Abstract
Both serotonin-1B (5-HT1B) receptors and stress modulate the behavioral and neurobiological effects of psychostimulant drugs. In order to examine how these factors interact to influence the development of behaviors associated with addiction, we used viral-mediated gene transfer to transiently increase expression of 5-HT1B receptors in the nucleus accumbens (NAc) shell along with exposure to repeated mild stress (novelty + saline injection) in rats. Once the viral-mediated increases in gene expression had dissipated, the resulting effects of this 5-HT1B-stress pairing on the acute locomotor response to amphetamine and on the development of psychomotor sensitization were examined. We report that increasing expression of 5-HT1B receptors on the terminals of NAc shell neurons that project to the VTA and repeatedly exposing rats to mild stress subsequently enhances the acute locomotor activating effects of amphetamine. In addition, the development of psychomotor sensitization–both locomotor activity and stereotypy components - is facilitated. These results suggest that serotonin signaling through NAc 5-HT1B heteroreceptors can interact with stress to increase susceptibility to enduring forms of drug-induced plasticity that are associated with addiction.
Introduction
Psychostimulant abuse and dependence has become a major epidemic with growing social and economic costs to society. Understanding the complex network of factors that mark the transition from casual drug use to addiction will be critical for combating this disease. This is not an easy task, however, as an individual’s initial response to psychostimulant drugs varies widely, as does susceptibility to addiction. Both genetic and environmental factors are thought to play essential roles in addiction vulnerability, but how interactions between these components influence the development of addiction is not yet well understood (Palomo et al., 2004; Kreek et al., 2005).
The serotonin-1B (5-HT1B) receptor, which can act as an inhibitory heteroreceptor in the axon terminals of GABAergic nucleus accumbens (NAc) neurons that project to the ventral tegmental area (VTA) (Johnson et al., 1992; Cameron and Williams, 1994; Morikawa et al., 2000), has received considerable attention regarding its role in the etiology of drug addiction. For example, genetic studies in humans have found associations between 5-HT1B receptor polymorphisms and substance abuse, suggesting that modified 5-HT1B receptor activity may be a contributing factor for increasing susceptibility to drug addiction (Fang et al., 2002; Huang et al., 2003; Proudnikov et al., 2006). In animals, pharmacological and genetic studies have found that 5-HT1B receptors can modulate the rewarding and psychomotor activating effects of psychostimulant drugs, although the direction of modulation can depend on the brain region manipulated and the type of 5-HT1B receptor that is effected (heteroreceptors versus autoreceptors) (Przegalinski et al., 2001; Fletcher et al., 2002; see Muller and Huston, 2006 for review). For example, systemic administration of a 5-HT1B receptor agonist facilitates the development of amphetamine sensitization in mice (Przegalinski et al., 2001), but 5-HT1B receptor knockout mice also show enhanced amphetamine sensitization compared to wild-type controls (Bronsert et al., 2001). In addition, intra-accumbens infusion of a 5-HT1B receptor agonist reduces amphetamine self-administration (Fletcher et al., 2002) whereas we have shown that increasing expression of 5-HT1B receptors in terminals of NAc shell neurons that project to the VTA enhances the rewarding effects of cocaine in a conditioned place preference test (Neumaier et al., 2002; Barot et al., 2007). It is thought that these 5-HT1B heteroreceptors inhibit GABA release in the VTA, thereby disinhibiting dopaminergic activity and amplifying drug reward mechanisms (Cameron and Williams, 1994, 1995; Yan, 2001; Yan and Yan, 2001; O’Dell and Parsons, 2004).
Exogenous factors, such as stress, can increase the likelihood of drug use and facilitate the development of addiction (Kalivas and Stewart, 1991; Sinha, 2001; Caprioli et al., 2007). Although 5-HT1B receptors can also modulate stress responses (see Clark and Neumaier, 2001 for review), how activity at 5-HT1B receptors and stress interact to influence behaviors associated with addiction has not been explored. In order to pursue this issue, we used viral-mediated gene transfer to transiently increase 5-HT1B receptor expression in the NAc shell and then exposed animals to repeated mild stress (novelty + saline injection). Once the viral-mediated increases in gene expression had dissipated, we examined the consequences of pairing this increase of 5-HT1B receptor expression with stress on the acute locomotor activating effects of amphetamine and on the development of psychomotor sensitization, a well-established animal model used to study the neural circuits thought to contribute to the development of addiction.
Experimental Procedures
Subjects
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 225–249 grams upon arrival were housed two per cage and given a one-week acclimation period prior to any experimental manipulation. The housing room was temperature- and humidity-controlled and maintained on a 12:12 h light:dark cycle, with food and water available ad libitum. All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee and were conducted in accordance with National Institutes of Health guidelines.
Drugs
Amphetamine (Sigma, St. Louis, MO) was dissolved in sterile 0.9% saline and administered by intraperitoneal (ip) injection in a volume of 1 ml/kg.
