Abstract
The growing importance of cascade reactions reflects and imparts advances in the state of the art of organic synthesis and underscores the desire of synthetic chemists to achieve higher levels of elegance and efficiency. Besides their aesthetic appeal, cascade processes offer economical and environmentally friendly means for generating molecular complexity. Because of their many advantages, these reactions have found numerous applications in the synthesis of complex molecules, both natural and designed. In this Tutorial Review, we highlight the design and execution of cascade reactions within the context of total synthesis as demonstrated with selected examples from these laboratories.
Introduction
Chemical synthesis is, at its core, an endeavor in composition, and, as such, is a creative field of almost limitless scope, especially when applied to complex molecules whose primary element is carbon, collectively known as organic compounds. Total synthesis refers to the laboratory construction of naturally occurring substances, often complex organic compounds with important biological activities, some of which eventually find their way into the clinic as useful medicines. In a broader sense, the term may also be applied to the synthesis of designed molecules of significant complexity for multifaceted intents and purposes, whether in biology and medicine, or materials science and nanotechnology. Total synthesis has been hailed as a highly demanding and exacting science, but it is also recognized in its finest form as an art. The artistic nature of total synthesis manifests itself in the selection of the synthetic maneuvers that lead to the target molecule (the strategy) and the design of the structural variations of the target that are synthesized (the analogues).
In the eyes of the connoisseur of the art of total synthesis, the emergence of molecular complexity from simpler molecular assemblies becomes a choreographed event wherein, although each move is appreciated, it is their combinations in sequence that solicit the louder applause. In chemical synthesis, such combinations, commonly known as cascade reactions,1 are often aesthetically pleasing and can be highly efficient. Cascade reactions are sequences of chemical transformations in which the starting substrate is designed so as to (or happens to) undergo a reaction whose product becomes the substrate for the next step, whose product again becomes the substrate for the next step, and so on, until a product stable to the reaction conditions is reached. In addition to the obvious benefits of reducing the number of manipulations required, cascade reactions also expand our repertoire of reactions and strategies by allowing the use of synthetically enabling intermediates that may or may not be practical or possible to isolate. Indeed, many catalytic processes, such as palladium-catalyzed cross coupling reactions2 and olefin metathesis reactions,3 are in their very nature cascade reactions and have become key components of the synthetic toolbox. Cascade reactions, besides being artistically appealing, are often associated with cost savings in terms of reagents, catalysts, and solvents, as well as time and effort. They are, therefore, a means to achieve green chemistry and a way to develop economical processes for the manufacture of pharmaceuticals and other fine chemicals.
In this article, we will attempt to demonstrate the present power of cascade reactions in total synthesis and point out their future potential, with particular emphasis on the art and science involved in achieving molecular complexity and diversity. As cascade reactions in total synthesis have been thoroughly reviewed elsewhere,1 we shall confine ourselves only to cascade sequences discovered during selected synthetic campaigns in these laboratories, namely those directed toward the total syntheses of the endiandric acids [see endiandric acid A (1, Figure 1)], hybocarpone (2), thiostrepton (3), the C2-symmetric bisanthraquinones [see 2,2'-epi-cytoskyrin A (4)], biyouyanagin A (5), and antibiotic BE-43472B (6).
Figure 1.
Molecular structures of endiandric acid A (1), hybocarpone (2), thiostrepton (3), 2,2′-epi-cytoskyrin A (4), biyouyanagin A (5), and BE-43472B (6).
Endiandric acids
Endiandric acids A–C (1, 7, 8, Figure 2) were discovered from the Australian tree Endiandra introrsa and reported by Black, Gatehouse, and coworkers in 1980.4,5 Interestingly, these polycyclic systems exist naturally as racemic mixtures. Based on this observation and by virtue of their molecular structures, Black proposed an intriguing biosynthetic hypothesis whereby the endiandric acids are formed from linear, achiral polyunsaturated precursors through a sequence of thermally allowed pericyclic reactions independent of the influence of any enzymes.5 Thus, as shown in Scheme 1, polyunsaturated carboxylic acids 10 and 11 might suffer spontaneous conrotatory 8π e electrocyclization to form cyclooctatriene systems 12 and 13, differing only in their ring conformation. In turn, these proposed intermediates (12 and 13) were expected to readily undergo a disrotatory 6π e electrocyclization to furnish the bicyclic structures shown (9, 14–16). The putative compounds 14–16 (since named endiandric acids E, F, and G, respectively) could further react to generate endiandric acids A, B, and C (1, 7, and 8, respectively) through intramolecular Diels–Alder cycloadditions,6 as indicated in Scheme 1. In contrast, compound 9 (named endiandric acid D), being incapable of a further pericyclic reaction, was predicted to be a stable chemical entity,5 a fact that was later confirmed when it was both synthesized in the laboratory7 and isolated from nature.8
Figure 2.
Molecular structures of endiandric acids A–D (1, 7–9).
Scheme 1.
Black’s hypothesis for the biosynthesis of the endiandric acids.
Intrigued by these reports, we set out to test Black’s intriguing hypothesis, and, in 1982, published our results that provided its experimental verification.7 We first synthesized each of the polycyclic frameworks through sequential pericyclic reactions in order to test the viability of each step in the proposed cascade process. Having successfully prepared all the endiandric acids through stepwise syntheses, we then turned our attention to a more direct mimickery of the proposed biosynthesis. Polyunsaturated methyl ester 17 (Scheme 2), possessing a conjugated diyne moiety and the proper olefinic geometries, was subjected to Lindlar reduction conditions in order to form the highly conjugated polyene system 18 with the requisite cis-disposed central olefinic bonds. The latter intermediate proved transient, undergoing a spontaneous 8π e electrocyclization followed by a second 6π e electrocyclization9 to yield a mixture of endiandric acids D and E methyl esters (19 and 20). As expected, endiandric acid A methyl ester (21) was obtained upon heating, but surprisingly, endiandric acid D methyl ester (19) was no longer observed. Rather than being the dead end product predicted in Black’s hypothesis, endiandric acid D methyl ester (19) was evidently capable of undergoing a 6π e ring opening, ring flip, and subsequent 6π e electrocyclization (all reversible processes), finally being trapped irreversibly through an intramolecular Diels–Alder cycloaddition to deliver endiandric acid A methyl ester (21), as illustrated by the sequence 9→13→12→14→1 (see Scheme 1). Indeed, this proposed explanation was verified by following the fate of pure endiandric acid D methyl ester (19) under the same reaction conditions through 1H NMR spectroscopic analysis. Thus, heating a solution of endiandric acid D methyl ester (19) led to the formation of endiandric acid A methyl ester (21) via endiandric acid E methyl ester (20) (see Scheme 2).
