Abstract
Background
Multiple sclerosis (MS) is characterized by a polyspecific B-cell response to neurotropic viruses such as measles, rubella and varicella zoster, with the corresponding antibodies measurable in CSF as the so-called “MRZ reaction” (MRZR). We aimed to evaluate the relevance of MRZR to predict conversion of patients with clinically isolated syndrome (CIS) to MS, and to compare it to oligoclonal bands (OCB) and MRI.
Methodology/Principal Findings
MRZR was determined in a prospective study over 2 years including 40 patients that remained CIS over follow-up (CIS-CIS) and 49 patients that developed MS (CIS-RRMS) using ELISA. Using logistic regression, a score (MRZS) balancing the predictive value of the antibody indices included in MRZR was defined (9 points measles, 8 points rubella, 1 point varicella zoster, cutpoint: sum of scores greater 10).
MRZR and MRZS were significantly more frequent in CIS-RRMS as compared to CIS-CIS (p = 0.04 and p = 0.02). MRZS showed the best positive predictive value (PPV) of all parameters investigated (79%, 95%-CI: 54–94%), which could be further increased by combination with MRI (91%, 95%-CI: 59–99%).
Conclusions/Significance
Our data indicate the relevance of MRZR to predict conversion to MS. It furthermore shows the importance of weighting the different antibody indices included in MRZR and suggest that patients with positive MRZR are candidates for an early begin of immunomodulatory therapy.
Introduction
In over 80% of patients who later develop multiple sclerosis (MS), the disease initially presents with an episode of neurological symptoms due to a single demyelinating lesion known as clinically isolated syndrome (CIS) [1]. Given the importance of an early treatment of MS, the clinical challenge in patients with CIS is to identify patients at risk of future events that would confirm the diagnosis of definite MS [2], [3]. Consequently, there is an ongoing search for biomarkers that could help to evaluate the prognosis in CIS. Several magnetic resonance imaging (MRI) criteria as well as biochemical markers have been investigated as possible predictors of conversion from CIS to definite MS [4]–[9]. Besides history and clinical findings, magnetic resonance imaging (MRI) has become the most important tool to establish the diagnosis of MS. However, as shown in a meta-analysis evaluating the use of MRI in the diagnosis of MS, MRI studies tend to produce higher estimates of sensitivity and lower estimates of specificity particularly in short-term studies [10]. Cerebrospinal fluid (CSF) is a promising source of biochemical markers in MS, since the CSF compartment is in close anatomical contact with the brain interstitial fluid, where biochemical changes related to the disease are reflected [11], [12]. It has recently been demonstrated that oligoclonal bands (OCB) are an independent risk factor in CIS implementing an almost two-fold increased risk of having a second attack in all patients independent of MRI [9]. CSF of patients with MS is characterized by a polyspecific, intrathecal B-cell response with prominent antibody production against neurotropic viruses such as measles, rubella and varicella zoster, the so-called “MRZ reaction” (MRZR), which was shown to be detectable in CSF of 80–100% of patients with MS [13]–[20]. As shown in a small study on optic neuritis, MRZR appears to be more specific than detection of OCB for conversion to MS [19]. In the present study, we used an assay optimized for CSF measurement in a highly standardized manner to determine the prevalence and prognostic relevance of MRZR in CIS regarding conversion to MS in comparison to other markers like OCB and MRI lesion load [4].
Methods
Patients
In a prospective study of the Department of Neurology, University of Ulm (Germany), we collected CSF samples from patients with CIS that remained CIS (CIS-CIS) over a follow-up of 2 years and from patients with CIS that developed definite MS of the relapsing-remitting subtype (CIS-RRMS) over the same period [2] (Table 1). Informed consent was obtained from all patients, and the study was approved by the local ethics committee.
Table 1. Demographic data, CSF and MRI findings in patients with clinically isolated syndrome (CIS).
| CIS all | CIS-CIS | CIS-RRMS | S* | ||
| n (female/male) | 89 (56/33) | 40 (22/18) | 49 (34/15) | NS | |
| Age [years] | Median (Range) | 38.5 (13.1–70.9) | 38.4 (16.8–70.9) | 39.4 (13.1–63.6) | NS |
| EDSS | Median (Range) | 2 (0–5) | 2 (0–5) | 3 (0–5) | NS |
| Measles AI≥1.5 | n (%) | 37 (42) | 11 (33) | 26 (59) | NS |
| Rubella AI ≥1.5 | n (%) | 32 (36) | 9 (24) | 23 (49) | p = 0.03 |
| Zoster AI ≥1.5 | n (%) | 32 (36) | 12 (32) | 20 (42) | NS |
| MRZR | n (%) | 33 (37) | 10 (25) | 23 (47) | p = 0.04 |
| MRZS | n (%) | 19 (21) | 4 (10) | 15 (31) | p = 0.018 |
| OCB | n (%) | 74 (83) | 27 (68) | 47 (96) | p = 0.001 |
| MRI | n (%) | 59 (66) | 21 (53) | 38 (78) | p = 0.02 |
| Barkhof criteria | n (%) | 25 (28) | 8 (20) | 17 (35) | p = 0.125 |
CIS all = all patients with CIS, CIS-CIS = patients with CIS that remained CIS over follow-up, CIS-RRMS = CIS patients with conversion to MS over follow-up, EDSS = Kurtzke Expanded Disability Status Scale, AI = antibody index, MRZR = AI for measles, rubella, zoster, two or more AI ≥1.5, MRZS = MRZ score >10, OCB = oligoclonal bands in cerebrospinal fluid, MRI = two or more lesions in T2-weighted magnetic resonance imaging of the brain. NS = not significant, S = statistical significance. * CIS-CIS vs. CIS-RRMS
CSF Basic Analysis and Determination of MRZR
CSF leukocyte count (cells/cu.mm), total protein (g/L), lactate (mmol/L), the albumin CSF/serum concentration ratio (Qalb), immunoglobulin G, A and M and OCB were obtained as previously described [29], [30], [31].