Viral Vector
A modified herpes simplex virus (HSV) amplicon was used to express the 5-HT1B receptor transgene and green fluorescent protein (GFP) as described in detail previously (Clark et al., 2002). Briefly, the pHSV-HA1B/GFP (1B; Fig. 1A) viral vector expresses both hemagglutinin-tagged 5-HT1B receptors and GFP from separate transcriptional cassettes whereas the pHSV-GFP (GFP) viral vector only expresses GFP, and is used as a control. Previous studies have found that the HSV vector system does not alter drug-related behaviors compared with sham or vehicle injections (Carlezon et al., 2000; Neumaier et al., 2002). The HA-5-HT1B receptor sequence was confirmed in its entirety with PCR and the vector was packaged using replication-deficient helper virus as described previously (Clark et al., 2002), yielding approximately 1 × 108 infective units/ml.
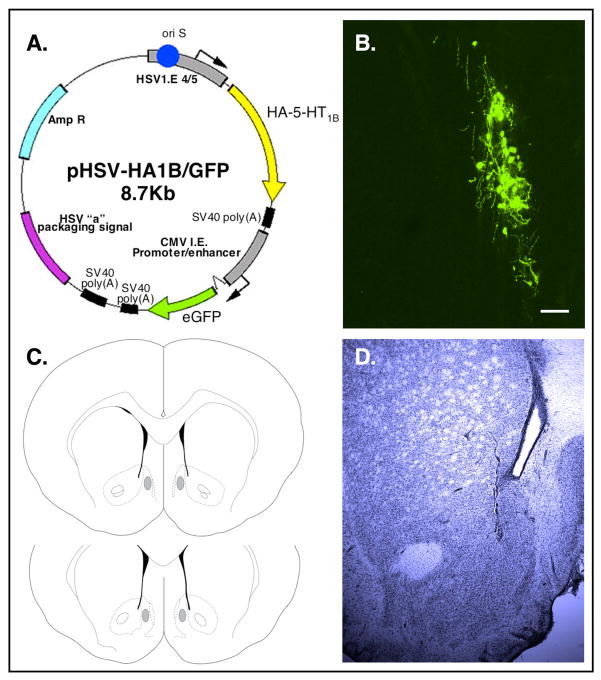
Figure 1.
The HA-5-HT1B receptor transgene. (A) Illustration of the pHSV-HA-5-HT1B transgene amplicon. (B) Representative histological plate of GFP expression from a section of the NAc shell 4 days following viral infusion of the 5-HT1B receptor transgene. Scale bar, 50 μm. (C) Injection needle tracts that ended within the grey-shaded areas indicate correctly targeted injections into the NAc shell. (D) Representative section of cresyl violet staining of an injection needle tract.
Surgery and viral gene transfer
Rats received bilateral infusions of viral vector into the medial NAc shell as described in detail previously (Neumaier et al., 2002; Barot et al., 2007). Briefly, rats were anesthetized with 2–4% isoflurane (Webster Veterinary Supply, Sterling, MA). Using standard stereotaxic procedures, 27-gauge stainless steel injectors were angled 10° from midline and placed bilaterally above the NAc shell (coordinates from bregma (mm): A/P 1.7; M/L ± 2.3; D/V; −6.8 from dura). Then, 2 μl of either GFP (control) or 1B viral vector (~200,000 infectious units in 10% sucrose) was infused on each side over a 10 min period at a flow rate of 0.2 μl/min. The injector was left in place an additional 5 min to minimize diffusion up the injector tract. We have previously shown that following viral infusion into the NAc shell transgenic 5-HT1B receptor expression is limited to GABAergic medium spiny projection neurons in a small region (the viral particles do not diffuse extensively) and that the transgenic receptors are transported to the VTA (Barot et al., 2007). In addition, peak levels of transgene expression occur 3–5 days following viral infusion and have dissipated by 10–14 days post-infusion (Barot et al., 2007; Ferguson et al., 2008). In the present set of experiments testing occurred once the viral-mediated gene expression had dissipated. Thus, the accuracy of injection coordinates was confirmed by cresyl violet staining of the injection needle tracts in 60 μm tissue sections by light microscopy (Fig. 1C), rather than by location of GFP expression. Rats with injection sites outside of the NAc shell (3 out of 72) were excluded from the acute locomotor response and no stress experiments. Brains from the sensitization study were unavailable for histological staining so all of the rats were included in the analysis. A representative histological plate of GFP expression in the NAc shell 4 days following infusion of the 5-HT1B virus is shown in Fig. 1B and a representative section of cresyl violet staining of the injection tract is shown in Fig. 1D.
Locomotor sensitization
The effect of mild stress exposure during increased 5-HT1B receptor expression in the NAc on the acute psychomotor activating effects of amphetamine and the development of locomotor sensitization were measured using locomotor activity boxes (22 × 45 × 23 cm; San Diego Instruments, San Diego, CA). Briefly, 24 h following viral infusion rats received a 4-day stress treatment. It is well-established that the mild stressors used for this treatment (exposure to novelty and saline injections) elicit HPA responses. On Day 1 and Day 4, all rats were placed in a novel test cage for a 1 h habituation period, followed by an injection of saline. Behavior was recorded for an additional 90 min and then all rats were returned to their home cage. On Day 2 and Day 3, rats received an injection of saline in their home cage. For the acute locomotor dose response experiment, ten days following the last day of stress treatment (i.e., Day 14) rats received an escalating dose regimen of amphetamine (0, 0.5 and 2 mg/kg ip). For this acute dose response test, all rats were given a 1 h habituation period to the test cage, followed by an injection of saline. Sixty min later rats were given an injection of 0.5 mg/kg amphetamine, followed 90 min later by an injection of 2 mg/kg amphetamine. Behavior was recorded for an additional 90 min. Additional control groups that received 5-HT1B or GFP viral infusions but not stress treatment were also given the acute locomotor dose response test. For the locomotor sensitization experiment, ten days following the last day of stress treatment (i.e., Day 14) rats received a 4-day drug treatment designed to induce mild locomotor sensitization to amphetamine. On Day 1 and Day 4, all rats were placed into the locomotor activity cages for a 1 h habituation period, followed by an injection of 0.5 mg/kg amphetamine or saline. Behavior was recorded for an additional 90 min and then all rats were returned to their home cage. On Day 2 and Day 3, rats received an injection of 5 mg/kg amphetamine or saline in their home cage. One week after the last amphetamine treatment, rats were given an escalating dose challenge of amphetamine (saline, 0.5 and 2 mg/kg) in order to test for locomotor sensitization. The protocol used was identical to that for the acute dose response test. The number of cage crossovers, defined as two consecutive beam breaks; photobeams spaced 2″ apart, was used as an index of locomotor activity.