Scheme 2.
Biomimetic cascade total synthesis of endiandric acids A (21), D (19), and E (20) methyl esters.
As shown in Scheme 3, polyunsaturated methyl ester 22 was subjected to the same Lindlar reduction conditions to effect formation of endiandric acids F and G methyl esters (23 and 24). As anticipated, endiandric acids B and C methyl esters (25 and 26) were produced upon heating. As in the formation of endiandric acid A methyl ester (21, Scheme 2), at elevated temperatures, endiandric acids F and G methyl esters (23 and 24) interconverted reversibly and, finally, trapped irreversibly, as shown by the sequences 15→12→13→16→8 and 16→13→12→15→7 (see Scheme 1). Thus, exposure of a pure sample of either compound (23 or 24) to heat led to the formation of both endiandric acids B and C methyl esters (25 and 26).
Scheme 3.
Biomimetic cascade total synthesis of endiandric acids B (25), C (26), F (23), and G (24) methyl esters.
In the endiandric acid cascades, the simplest of reagents (H2 gas, Lindlar catalyst, and heat) effected formation of up to four rings and generation of up to eight contiguous stereogenic centers with complete stereocontrol. Considering the striking operational simplicity of these cascade reactions and the impressive molecular complexity so formed, it is indeed difficult to imagine a more efficient strategy to synthesize the endiandric acids and other molecules like them.
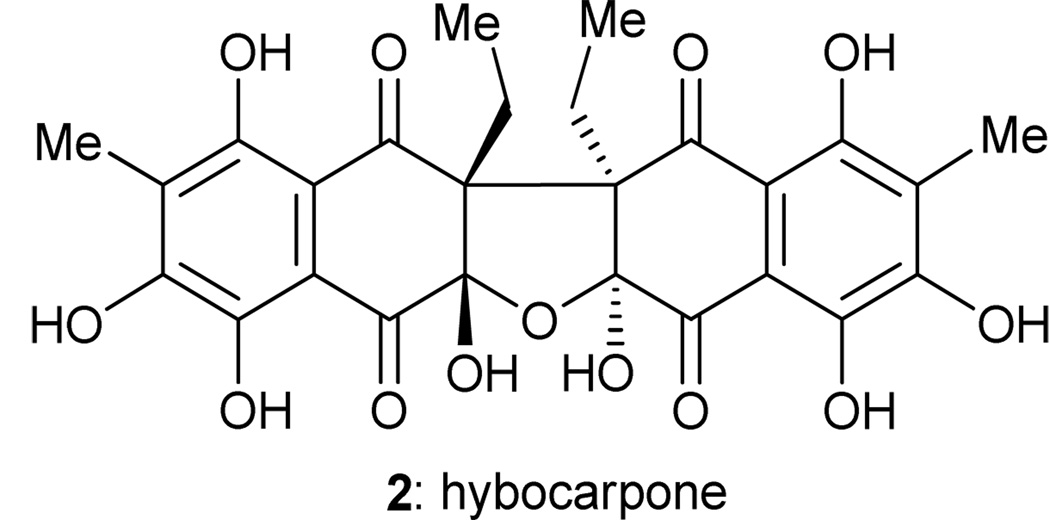
Hybocarpone
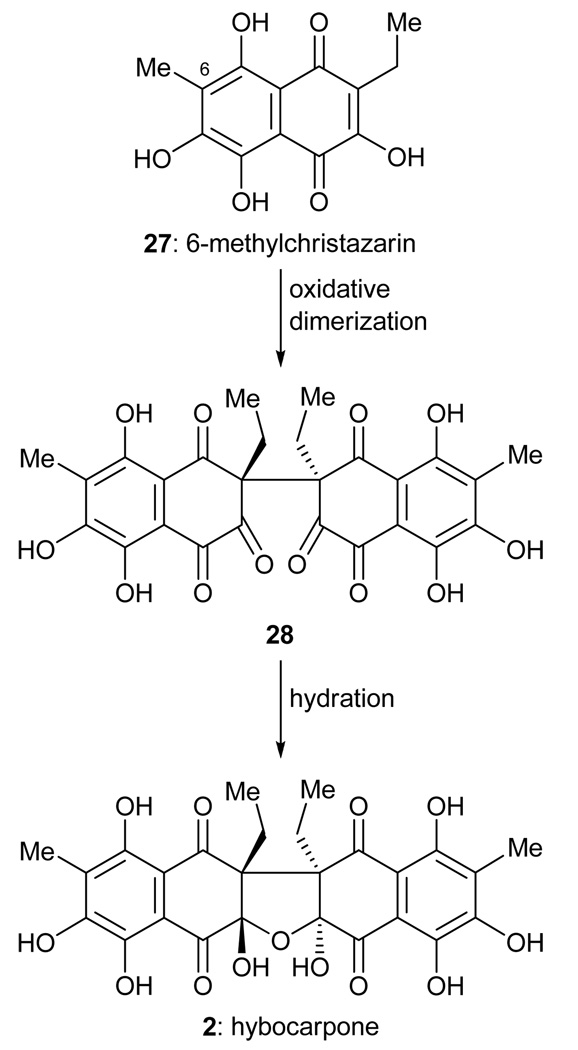
Hybocarpone (2, Figure 3) is a cytotoxic compound isolated from Lecanora hybocarpa, a lichen found in the Louisiana woodland, and reported by Elix and coworkers in 1999.10 Hybocarpone is a C2-symmetric compound, and the suspiciously related monomeric structure 27 (6-methylchristazarin, Scheme 4) is also naturally occurring.11 Although there is no proof of the biosynthetic proposal shown in Scheme 4, it seemed reasonable to us that nature may oxidatively dimerize 6-methylchristazarin (27) in an enantio- and diastereoselective manner to form a dimeric structure such as 28. We further reasoned that spontaneous hydration of 28 may yield hybocarpone (2) in a cascade fashion. It was after failing to synthesize hybocarpone through the use of a Claisen rearrangement to install its highly hindered all-carbon quaternary centers that we turned to this postulated, but highly speculative, biosynthetic hypothesis as a means to construct the molecule. In 2001, we reported a total synthesis of hybocarpone (2) based on this approach.12
Figure 3.
