotan R, Tamir A, Hochberg Z. Maternal hypothyroidism may affect fetal growth and neonatal thyroid function. Obstet.Gynocol. 2003;102:232–241. doi: 10.1016/s0029-7844(03)00513-1. [DOI] [PubMed] [Google Scholar]
- 28.Valentin-Blasini L, Mauldin JP, Maple D, Blount BC. Analysis of perchlorate in human urine using ion chromatography and electrospray tandem mass spectrometry. Anal.Chem. 2005;77:2475–2481. doi: 10.1021/ac048365f. [DOI] [PubMed] [Google Scholar]
- 29.Amitai Y, Winston G, Sack J, Wasser J, Lewis M, Blount BC, Valentin-Blasini L, Fisher N, Israeli A, Leventhal A. Gestational exposure to high perchlorate concentrations in drinking water and neonatal thyroxine levels. Thyroid. 2007;17:843–850. doi: 10.1089/thy.2006.0336. [DOI] [PubMed] [Google Scholar]
- 30.Valentin-Blasini L, Blount BC, Delinsky A. Quantification of iodide and sodium-iodide symporter inhibitors in human urine using ion chromatography tandem mass spectrometry. J Chromatogr.A. 2007;1155:40–46. doi: 10.1016/j.chroma.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Scherer G, Richter E. Biomonitoring exposure to environmental tobacco smoke (ETS): a critical reappraisal. Hum.Exp.Toxicol. 1997;16:449–459. doi: 10.1177/096032719701600806. [DOI] [PubMed] [Google Scholar]
- 32.Windham GC, Eaton A, Hopkins B. Evidence for an association between environmental tobacco smoke exposure and birthweight: a meta-analysis and new data. Paediatr.Perinat.Epidemiol. 1999;13:35–57. doi: 10.1046/j.1365-3016.1999.00150.x. [DOI] [PubMed] [Google Scholar]
- 33.Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am.J Epidemiol. 2007;165:734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- 34.Caldwell KL, Jones R, Hollowell JG. Urinary iodine concentration: United States National Health And Nutrition Examination Survey 2001–2002. Thyroid. 2005;15:692–699. doi: 10.1089/thy.2005.15.692. [DOI] [PubMed]
- 35.Chatterjee MS, Abdel-Rahman M, Bhandal A, Klein P, Bogden J. Amniotic fluid cadmium and thiocyanate in pregnant women who smoke. J Reprod.Med. 1988;33:417–420. [PubMed] [Google Scholar]
- 36.Blount BC, Valentin-Blasini L. Biomonitoring as a method for assessing exposure to perchlorate. Thyroid. 2007;17:837–841. doi: 10.1089/thy.2007.0106. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs J. A comparative toxicological assessment of perchlorate and thiocyanate based on competitive inhibition of iodide uptake as the common mode of action. Hum.Ecol.RiskAssess. 2006;12:157–173. [Google Scholar]
- 38.Magann EF, Doherty DA, Chauhan SP, Barrilleaux SP, Verity LA, Martin JN., Jr Obstet Gynecol. 2003;101(6):1261–1265. doi: 10.1016/s0029-7844(03)00344-2. [DOI] [PubMed] [Google Scholar]
- 39.Leung AM, Pearce EN, Braverman LE. Iodine content of prenatal multivitamins in the United States. N Engl. J Med. 2009;360(24):2582–2583. doi: 10.1056/NEJMc0807851. [DOI] [PubMed] [Google Scholar]
- 40.Rayburn WF, Robinson A, Braverman LE, He XM, Pino S, Gargas ML, Kinzell JH. Iodide concentrations in matched maternal serum, cord serum, and amniotic fluid from preterm and term human pregnancies. Reprod.Toxicol. 2008;25:129–132. doi: 10.1016/j.reprotox.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Crone M, Waago G. Transfer of radioactive iodide between mother and foetus in the rabbit. Acta Physiol Scand. 1961;51:84–93. doi: 10.1111/j.1748-1716.1961.tb02116.x. [DOI] [PubMed] [Google Scholar]
- 42.Kirk AB, Martinelango PK, Tian K, Dutta A, Smith EE, Dasgupta PK. Perchlorate and iodide in dairy and breast milk. Environ.Sci.Technol. 2005;39:2011–2017. doi: 10.1021/es048118t. [DOI] [PubMed] [Google Scholar]
- 43.Pearce EN, Leung AM, Blount BC, Bazrafshan HR, He X, Pino S, Valentin-Blasini L, Braverman LE. Breast milk iodine and perchlorate concentrations in lactating Boston-area women. J Clin Endocrinol Metab. 2007;92:1673–1677. doi: 10.1210/jc.2006-2738. [DOI] [PubMed] [Google Scholar]
- 44.Schier J, Wolkin A, Valentin-Blasini L, Belson M, Kieszak S, Rubin C, Blount B. Perchlorate exposure from infant formula and comparisons with the perchlorate reference dose. J Expo.Sci.Environ.Epidemiol. 2009 doi: 10.1038/jes.2009.18. in press. doi:10.1038/jes.2009.18. [DOI] [PubMed] [Google Scholar]
- 45.Baier-Anderson C, Blount BC, LaKind JS, Naiman DQ, Wilbur SB, Tan S. Estimates of exposures to perchlorate from consumption of human milk, dairy milk, and water, and comparison to current reference dose. J Toxicol. Environ. Health Part A. 2006;69:319–330. doi: 10.1080/15287390500323420. [DOI] [PubMed] [Google Scholar]
- 46.Ginsberg G, Rice D. The NAS perchlorate review: questions remain about the perchlorate RfD. Environ.Health Perspect. 2005;113:1117–1119. doi: 10.1289/ehp.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.