Stereotypy
The effect of mild stress exposure during increased 5-HT1B receptor expression in the NAc on amphetamine-induced stereotypy was evaluated in the locomotor activity boxes using a 9-point rating scale adapted from Dougherty and Ellinwood (1983). During the challenge test of the locomotor sensitization experiment, an experimenter blind to the experimental conditions observed the rats for 30 sec every 5 min over a 90 min period following injection of 2 mg/kg amphetamine. Rats were given a stereotypy rating score during each observation (1–asleep; 2–inactive; 3–normal in place activity; 4–normal, alert, active; 5–hyperactive; 6–slow patterned stereotyped behaviors; 7–fast patterned stereotyped behaviors; 8–restricted stereotyped behaviors; 9–dyskinetic-reactive).
Statistical Analysis
Group differences in locomotor activity and stereotypy were tested using two-way analyses of variance (ANOVAs) with repeated measures followed by Bonferroni’s post-hoc tests or t-tests. For the locomotor activity dose response curves (Fig. 3A, Fig. 4A and Fig. 6B), statistical analysis of the two amphetamine doses was restricted to the first 60 min in order to permit comparisons to saline. For all comparisons, α = 0.05.
Figure 3.
Stress exposure during increased NAc 5-HT1B receptor expression subsequently enhances the acute locomotor response to amphetamine. (A) The mean (± SEM) number of crossovers made during the first 60 min of each treatment of the multiple-dose acute locomotor response test (habituation (Hab), followed 60 min later by saline treatment (Sal), followed 60 min later by 0.5 and 2 mg/kg amphetamine, injections spaced 90 min apart). Grey circles represent the GFP group and black squares represent the 5-HT1B group. *, differs from the GFP group (p < 0.05, Two-way repeated measures ANOVA, Bonferroni’s test). (B) Illustration of experimental design. (c) The mean (± SEM) number of crossovers made over time (6-min intervals) during the acute locomotor response test. N = 9–11/group.
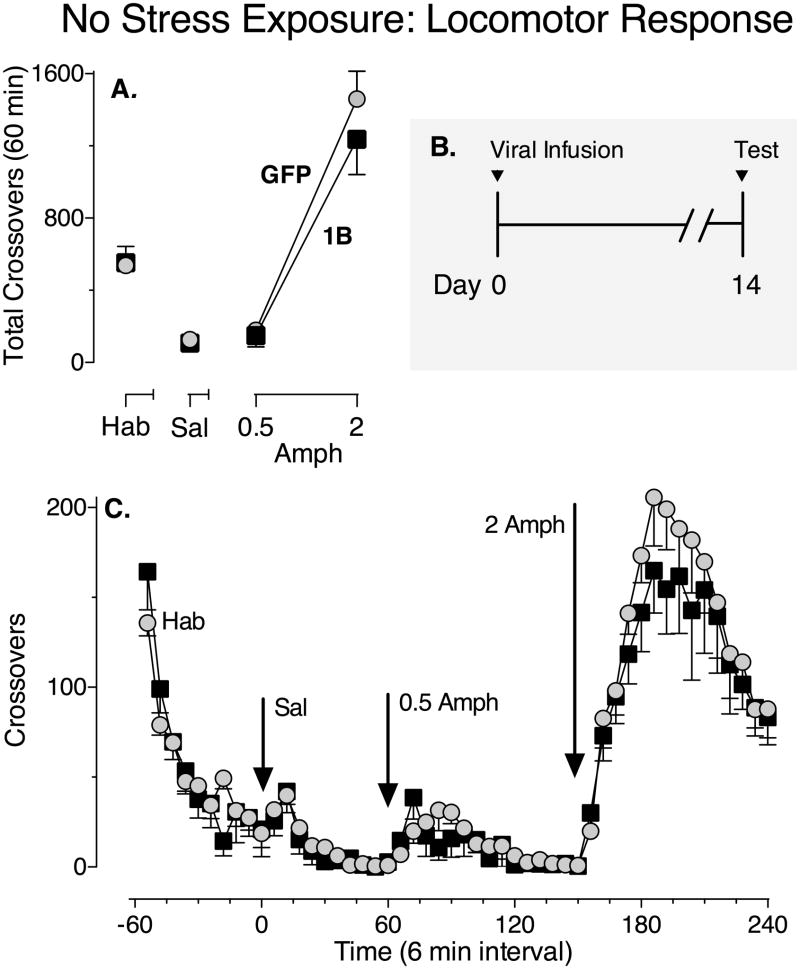
Figure 4.