Molecular structure of hybocarpone (2).
Scheme 4.
Proposed biosynthesis of hybocarpone (2) from 6-methylchristazarin (27).
Before we could attempt the proposed oxidative dimerization, we first had to prepare 6-methylchristazarin (27), or a masked equivalent of this monomeric unit. Toward this end, a mixture of aromatic aldehyde 29 (Scheme 5) and α,β-unsaturated ester 30 was irradiated with UV light to effect photoenolization13 of 29, thus providing access to the highly reactive o-quinonedimethide 31, which underwent rapid Diels–Alder cycloaddition6 with dienophile 30 to afford bicyclic system 32. A series of oxidations and a decarboxylation then yielded trimethoxy naphthoquinone 33, from which 6-methylchristazarin (27) was generated through the action of BBr3.
Scheme 5.
Highlights of the photoenolization/Diels–Alder synthesis of 6-methylchristazarin (27)
Initial attempts to effect the desired oxidative dimerization of 6-methylchristazarin (27) to directly yield hybocarpone (2) were unsuccessful. Experimentation with its protected form 33, however, proved more fruitful. Thus, as shown in Scheme 6, a solution of 33 was exposed to cerium(IV) ammonium nitrate (CAN) to effect a single electron oxidation, generating radical cation 34. The latter species proved to be transient, rapidly dimerizing with ease to afford hexaketone 35, selectively, with the trans stereochemistry shown. The high degree of stereocontrol observed in this carbon–carbon bond forming reaction is presumably due to minimization of severe steric interactions at this congested site. The resulting reaction mixture was rapidly quenched with a solution of aqueous 5 M KOH, a process that resulted in hydration of the reactive polyketone 35 (which acts as an anhydride), thus casting the oxygen ring of the molecule and furnishing a mixture of the desired product 37 and its diastereoisomer 36. This strongly basic quenching process was essential to the successful isolation of the dimeric products 36 and 37. Though the combined yield (19%) was modest, recycling of the starting material (35), which was recovered in 60% yield by simple acid–base extraction, greatly enhanced the material throughput of this dimerization process. Upon prolonged standing in CDCl3, the undesired product 36 was found to cleanly isomerize to the desired stereoisomer 37, a conversion that could be accelerated by the addition of acetic acid. The aryl methyl ether groups of 37 were finally cleaved (AlBr3, 60% yield) to complete the total synthesis of hybocarpone (2).
Scheme 6.
Oxidative radical dimerization of 33 and total synthesis of hybocarpone (2).
The ease and rapidity of formation of the dimeric structure of hybocarpone and the high degree of stereocontrol in this cascade sequence suggest this oxidative dimerization may indeed represent a biosynthetic pathway, as postulated. It represents an unusually mild method of forging a highly hindered carbon–carbon bond in a diastereoselective manner and achieves an impressive increase in molecular complexity through an attractive sequence of synthetic transformations.
Thiostrepton
Thiostrepton (3, Figure 4) is the flagship member of the thiopeptide family of antibiotics.14 First described in 1956 by scientists at The Squibb Institute for Medical Research (now a subsidiary of Bristol-Myers Squibb),15 thiostrepton has found use as a topical antibiotic in veterinary medicine. Floss and coworkers have elucidated many details of the biosynthesis of thiostrepton.16 Of particular interest to us was the putative biosynthesis of the central dehydropiperidine core of the molecule, summarized in Scheme 7. Thus, a biosynthetic intermediate such as 38, possessing a dehydroalanine-derived domain (formed by dehydration of a serine residue) and a serine-derived moiety, likely undergoes a dehydration and tautomerization to afford azadiene 39, which is proposed to undergo a spontaneous aza-Diels–Alder cycloaddition to form dehydropiperidine system 40. According to this biosynthetic hypothesis, subsequent reduction of the latter compound completes the formation of the dehydropiperidine core of thiostrepton (41).
Figure 4.
Molecular structure of thiostrepton (3).
Scheme 7.
Proposed biosynthesis of the dehydropiperidine core of thiostrepton.
A previously reported observation from the Wulff group17 provided an encouraging precedent for our proposed biomimetic dehydropiperidine core construction in the laboratory. Thus, azadiene 42 (Scheme 8), isolated as a crystalline solid at −90 °C, was conceived as a building block for α-amino acid synthesis.17 Upon warming, azadiene 42 spontaneously dimerized to give aza-Diels–Alder6 adduct 44, presumably through exo transition state 43. Under appropriate conditions, the aryl imine of 44 was preferentially hydrolyzed to afford primary amine 45.17 Although this precedent differed in key details from what we proposed to attempt (notably in that our proposed aza-Diels–Alder dimerization required an endo transition state), it nonetheless provided inspiration for our thiostrepton campaign, which we successfully completed in 2004.18
Scheme 8.
Precedent for the proposed aza-Diels–Alder dimerization reaction.
Scheme 9 depicts our thiostrepton cascade, developed for the construction of the dehydropiperidine core of the molecule. Thus, thiazolidine 46 (prepared from threonine and cysteine) was exposed to Ag2CO3 and DBU in pyridine at −12 °C (conditions A, Scheme 9) to effect extrusion of the sulfur atom and generate transient azadiene 47.19 The latter species proceeded to dimerize in a Diels–Alder fashion, as expected. The yield of the desired imine hydrolysis product 49, however, was a modest 22%, with bridged polycyclic byproduct 52 representing the lion’s share of the isolated material (63% yield). This rather annoying side reaction was the result of a tautomerization of the initially formed endo aza-Diels–Alder dimerization product 48 to provide enamine 51, which apparently underwent an aza-Mannich cyclization reaction to afford the undesired product 52. After considerable experimentation, we discovered that the imine hydrolysis reaction manifold could be facilitated by simple addition of BnNH2 (conditions B, Scheme 9), which raised the yield of dehydropiperidine 49 to a satisfying 60%. Aldehyde 50 was also recovered from this process in 68% yield, and could be recycled.
Scheme 9.
Biomimetic aza-Diels–Alder dimerization cascade and construction of the dehydropiperidine core of thiostrepton (49).
This would not be the only instance of an undesired cascade pathway in our thiostrepton campaign that had to be suppressed. Indeed, as shown in Scheme 10, in the subsequent manipulation, coupling of dehydropiperidine 49 with protected alanine 53 (EDC, HOAt) gave not the expected amide product, but rather imine ring contracted system 55. Presumably, this transformation proceeded through the intermediacy of bridged bicyclic aminal 54. It was after considerable experimentation that we discovered that the use of the sterically small acylating agent 56 in the absence of any activating agents (e.g. 4-DMAP) could effect direct amidation of the sterically hindered primary amine moiety of 49. The resulting amide product (57) was subsequently elaborated to synthetic thiostrepton (3).
Scheme 10.
Competing amide formation pathways and completion of the total synthesis of thiostrepton (3).
The cascade reactions of the thiostrepton total synthesis discussed above had to be carefully optimized since minor changes to the reaction conditions induced wildly different reactions yielding unexpected products. Indeed, this is one of the challenges faced in developing and executing such complex sequences. Thorough and exquisite experimentation is, therefore, required to achieve and control the desired reaction pathways. However, as demonstrated here, such efforts can reap rich dividends.
C2-Symmetric bisanthraquinone natural products
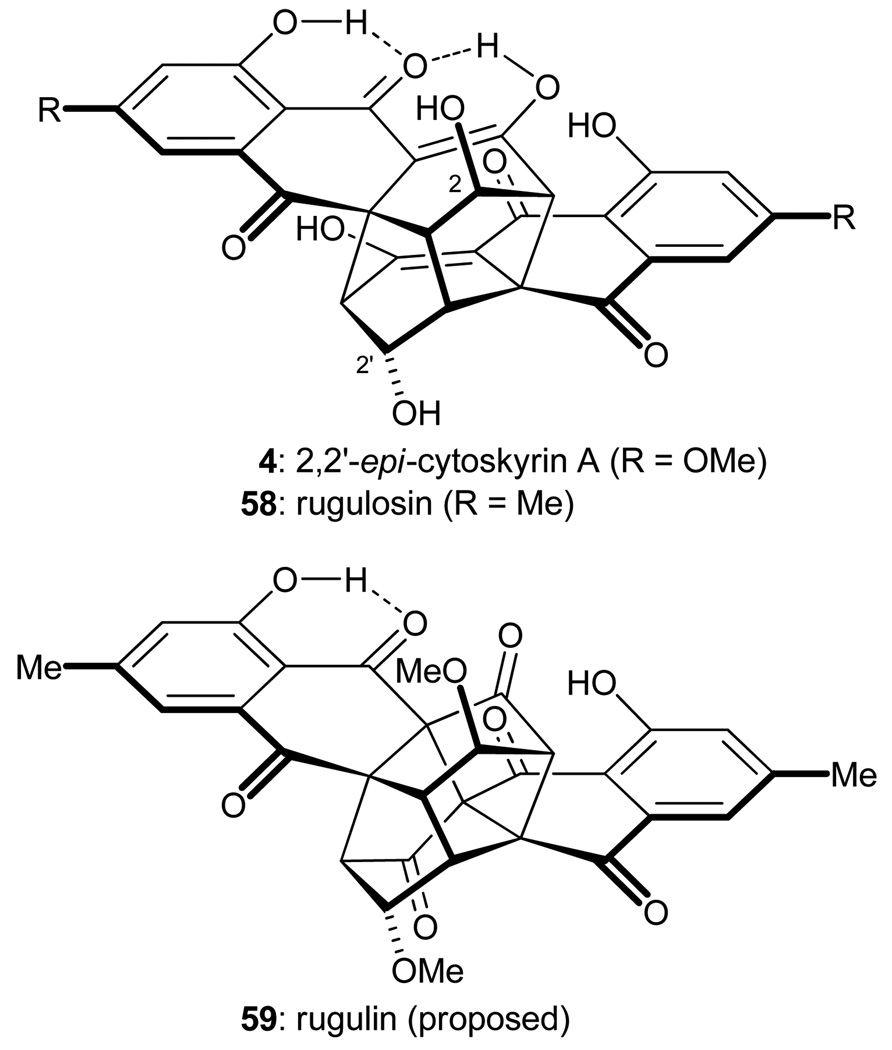
The bisanthraquinone natural products are a large class of complex secondary metabolites possessing a broad array of biological activities. Our attention was drawn to the cytoskyrin family of C2-symmetric bisanthraquinone compounds, such as 2,2'-epi-cytoskyrin A (4, Figure 5),20 rugulosin (58),21 and rugulin (59).22 Although anthraquinone monomers are readily identified within the polycyclic molecular architectures of these compounds, it was not evident at first glance how these structures might arise from their monomeric precursors. Careful analysis of these cage-like structures, however, revealed a logical pathway for their construction from simple monomeric anthraquinones. Thus, and as shown in Figure 6, retrosynthetic disconnection of the C4a–C4a' bond of rugulin (59) suggested structure 60, possessing the same ring system as that of 2,2'-epi-cytoskyrin A (4) and rugulosin (58), as a possible advanced subtarget. Two of the three carbon–carbon bonds that join the two monomeric units within this carbon skeleton may be disconnected through retrosynthetic application of two sequential Michael additions, thus revealing simplified dimers 61–63 as potential intermediates. The latter structures may be formed through oxidation and dimerization of monomeric anthraquinones 64–66, which, in turn, may arise from the oxidation of dihydroanthraquinones 67–69. Indeed, Shibata and coworkers had previously demonstrated the feasibility of such transformations, albeit in low yield.23 It seemed reasonable that nature may employ a similar sequence of events for the construction of these fascinating compounds.
Figure 5.
Molecular structures of 2,2′-epi-cytoskyrin A (4), rugulosin (58), and the proposed structure of rugulin (59).
Figure 6.
Retrosynthetic analysis of 2,2′-epi-cytoskyrin A (4), rugulosin (58), and the proposed structure of rugulin (59).