Increased expression of 5-HT1B receptors in the NAc without stress exposure does not alter the acute locomotor response to amphetamine. (A) The mean (± SEM) number of crossovers made during the first 60 min of each treatment of the multiple-dose acute locomotor response test (habituation (Hab), followed 60 min later by saline treatment (Sal), followed 60 min later by 0.5 and 2 mg/kg amphetamine (Amph), injections spaced 90 min apart). Grey circles represent the GFP group and black squares represent the 5-HT1B group. (B) Illustration of experimental design. (C) The mean (± SEM) number of crossovers made over time (6-min intervals) during the acute locomotor response test. (p > 0.05, Two-way repeated measures ANOVA). N = 7–9/group.
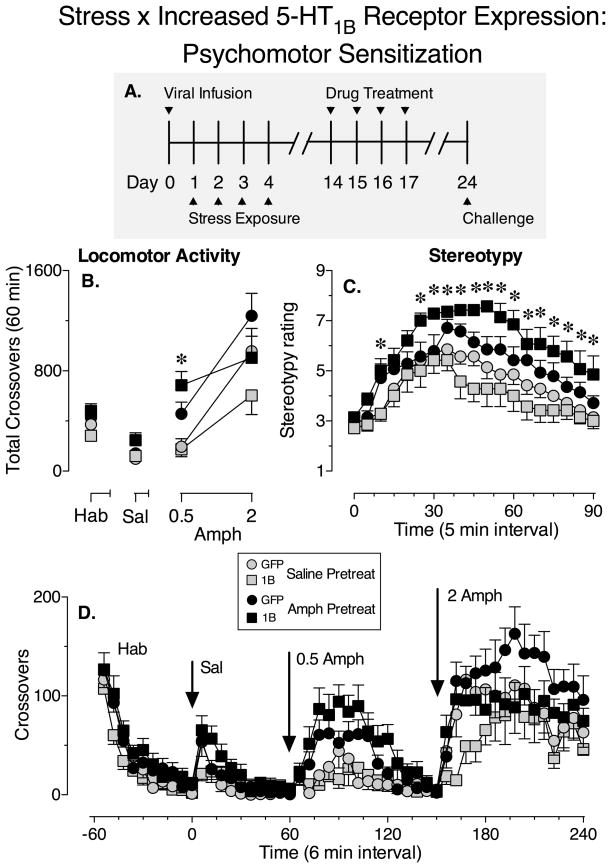
Figure 6.
Stress exposure during increased NAc 5-HT1B receptor expression subsequently facilitates the development of psychomotor sensitization to amphetamine. (A) Illustration of experimental design. (B) The mean (± SEM) number of crossovers made during the first 60 min of each treatment of the multiple-dose challenge test for sensitization (habituation (Hab), followed 60 min later by saline treatment (Sal), followed 60 min later by 0.5 and 2 mg/kg amphetamine, injections spaced 90 min apart). Grey symbols represent animals that received saline pretreatment and black symbols represent animals that received amphetamine pretreatment. Circles represent GFP groups and squares represent 5-HT1B groups. (C) The mean (± SEM) stereotypy rating (using a 9-point rating scale) over time (one rating every 5 min) for the 90 min period following treatment with 2 mg/kg amphetamine. (D) The mean (± SEM) number of crossovers made over time (6-min intervals) during the challenge test. *, differs from the saline pretreated/1B group (p < 0.05, Two-way repeated measures ANOVA, Bonferroni’s test). N = 7–10/group.
Results
Increasing 5-HT1B receptor expression in the NAc does not alter stress-induced locomotor activity
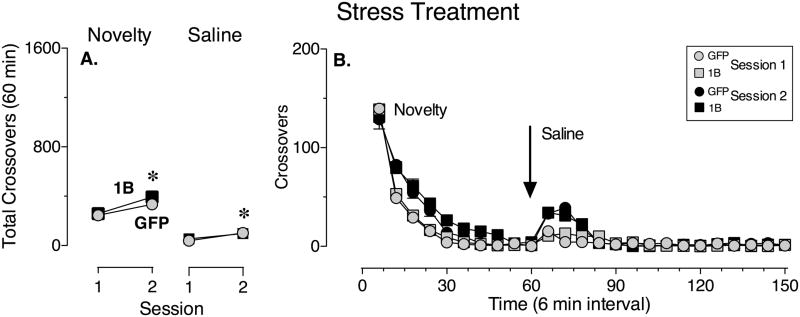
Figure 2 shows the effect of viral-mediated increased expression of 5-HT1B receptors in the NAc on the locomotor response to a compound mild stressor (i.e., exposure to novelty followed by an injection of saline) over test sessions. Repeated exposure to each stressor produced an increase in locomotor activity over sessions that was significantly greater on Session 2 compared to Session 1 (Fig. 2A; main effect of Session: Novelty, F1,51 = 39.82, P < 0.0001; Saline, F1,51 = 37.36, p < 0.0001), indicating that all of the rats had a greater level of arousal on Session 2 and did not habituate to the stressors across the two test sessions. However, there were no significant differences between the GFP control group and the 5-HT1B group in the number of crossovers on Session 1 or on Session 2 (Fig. 2A; main effect of Virus: Novelty, F1,51 = 1.51, P = 0.22; Saline, F1,51 = 0.15, P = 0.70 and interaction between Session and Virus factors: Novelty, F1,51 = 1.61, P = 0.21; Saline, F1,51 = 1.15, P = 0.29 not significant). Thus, the locomotor response to mild stress exposure was similar for each group. Figure 2 (panel B) illustrates the time course of the effect of mild stress exposure on locomotor activity in GFP control and 5-HT1B groups on Session 1 and Session 2.
Figure 2.
Increased expression of 5-HT1B receptors in the NAc has no effect on stress- induced locomotor activity. (A) The mean (± SEM) number of crossovers made during Session 1 and Session 2 of the stress treatment following exposure to novelty (left panel) and after a saline injection (right panel). Grey circles represent animals that received GFP-only control viral infusions in the NAc shell (GFP) and black squares represent animals that received viral infusions of the HA-5-HT1B receptor transgene (1B). (B) The mean (± SEM) number of crossovers made over time (6-min intervals) during Session 1 (grey symbols) and Session 2 (black symbols) of the stress treatment. Circles represent the GFP group and squares represent the 1B group. *, differs from Session 1 (p < 0.05, Two-way repeated measures ANOVA, Bonferroni’s test). N = 26–27/group.
Stress exposure during increased NAc 5-HT1B receptor expression subsequently enhances the acute locomotor response to amphetamine
Ten days following the stress treatment, the acute locomotor response to a multiple dose regimen of amphetamine (saline, 0.5 and 2 mg/kg) was assessed in GFP and 5-HT1B animals (Fig. 3, see panel B for illustration of experimental design). The timing of the amphetamine test coincides with dissipation of the viral-mediated increases in gene expression and allows for the determination of whether increasing 5-HT1B receptor expression during stress exposure impacts the subsequent reactivity to amphetamine. Administration of amphetamine produced a significant increase in crossovers following the highest dose tested (2 mg/kg) compared to saline (Fig. 3A; main effect of Dose: F2,34 = 101.9, P < 0.0001). In addition, the increase in crossovers was greater in the 5-HT1B group compared to the GFP control group (Fig. 3A; main effect of Virus: F1,34 = 5.98, P = 0.03; interaction between Dose and Virus factors: F2,34 = 6.87, P = 0.003) indicating that pairing mild stress with increased 5-HT1B receptor expression made rats more susceptible to amphetamine after the transgene expression had dissipated. Figure 3 (panel C) illustrates the time course of the effect of amphetamine on locomotor activity in GFP control and 5-HT1B groups during the acute dose response test.
Since it is possible that the transient increase in 5-HT1B receptor expression could account for the subsequent enhanced responsiveness to amphetamine, we next tested whether infusion of GFP or 5-HT1B viral vectors without stress exposure affected the acute locomotor response to amphetamine 14 d later (after transgene expression dissipated; Fig. 4, see panel B for illustration of experimental design). Administration of amphetamine produced a significant increase in crossovers following the highest dose tested (2 mg/kg) compared to saline (Fig. 4A; main effect of Dose: F2,28 = 125.6, P < 0.0001). However, there were no significant differences between the GFP group and the 5-HT1B group in the number of crossovers made following amphetamine administration (Fig. 4A; main effect of Virus: F1,28 = 0.72, P = 0.41 and interaction between Dose and Virus factors: F2,28 = 0.86, P = 0.44 not significant). Figure 4 (panel C) illustrates the time course of the effect of amphetamine on locomotor activity during the acute dose response test in GFP control and 5-HT1B groups that did not receive stress exposure. Since baseline activity can vary across experiments and prior stress exposure can alter behavioral responses in control groups, in order to permit direct comparison of the effects of combined stress and increased 5-HT1B receptor expression (1B/Stress) with those of increased 5-HT1B receptor expression alone (1B/No stress), the data from the 5-HT1B groups were normalized to their respective GFP control groups (Fig. 5).
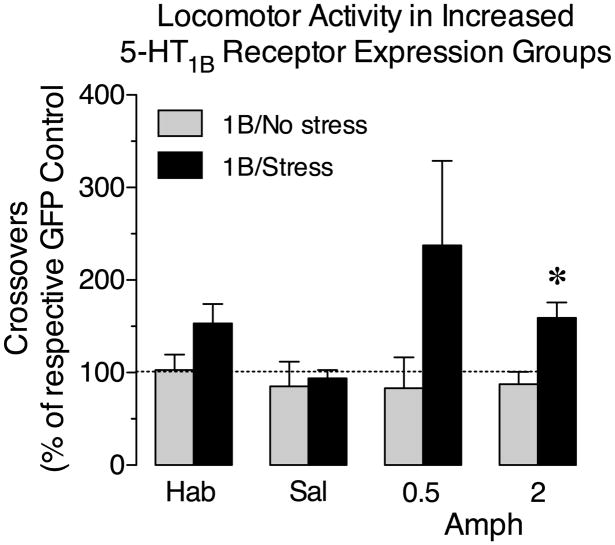
Figure 5.