Intrigued by the relatively simple chemistry postulated to lead to these complex cage-like systems, we set out to discover an efficient cascade pathway to cytoskyrin-type molecules in the laboratory, and reported the so-called cytoskyrin cascades in 2005.24 In our model study with a substrate lacking the C2-hydroxyl group (see 67, Figure 6), we discovered that each of the requisite transformations could be accomplished through judicious use of only the simplest of reagents, namely MnO2, CSA, and/or Et3N. Furthermore, through careful tuning of reaction conditions, any or all of the synthetic steps could be combined in a cascade sequence. In our ultimate synthetic strategy toward these natural products, we employed protected C2-hydroxyl groups in spite of the potential complication of elimination reactions. As shown in Scheme 11, dihydroanthraquinone 67 was exposed to the action of MnO2, which initially effected oxidation to anthraquinone 64. Tautomerization of this intermediate gave access to enol 70, which dimerized through two sequential Michael additions to give, through the intermediacy of 73, isolable oxygen-bridged intermediate 76. Further oxidation (see 79) effected collapse of the oxygen bridge, giving rise to compound 61. An intramolecular Michael addition within the latter molecule then generated intermediate 79, lacking only one carbon–carbon bond of the cage-like framework of the targeted molecules. Upon addition of Et3N and gentle warming, a second Michael addition effected the closure of the cage, furnishing protected 2,2'-epi-cytoskyrin A (82) in a highly gratifying 60% overall yield for the entire cascade starting from simple dihydroanthraquinone 67. Subjecting dihydroanthraquinone 68 to the same reaction conditions afforded protected rugulosin (83) in 50% overall yield. Similarly, exposure of dihydroanthraquinone 69 to these reaction conditions provided cage system 60 in 40% overall yield.
Scheme 11.
Cascade synthesis of cage-like compounds 82, 83, and 60.
The total syntheses of the targeted natural products were completed as shown in Scheme 12. Thus, cleavage of the MOM ethers from intermediates 82 and 83 revealed 2,2'-epi-cytoskyrin A (4) and rugulosin (58), respectively. Cage compound 60 was further oxidized with MnO2 to give rugulin derivative 84. Removal of the two MOM protecting groups from the latter compound afforded a substance with the alleged structure of rugulin (i.e. 59), but its NMR spectral data did not match those reported for natural rugulin. The structure assigned to our synthetic material was verified by X-ray crystallographic analysis and, therefore, we concluded that the molecular structure of natural rugulin is not 59. Unfortunately, as an authentic sample was not available, the true structure of rugulin still remains an unsolved mystery.
Scheme 12.
Completion of the total syntheses of 2,2′-epi-cytoskyrin A (4), rugulosin (58), and the proposed structure of rugulin (59).
The cytoskyrin cascades are striking for both the expedient introduction of significant molecular complexity and the ease with which the reactions were induced. In total, the cytoskyrin cascade involves four bond-forming reactions and one bond-breaking event, and sets six stereogenic centers with complete stereochemical fidelity. Yet, the only reagents required to bring about this significant increase in molecular complexity were a common oxidant (MnO2), a simple base (Et3N), and a little warmth. It is indeed difficult to imagine a more direct means of accessing these complex cage-like molecules.
Biyouyanagin A
The anti-HIV agent biyouyanagin A was isolated from Hypericum chinense L. var. salicifolium (biyouyanagi in Japanese), a plant used in traditional Japanese medicine and reported in 2005, by Takaishi and colleagues.25 Biyouyanagin A was assigned the molecular structure 85 or 86 (Figure 7) by NMR spectroscopy. This structure contains a striking and unusual all-cis cyclobutane ring system, which was proposed to arise biosynthetically from a [2+2] photocycloaddition reaction. Drawn to the promising biological activity, intriguing molecular architecture, and appealing biosynthetic hypothesis for biyouyanagin A, we evaluated the proposed [2+2] photocycloaddition through manual molecular modeling. We reasoned that the endo product (85 or 86) was disfavored because of significant steric repulsion in the transition state. Therefore, in our view, the exo product (5 or 87, Figure 7) represented the most likely structure of biyouyanagin A. As shown in Figure 8, we envisioned a synthesis of both 5 and 87 through a biomimetic [2+2] photocycloaddition reaction between hyperolactone C (90, naturally occurring)26,27 and either ent-zingiberene (88; ent-88 is naturally occurring),28 or ent-7-epi-zingiberene (89; ent-89 is naturally occurring),28 respectively. In 2007, we completed total syntheses of both 5 and 87, and demonstrated conclusively that the true structure of biyouyanagin A is 5.29
Figure 7.
Originally proposed (a) and revised (b) structures of biyouyanagin A.
Figure 8.
Retrosynthetic analysis of the proposed structures for biyouyanagin A.
Total syntheses of hyperolactone C (90) had previously been reported, but we hoped to improve on the Kinoshita synthesis,27 which was not stereoselective with regards to C4. Our efforts to install the C4 stereogenic center in a selective manner led us to apply an interesting palladium-mediated cascade reaction first reported by Inuoe and coworkers30 that had not previously seen use in total synthesis. Thus, as shown in Scheme 13, tertiary alcohol 91 (readily prepared by an adaptation of the Kinoshita route) was heated with iodobenzene and catalytic amounts of Pd(PPh3)4 in Et3N under an atmosphere of CO and CO2 to deliver the desired product 98 in 79% yield. This cascade process presumably proceeded through an initial carbonylative Sonogashira-type cross coupling2 to afford ynone 92, which then engaged a molecule of CO2 to form carbonic acid 93. Intramolecular Michael addition then gave cyclic carbonate 94, which reacted with the palladium catalyst to generate palladium–allyl species 95. The η1 complex 96, in equilibrium with η3 complex 95, underwent ring closure to palladacycle 97. Reductive elimination from palladacycle 97 delivered the observed product 98 and regenerated the active Pd(0) catalyst. By virtue of the above mechanism, the formation of spirocycle 98 was stereospecific, with complete retention of stereochemistry at C4, as verified by X-ray crystallographic analysis.
Scheme 13.
Palladium-catalyzed cascade synthesis of spirocycle 98.
Spirocycle 98 was smoothly converted into hyperolactone C (90) through a standard sequence of manipulations as shown in Scheme 14. A biomimetic [2+2] photocyclization reaction of hyperolactone C (90) and ent-zingiberene (88) then gave synthetic biyouyanagin A (5), which was identical to the natural substance. X-Ray crystallographic analysis confirmed the assigned stereochemistry of 5, validating our structural reassignment of this secondary metabolite. 24-epi-Biyouyanagin A (87, Figure 7) was also synthesized in the same manner during this study.