Exposure to stress during increased expression of 5-HT1B receptors in the NAc is necessary for increasing susceptibility to the acute locomotor effects of amphetamine. Data are from the increased 5-HT1B receptor expression groups following each treatment of the multiple-dose acute locomotor response test (habituation (Hab), saline (Sal), 0.5 and 2 mg/kg amphetamine (Amph)) and are plotted as the mean (± SEM) percent of crossovers of the respective GFP control groups. Grey bars represent the 1B group that did not receive exposure to stress (1B/No stress) and black bars represent the 5-HT1B group that received the stress treatment (1B/Stress). *, differs from the 1B/No stress group (p < 0.05, two-tailed t-test). N = 7–10/group.
Although the locomotor response to amphetamine in the 5-HT1B/No stress group was not different from the GFP controls, the locomotor response to amphetamine in the 5-HT1B/Stress group was dramatically increased. In addition, the 5-HT1B/stress group made significantly more crossovers following treatment with 2 mg/kg amphetamine compared to the 5-HT1B/No stress group (t15 = 3.10, P = 0.007). The difference in crossovers between the two groups was not statistically significant during habituation (t15 = 1.40, P = 0.18), following saline (t15 = 0.34, P = 0.74), or following treatment with 0.5 mg/kg amphetamine (t15 = 1.36, P = 0.19). Thus, these data suggest that exposure to stress during increased 5HT1B receptor expression in the NAc is critical for alterations in vulnerability to the behavioral effects of amphetamine.
Stress exposure during increased 5-HT1B receptor expression in the NAc subsequently facilitates the development of psychomotor sensitization to amphetamine
Ten days following the stress treatment, a subset of the GFP and 5-HT1B animals received a 4-day treatment regimen of amphetamine or saline. This mild amphetamine dosing procedure is on the threshold for producing sensitization in control rats, and was used to increase sensitivity for detecting enhancement of sensitization in the experimental group. One week later, psychomotor sensitization (i.e., locomotor activity and stereotypy) was assessed with a multiple dose challenge test of amphetamine (saline, 0.5 and 2 mg/kg) (Fig. 6; see panel A for illustration of experimental design). At the lower dose of amphetamine tested (0.5 mg/kg), there was a significant increase in crossovers in animals that had been pretreated with amphetamine compared to the saline pretreatment groups (Fig. 6B; main effect of Pretreatment: F1,30 = 16.73, P = 0.0003); however, there were no significant differences in crossovers between the saline-pretreated or amphetamine-pretreated GFP and 5-HT1B groups (Fig. 6B; main effect of Virus: F1,30 = 1.20, P = 0.28 and interaction between Pretreatment and Virus factors: F1,30 = 1.72, P = 0.2 not significant). Bonferroni’s post-hoc tests revealed that there were no significant differences between the GFP groups, however, 5-HT1B animals that had previously been treated with amphetamine made significantly more crossovers compared to the 5-HT1B animals that had previously been treated with saline indicating that locomotor sensitization had developed in this group.
At the higher dose of amphetamine tested (2 mg/kg) there were no significant differences in locomotor activity between groups (Fig. 6B; main effect of Pretreatment: F1,30 = 2.30, P = 0.09; main effect of Virus: F1,30 = 3.35, P = 0.08 and interaction between Pretreatment and Virus (F1,30 = 0.02, P = 0.88 not significant). However, repeated treatment with low to moderate doses of psychostimulant drugs can produce stereotyped behaviors, which would decrease locomotion and mask locomotor sensitization to a moderate challenge dose of amphetamine (Post and Rose, 1976; Robinson and Becker, 1986). Indeed, 2 mg/kg amphetamine induced stereotypy in animals that had been previously treated with amphetamine compared to the saline pretreatment groups for all time points except three (0, 5 and 20 min post drug injection) (Fig. 6C; main effect of Pretreatment: F1,24 = 4.53–22.53, P < 0.05). Although there were no differences between GFP groups, at the 10 min time interval and intervals ranging from 25–90 min the 5-HT1B animals that had previously been treated with amphetamine scored significantly higher stereotypy ratings compared to the 5-HT1B animals that had previously been treated with saline (Fig. 6C; interaction between Pretreatment and Virus factors: F1,24 = 4.63–10.8, P < 0.05). These data suggest that sensitization in the 5-HT1B group was also evident at 2 mg/kg amphetamine challenge dose. The differences seen following the amphetamine treatments are unlikely to be a result of overall changes in locomotor activity produced by the amphetamine history or viral injection because there were no significant differences between groups following saline treatment (Fig. 6B; main effect of Virus: F1,30 = 1.90, P = 0.17; main effect of Pretreatment: F1,30 = 3.45, P = 0.07; interaction between Virus and Pretreatment factors: F1,30 = 0.83, P = 0.37) not significant. Thus, these data indicate that stress exposure during increased NAc 5HT1B receptor expression facilitates the development of psychomotor sensitization to amphetamine. Figure 6 (panel D) illustrates the time course of the effect of amphetamine on locomotor activity in GFP and 1B groups during the challenge dose response test.