Scheme 14.
Highlights of the completion of the total synthesis of biyouyanagin A (5).
The application of Inuoe’s palladium-mediated cascade sequence to the synthesis of hyperolactone C (90) provided exquisite stereocontrol in a situation where no such control was previously observed. The elimination of a tedious chromatographic separation of diastereoisomers significantly improved the practicality of the total synthesis of hyperolactone C (90) and, in turn, biyouyanagin A (5). Indeed, several biyouyanagin A analogues were synthesized for chemical biology studies through the developed synthetic technology.
Bisanthraquinone antibiotic BE-43472B
BE-43472B (6, Figure 9) is a structurally unique bisanthraquinone natural product first described (with no stereochemical details) in a Japanese patent in 1996 as an antitumor agent.31 It was rediscovered in 2006 by Rowley and coworkers32 as a bactericidal antibiotic with potency against a broad panel of drug-resistant bacterial strains, including methicillin-susceptible, methicillin-resistant, and tetracycline-resistant Staphylococcus aureus (MSSA, MRSA, and TRSA, respectively) and vancomycin-resistant Enterococcus faecium (VRE). The relative stereochemistry of the substance was elucidated, but the absolute configuration was left undetermined. Drawn to this combination of promising biological activity and exquisite molecular architecture, we initiated a program directed towards the laboratory construction of this molecule. We recently reported the total synthesis of both enantiomeric forms of BE-43472B and the assignment of its absolute configuration as that depicted in structure 6.33 Our synthetic strategy focused on a Diels–Alder-based assembly of the bisanthraquinone system. Thus, and as shown in Figure 10, advanced intermediate 99, containing the complete octacyclic skeleton of BE-43472B, was envisioned as the product of an SN(Ar) reaction of hemiketal 100, which was anticipated to arise spontaneously from its ketone isomer (i.e. 101). The latter structure was proposed to be formed through a Diels–Alder cycloaddition6 of silyl enol ether 103 and naphthoquinone system 102.
Figure 9.
Molecular structure of BE-43472B (6).
Figure 10.
Retrosynthetic analysis of BE-43472B (6).
Although the sequence of events anticipated by our retrosynthetic analysis likely could be achieved in a sequential manner, we were pleased to find that under optimized conditions, the formation of octacyclic intermediate 99 could be effected in a single operation by simple heating. Thus, and as shown in Scheme 15, a solution of diene 103 and naphthoquinone system 102 in CH2Cl2 was initially heated at 85 °C in a sealed tube, conditions that induced the expected Diels–Alder cycloaddition reaction, presumably by way of endo transition state 104, to provide an equilibrating mixture of adduct 101 and its hemiketal isomer (i.e. 100). Upon further heating in toluene at reflux in a reaction vessel equipped with a Dean–Stark trap (to azeotropically remove the generated MeOH), an intramolecular Michael-type addition occurred to afford 105, which spontaneously proceeded to furnish the targeted octacyclic product in a gratifying 98% overall yield for the entire cascade sequence. Interestingly, the resulting product was obtained as a ca. 2:1 mixture of inconsequential C9a epimers 107 and 99. The unexpected isomer 107 probably formed through a spontaneous isomerization of hemiketal 100 to the epimeric system 106.
Scheme 15.
Diels–Alder/SN(Ar) cascade synthesis of the BE-43472B skeleton (99 and 107).
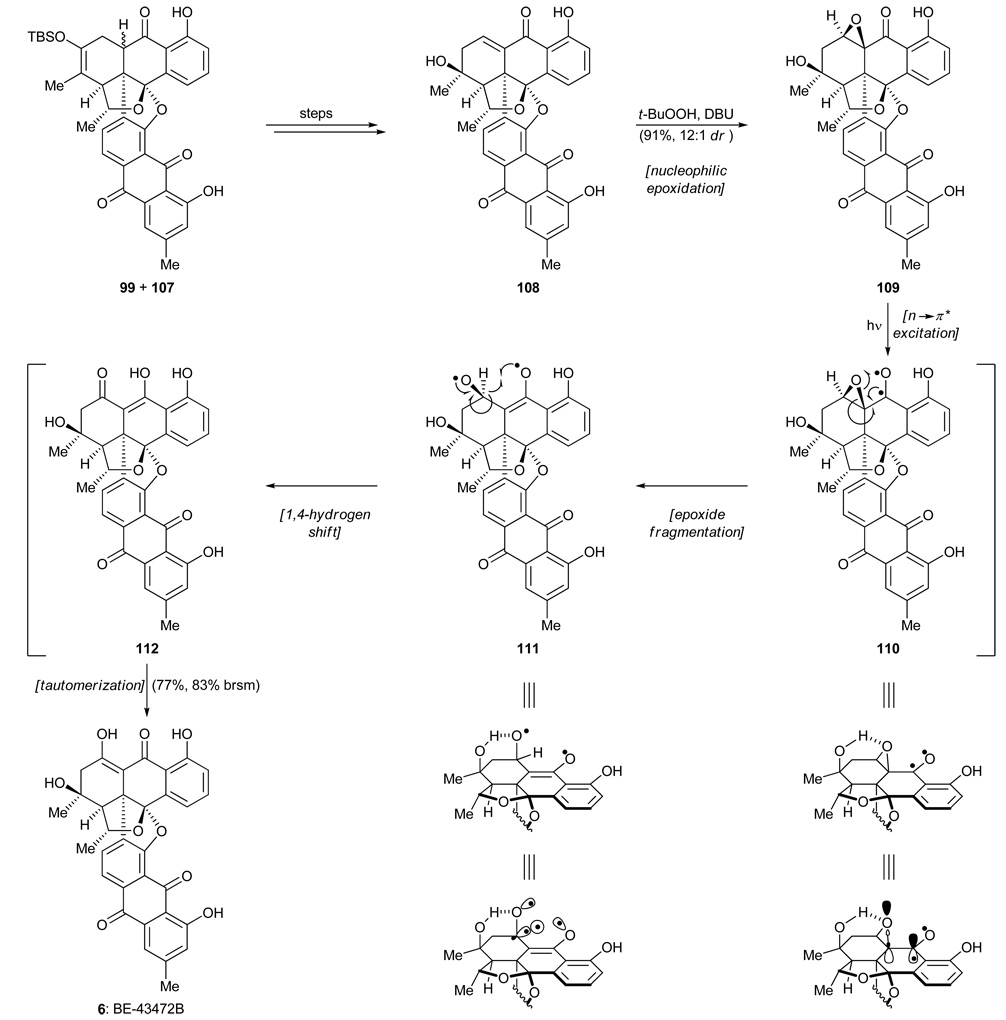
The mixture of 99 and 107 was transformed into enone 108 (Scheme 16) through a standard sequence of manipulations. All that now remained for the completion of the total synthesis of BE-43472B (6) was the installation of one oxygen atom at the proper position. After considerable experimentation, we discovered that the requisite oxygen could be installed through nucleophilic epoxidation (t-BuOOH, DBU) to afford epoxide 109 as the predominant diastereoisomer (12:1 dr). Distressingly, all attempts at thermal or acid-promoted isomerization of this epoxide to antibiotic BE-43472B (6) yielded either no reaction, or decomposition. We finally called upon the rarely used photoinduced isomerization of α,β-epoxy ketones34 as a means to achieve the desired rearrangement. Gratifyingly, irradiating a benzene solution of epoxide 109 with UV light effected the desired isomerization and delivered BE-43472B (6) in a satisfying 83% yield (based on 92% conversion). This solution to the problem at hand was striking and pleasing for both its simplicity and efficiency. The reaction was proposed to proceed through initial photo-excitation (n→π*) of the carbonyl moiety adjacent to the epoxide unit of 109 to generate diradical 110. As shown in the partial structure of 110, the neighboring epoxide C–O bond is appropriately aligned with the benzylic radical so as to undergo a facile bond cleavage to afford ring-opened intermediate 111. A subsequent 1,4-shift of the pseudoequatorial β-hydrogen, favored by good n,σ-orbital overlap, then results in enol 112, tautomerization of which provides the targeted molecule, antibiotic BE-42472B (6). Though none of the putative intermediates (110–112) are isolable, treatment of this reaction as a cascade sequence was useful to our understanding of the outcome, especially in view of the fact that the α-epoxide diastereoisomer of 109 refused to undergo the same rearrangement, presumably due to poor orbital overlap in the transient species corresponding to 110.
Scheme 16.
Photoisomerization and completion of the total synthesis of BE-43472B (6).
The total synthesis of bisanthraquinone antibiotic BE-43472B highlights the utility of cascade reactions in both planned and unplanned situations. The developed Diels–Alder cascade sequence resulted in the formation of three rings and the controlled installation of three stereogenic centers. The unusual photoisomerization process employed in the final step was adopted in response to an unanticipated challenge, and its successful execution enabled the completion of this synthetic campaign in a highly rewarding manner. Employing only heat or light, both cascade processes are shining examples of green chemistry.
Conclusion
The cascade reactions employed in the case studies presented herein are not the only conceivable means of synthesizing the target molecules under construction. However, these sequences possess many practical advantages in addition to their undeniable aesthetic appeal. Well designed and executed cascades are effective solutions to challenging problems in organic synthesis, and, in certain cases, only the simplest of conditions and reagents, such as heat, light, acid, or base, are needed. The significant molecular complexity generated, combined with a remarkable simplicity of operation, renders cascade reactions potentially cost-effective and environmentally friendly. As synthetic chemists take on ever more ambitious targets, the design and execution of cascade sequences will no doubt play a crucial role in reaching their goals of elegance and practicality.
Acknowledgments
We acknowledge the contributions of the many coworkers with whom we have had the privilege of working, and whose names appear in the cited papers. Without their bold creativity and exquisite experimentation, the synthetic campaigns highlighted in this article would not have been as rewarding as they turned out to be. We also thank Professor Albert Eschenmoser for insightful discussions. Financial support was provided by the National Institutes of Health (U.S.A.), the Skaggs Institute for Research, and the National Science Foundation (U.S.A.).
Biographies
K. C. Nicolaou, born and raised in Cyprus, studied chemistry in England at the University of London (B.Sc., 1969, Bedford College, First Class Honors; Ph.D., 1972, University College, with Professors Sondheimer and Garratt). After postdoctoral appointments at Columbia University (1972–1973, Professor Katz) and Harvard University (1973–1976, Professor Corey), he joined the faculty at the University of Pennsylvania, where he eventually became the Rhodes–Thompson Professor. In 1989, he accepted joint appointments at the University of California, San Diego, where he is Distinguished Professor of Chemistry, and The Scripps Research Institute, where he is Chemistry Department Chairman and holds the Skaggs Professorship of Chemical Biology and the Darlene Shiley Chair in Chemistry.
Jason S. Chen received his A.B. and A.M. degrees in 2001 from Harvard University, where he performed research under the supervision of Professor Matthew D. Shair. He then joined Enanta Pharmaceuticals (Watertown, MA) as a medicinal chemist studying novel cyclosporine A analogues. He joined Professor K. C. Nicolaou’s group at The Scripps Research Institute in 2003, where he was a National Defense Science and Engineering Graduate (NDSEG) Fellow. In 2008, he completed his Ph.D. studies on the total synthesis and biological evaluation of uncialamycin. He is currently a research associate in Professor Nicolaou’s laboratory.