Discussion
Both genetic and environmental factors can have a huge impact on the effects of psychostimulant drugs, but the way in which interactions between these factors influence addiction remains to be elucidated (Palomo et al., 2004; Kreek et al., 2005). In the present study we found that increasing expression of 5-HT1B receptors on the terminals of NAc shell neurons that project to the VTA and repeatedly exposing rats to mild stress (novelty + saline injection) subsequently enhances the acute locomotor activating effects of amphetamine. In addition, the development of psychomotor sensitization–both locomotor activity and stereotypy components - is facilitated. Importantly, these results are not simply due to an overall increase in activity of the 5-HT1B receptor overexpression groups because their locomotor response to both novelty and saline stress during the test sessions did not differ from the GFP controls. These findings are consistent with earlier reports from our laboratory that increased expression of 5-HT1B receptors in the NAc augments the acute locomotor activating effects of cocaine and modulates drug reward (Neumaier et al., 2002; Barot et al., 2007). However, it is unlikely that the increased susceptibility to the behavioral effects of amphetamine seen in the present experiments was simply due to an increase in 5-HT1B receptors on NAc projections because the amphetamine treatment and testing occurred once the viral-mediated increased expression in 5-HT1B receptors had dissipated. Furthermore, increasing gene expression without stress exposure was not sufficient to produce alterations in the behavioral effects of amphetamine. Thus, while the effects of our previous data were most likely due to the direct stimulation of transgenic 5-HT1B receptors following cocaine’s blockade of the serotonin transporter, the present results suggest that serotoninergic signaling through 5-HT1B receptors can also play an important role in mediating the behavioral effects of psychostimulant drugs indirectly via interactions with stress responses.
Repeated exposure to stress can lead to a cross-sensitivity to the psychomotor activating effects of drugs, including a facilitation of behavioral sensitization (Kalivas and Stewart, 1991). Nonetheless, in the present study not only were the GFP control groups and the 5-HT1B groups exposed to the same level of stress, but the behavioral response to the stress did not differ between these groups (i.e., both groups showed a small but significant increase in the locomotor response to the stress treatment itself). In addition, the GFP animals that received this relatively mild stress exposure prior to amphetamine treatment did not show a sensitized response during the amphetamine challenge test compared to GFP animals that were receiving amphetamine for the first time. Thus, the augmentation in amphetamine-induced behaviors seen in the 5-HT1B group also cannot be attributed to the stress experience alone. Instead, the data suggests that the critical variable for facilitating amphetamine-induced behavioral plasticity was the interaction between increasing 5-HT1B receptors in the NAc and the stress experience.
The mechanism by which 5-HT1B-stress interactions can influence vulnerability to drugs is not yet known. However, activation of 5-HT1B heteroreceptors in the VTA (i.e., the population of receptors that was transiently increased in the present experiments) inhibits GABA release, which leads to a disinhibition of dopaminergic neurons and a subsequent increase in dopamine release into the NAc (Cameron and Williams, 1994, 1995; Yan, 2001a,b; O’Dell and Parsons, 2004). Interestingly, glucocorticoids secreted following stress can potentiate psychostimulant-induced increases in NAc dopamine levels, an effect that is blocked by antagonism of 5-HT1B receptors in the VTA (Amato et al., 2007). Moreover, both chronic stress and repeated psychostimulant treatment upregulate mRNA levels of 5-HT1B receptors in the NAc (Hoplight et al., 2007). Exposure to stress can also increase serotonin levels in the striatum (Kirby et al., 1997; Noguchi et al., 2001). We hypothesize, therefore, that in the present experiments the viral-mediated amplification in 5-HT1B receptor levels increased the responsitivity of GABAergic NAc projection neurons to stress-induced serotonin release. This would reduce GABA release in the VTA, resulting in increased dopamine neuron activation and augmented dopamine release in the NAc. Activation of this mesolimbic dopamine system has been associated with some of the key neuroadaptations thought to underlie addiction (Robinson and Berridge, 2000; Nestler, 2001; Saal et al., 2003). It is probable, then, that this 5-HT1B receptor-mediated magnification of the normal neurobiological response to stress led to adaptive changes in the brain that subsequently enhanced the initial response to psychostimulant drugs and facilitated behaviors associated with addiction. Future studies that directly measure dopamine levels are warranted to confirm this hypothesis.
In humans, there are multiple risk factors that can lead to the development of drug addiction. Although studies have linked 5-HT1B receptor polymorphisms to substance abuse (Huang et al., 2003; Proudnikov et al., 2006), this is not always the case (Cigler et al., 2001). While these discrepancies in the literature could be due to the multitude of polymorphisms that exist, another intriguing possibility is that other risk factors, one of which is stress, could determine the impact that a genetic predisposition can have on vulnerability to addiction. Just as with the risk for depression and suicidality (Caspi et al., 2003), evaluating the genetics of serotonin neurotransmission on addiction without considering the history of stress exposure may not fully reflect the impact of individual differences in serotonergic function. Our results are consistent with this idea because only when increased NAc 5-HT1B receptor expression was combined with mild stress exposure did enhanced susceptibility to the behavioral effects of psychostimulant drugs ensue. Nonetheless, future studies examining how stable alterations in 5-HT1B receptor activity interact with stress to change addiction liability will be necessary to directly examine this hypothesis and determine whether coupling these factors increases the likelihood of becoming an addict.
Acknowledgments
This research was supported by a NIDA grant to JFN (DA16432). SMF was supported by a NIDA individual NRSA (DA210090).