References
- 1.Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem., Int. Ed. 2006;45:7134. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem., Int. Ed. 2005;44:4442. doi: 10.1002/anie.200500368. [DOI] [PubMed] [Google Scholar]
- 3.Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem., Int. Ed. 2005;44:4490. doi: 10.1002/anie.200500369. [DOI] [PubMed] [Google Scholar]
- 4.Bandaranayake WM, Banfield JE, St D, Black C, Fallon GD, Gatehouse BM. J. Chem. Soc., Chem. Commun. 1980:162. [Google Scholar]
- 5.Bandaranayake WM, Banfield JE, St D, Black C. J. Chem. Soc., Chem. Commun. 1980:902. [Google Scholar]
- 6.Corey EJ. Angew. Chem., Int. Ed. 2002;41:1650. doi: 10.1002/1521-3773(20020517)41:10<1650::aid-anie1650>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]; Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis GE. Angew. Chem., Int. Ed. 2002;41:1668. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Nicolaou KC, Petasis NA, Zipkin RE, Uenishi J. J. Am. Chem. Soc. 1982;104:5555. [Google Scholar]; Nicolaou KC, Petasis NA, Uenishi J, Zipkin RE. J. Am. Chem. Soc. 1982;104:5557. [Google Scholar]; Nicolaou KC, Zipkin RE, Petasis NA. J. Am. Chem. Soc. 1982;104:5558. [Google Scholar]; Nicolaou KC, Petasis NA, Zipkin RE. J. Am. Chem. Soc. 1982;104:5560. [Google Scholar]
- 8.Banfield JE, St D, Black C, Johns SR, Willing RI. Aust. J. Chem. 1982;35:2247. [Google Scholar]; Banfield JE, St D, Black C, Fallon GD, Gatehouse BM. Aust. J. Chem. 1983;36:627. [Google Scholar]
- 9.Meister H. Chem. Ber. 1963;96:1688. [Google Scholar]
- 10.Ernst-Russel M, Elix J, Chai C, Willis A, Hamada N, Nash T. Tetrahedron Lett. 1999;40:6321. [Google Scholar]
- 11.Yamamoto Y, Matsubara H, Kinoshita Y, Kinoshia K, Koyama K, Takahashi K, Ahmadjiam V, Kurokawa T, Yoshimura I. Phytochemistry. 1996;43:1239. [Google Scholar]
- 12.Nicolaou KC, Gray D. J. Am. Chem. Soc. 2004;126:607. doi: 10.1021/ja030497n. [DOI] [PubMed] [Google Scholar]
- 13.Fieser L, Ardao M-I. J. Am. Chem. Soc. 1956;78:774. [Google Scholar]
- 14.Bagley MC, Dale JW, Merritt EA, Xiong X. Chem. Rev. 2005;105:685. doi: 10.1021/cr0300441. [DOI] [PubMed] [Google Scholar]; Hughes RA, Moody CJ. Angew. Chem., Int. Ed. 2007;46:7930. doi: 10.1002/anie.200700728. [DOI] [PubMed] [Google Scholar]; Nicolaou KC, Chen JS, Edmonds DA, Estrada AA. Angew. Chem., Int. Ed. 2009;48:660. doi: 10.1002/anie.200801695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pagano JF, Weinstein MJ, Stout HA, Donovick R. Antibiot. Annu. 1955–1956:554. [PubMed] [Google Scholar]; Vandeputte J, Dutcher JD. Antibiot. Annu. 1955–1956:560. [PubMed] [Google Scholar]; Steinberg BA, Jambor WP, Suydam LO, Soriano A. Antibiot. Annu. 1955–1956:562. [PubMed] [Google Scholar]
- 16.Mocek U, Zeng Z, O’Hagan D, Zhou P, Fan L-DG, Beale JM, Floss HG. J. Am. Chem. Soc. 1993;115:7992. [Google Scholar]
- 17.Wulff G, Klinken HT. Tetrahedron. 1992;48:5985. [Google Scholar]
- 18.Nicolaou KC, Safina BS, Zak M, Lee SH, Nevalainen M, Bella M, Estrada AA, Funke C, Zécri FJ, Bulat S. J. Am. Chem. Soc. 2005;127:11159. doi: 10.1021/ja0529337. [DOI] [PubMed] [Google Scholar]; Nicolaou KC, Zak M, Safina BS, Estrada AA, Lee SH, Nevalainen M. J. Am. Chem. Soc. 2005;127:11176. doi: 10.1021/ja052934z. [DOI] [PubMed] [Google Scholar]
- 19.Öhler E, Schmidt U. Chem. Ber. 1979;112:107. [Google Scholar]
- 20.Agusta A, Ohashi K, Shibuya H. Chem. Pharm. Bull. 2006;54:579. doi: 10.1248/cpb.54.579. [DOI] [PubMed] [Google Scholar]
- 21.Takeda N, Seo S, Ogihara Y, Sankawa U, Iitaka I, Kitagawa I, Shibata S. Tetrahedron. 1973;29:3703. [Google Scholar]
- 22.Sedmera P, Podojil M, Vokoun J, Betina V, Nemec P. Folia Microbiol. 1978;23:64. doi: 10.1007/BF02876598. [DOI] [PubMed] [Google Scholar]
- 23.Yang D-M, Sankawa U, Ebizuka YS. Shibata, Tetrahedron. 1976;32:333. [Google Scholar]
- 24.Nicolaou KC, Lim YH, Piper JL, Papageorgiou CD. J. Am. Chem. Soc. 2007;129:4001. doi: 10.1021/ja0685708. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka N, Okasaka M, Ishimaru Y, Takaishi Y, Sato M, Okamoto M, Oshikawa T, Ahmed SU, Consentino LM, Lee K-H. Org. Lett. 2005;7:2997. doi: 10.1021/ol050960w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tada M, Nagai M, Okumura C, Osano Y, Matsuzaki T. Chem. Lett. 1989:683. [Google Scholar]
- 27.Ueki T, Doe M, Tanaka R, Morimoto Y, Yoshihara K, Kinoshita T. J. Heterocycl. Chem. 2001;38:165. [Google Scholar]
- 28.Eschenmoser A, Schinz H. Helv. Chim. Acta. 1950;33:171. [Google Scholar]; Breeden DC, Coates RM. Tetrahedron. 1994;50:11123. [Google Scholar]
- 29.Nicolaou KC, Wu TR, Sarlah D, Shaw DM, Rowcliffe E, Burton DR. J. Am. Chem. Soc. 2008;130:11114. doi: 10.1021/ja802805c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue Y, Ohuchi K, Yen I-F, Imaizumi S. Bull. Chem. Soc. Jpn. 1989;62:3518. [Google Scholar]
- 31.JP Pat. 1996 8 143 569. [Google Scholar]
- 32.Socha AM, LaPlante KL, Rowley DC. Bioorg. Med. Chem. 2006;14:8664. doi: 10.1016/j.bmc.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaou KC, Lim YH, Becker J. Angew. Chem., Int. Ed. DOI: 10.1002/anie.200900058. [Google Scholar]
- 34.Bodforss S. Chem. Ber. 1918;51:214. [Google Scholar]; Kim H, Kim TG, Hahn J, Jang D-J, Chang DJ, Park BS. J. Phys. Chem. A. 2001;105:3555. [Google Scholar]