References
- Amato JL, Bankson MG, Yamamoto BK. Prior exposure to chronic stress and MDMA potentiates mesoaccumbens dopamine release mediated by the 5-HT(1B) receptor. Neuro psychopharmacology. 2007;32:946–954. doi: 10.1038/sj.npp.1301174. [DOI] [PubMed] [Google Scholar]
- Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25:3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- Ronsert MR, Meade AN, Hen R, Rocha BA. Amphetamine-induced locomotor activation in 5-HT1B knockout mice: effects of injection route on acute and sensitized responses. Behav Pharm. 2001;12:549–555. doi: 10.1097/00008877-200111000-00017. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J Neurosci. 1994;14:6763–6767. doi: 10.1523/JNEUROSCI.14-11-06763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Opposing roles for dopamine and serotonin at presynaptic receptors in the ventral tegmental area. Clin Exp Pharmacol Physiol. 1995;22:841–845. doi: 10.1111/j.1440-1681.1995.tb01947.x. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Paolone G, Badiani A. Modeling the role of environment in addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1639–1653. doi: 10.1016/j.pnpbp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ, Neve RL. Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit Rev Neurobiol. 2000;14:47–67. doi: 10.1080/08913810008443546. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cigler T, LaForge KS, McHugh PF, Kapadia SU, Leal SM, Kreek MJ. Novel and previously reported single-nucleotide polymorphisms in the human 5-HT(1B) receptor gene: no association with cocaine or alcohol abuse or dependence. Am J Med Genet. 2001;105:489–497. doi: 10.1002/ajmg.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Neumaier JF. The 5-HT1B receptor: behavioral implications. Psychopharmacol Bull. 2001;35:170–85. [PubMed] [Google Scholar]
- Clark MS, Sexton TJ, McClain M, Root D, Kohen R, Neumaier JF. Overexpression of 5-HT1B receptor in dorsal raphe nucleus using Herpes Simplex Virus gene transfer increases anxiety behavior after inescapable stress. J Neurosci. 2002;22:4550–4562. doi: 10.1523/JNEUROSCI.22-11-04550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty GG, Ellinwood EH., Jr Influence of gamma-butyrolactone on behavior due to dopaminergic drugs. Physiol Behav. 1983;30:607–612. doi: 10.1016/0031-9384(83)90228-7. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Mitchell ES, Neumaier JF. Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol Psychiatry. 2008;63:207–213. doi: 10.1016/j.biopsych.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Azampanah A, Korth KM. Activation of 5-HT(1B) receptors in the nucleus accumbens reduces self-administration of amphetamine on a progressive ratio schedule. Pharmacol Biochem Behav. 2002;71:717–725. doi: 10.1016/s0091-3057(01)00717-1. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF. Cocaine increases 5-HT1B mRNA in rat nucleus accumbens shell neurons. Neuropharmacology. 2007;52:444–449. doi: 10.1016/j.neuropharm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Huang Y, Oquendo M, Friedman J, Greenhill L, Brodsky B, Malone K, Khait V, Mann J. Substance abuse disorder and major depression are associated with the human 5-HT1B receptor gene (HTRIB) G861C Polymorphism. Neuropsychopharmacology. 2003;28:163–169. doi: 10.1038/sj.npp.1300000. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Mercuri NB, North RA. 5-hydroxytryptamine1B receptors block the GABAB synaptic potential in rat dopamine neurons. J Neurosci. 1992;12:2000–2006. doi: 10.1523/JNEUROSCI.12-05-02000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT(1B) auto- and heteroreceptors. Mol Pharmacol. 2000;58:1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- Muller CP, Huston JP. Determining the region-specific contributions of 5-HT receptors to the psychostimulant effects of cocaine. Trends Pharmacol Sci. 2006;27:105–112. doi: 10.1016/j.tips.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA., Jr Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Yoshida Y, Chiba S. Effects of psychological stress on monoamine systems in subregions of the frontal cortex and nucleus accumbens of the rat. Brain Res. 2001;916:91–100. doi: 10.1016/s0006-8993(01)02868-2. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- Palomo T, Kostrzewa RM, Beninger RJ, Archer T. Gene-environment interplay in alcoholism and other substance abuse disorders: expressions of heritability and factors influencing vulnerability. Neurotox Res. 2004;6:343–361. doi: 10.1007/BF03033309. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–732. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Proudnikov D, LaForge KS, Hofflich H, Levenstien M, Gordon D, Barral S, Ott J, Kreek MJ. Association analysis of polymorphisms in serotonin 1B receptor (HTR1B) gene with heroin addiction: a comparison of molecular and statistically estimated haplotypes. Pharmacogenet Genomics. 2006;16:25–36. doi: 10.1097/01.fpc.0000182782.87932.d6. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Siwanowicz J, Nowak E, Papla I, Filip M. Role of 5-HT(1B) receptors in the sensitization to amphetamine in mice. Eur J Pharmacol. 2001;422:91–99. doi: 10.1016/s0014-2999(01)01079-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharm (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Yan QS, Yan SE. Serotonin-1B receptor-mediated inhibition of [(3)H]GABA release from rat ventral tegmental area slices. J Neurochem. 2001a;79:914–922. doi: 10.1046/j.1471-4159.2001.00643.x. [DOI] [PubMed] [Google Scholar]
- Yan QS, Yan SE. Activation of 5-HT(1B/1D) receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. Eur J Pharmacol. 2001b;418:55–64. doi: 10.1016/s0014-2999(01)00913-x. [DOI] [PubMed] [Google Scholar]