Antibody levels against measles (M), rubella (R) and zoster (Z) were determined using an ELISA according to the instructions as supplied by the manufacturer (Genzyme Virotech, Rüsselsheim, Germany; CE certificate). Of note is that CSF and serum were diluted into a similar protein concentration range allowing a measurement in a similar region of the standard curve. Quantitative expressions of the intrathecal immune response were based on calculation of the CSF/serum quotients (Q) of specific antiviral IgG antibodies (IgG[spec]) and total IgG (IgGtotal): QIgG[spec] = IgGspec[CSF]/IgGspec[serum], and QIgG[total] = IgGtotal[CSF]/IgGtotal[serum]). The intrathecal synthesis of antibodies to M, R and Z was detected by calculation of the corresponding antibody indices (AI): AI = QIgG[spec]/QIgG[total]. In case of an overall intrathecal synthesis of immunoglobulin above the reference range (Qlim), the Qlim was used instead of the QIgG[total]: AI = QIgG[spec]/Qlim, if QIgG[total] > Qlim [32]. The upper reference range of QIgG[total], Qlim, was calculated according to Reiber's formula [32]. AI values ≥1.5 were considered to be indicative of intrathecal IgG synthesis against the respective pathogen [17], [18], [32]. MRZR was considered positive if two or more AI values were ≥1.5.
MRI Analysis
MRI scans of the brain and spinal cord were performed on a 1.5 tesla whole-body MRI (Symphony Siemens, Erlangen, Germany) according to a previously fixed protocol including T1-weighted spin-echo (SE) axial slices with and without application of gadolinium-DTPA as well as T2-weighted SE axial slices. Hyperintense lesion on T2-weighted MRI were analyzed for lesions >3 mm2 and quantified on hardcopies. For this study, the presence of ≥2 lesions suggestive of MS in T2 weighted MRI (MRI ≥2 lesions) as well as Barkhof criteria [4] were applied as diagnostic criteria. Spinal lesions were accepted as infratentorial lesions in our application of MRI criteria.
Statistical Analysis
Absolute and relative frequencies were given for discrete variables, median and range for continuous variables. Differences between CIS-CIS and CIS-RRMS were analyzed by Chi-Square test and Mann-Whitney U-Test respectively on an univariate basis in an exploratory sense. P-values below 0.05 were considered to be significant.
A score of the effects of measles, rubella and varicella zoster antibodies (MRZS) was defined by logistic regression analysis with weights for the single antibodies calculated by using the regression coefficients [33]. A cutoff value for the score to achieve the diagnostic characteristics was chosen on the basis of a ROC-analysis.
Combinations of the variables will be examined for their diagnostic value. Sensitivity was calculated as (true-positive/[true-positive + false-negative]), specificity was calculated as (true-negative/[true-negative + false-positive]). The positive predictive value (PPV) was calculated as (true-positive/[true-positive + false-positive]), and the negative predictive value (NPV) as (true-negative/[true-negative + false-negative]). For all diagnostic values the exact 95% confidence intervals were given [34].
Results
Demographic Data Analysis
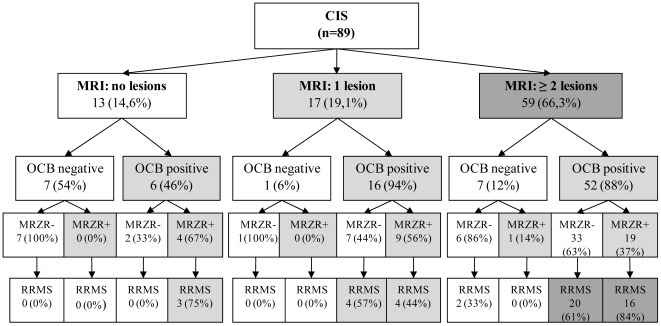
In total 89 patients with CIS were investigated (Table 1). Fourty-nine of these patients developed an MS within a time span of two years. The other 40 did not develop MS according to McDonald criteria (Figure 1) [2]. There was no significant difference in age, gender and EDSS distribution of these groups.
Figure 1. MRZR in patients with CIS.
Figure shows biomarkers that help to evaluate the risk of multiple sclerosis in patients with clinically isolated syndrome (CIS). MRI = lesion load in T2-weighted magnetic resonance imaging (lesions >3 mm2), OCB = oligoclonal bands in cerebrospinal fluid (CSF), MRZR = MRZ reaction, antibodies to measles, rubella and varicella zoster virus in CSF with two or more AI (antibody indices) ≥1.5. RRMS = relapsing-remitting multiple sclerosis.
Frequency of MRZ Antibodies in CIS
The conversion rate from CIS to definite MS over follow-up of 2 years was 55% (49/89). A positive MRZR was significantly more frequent in CIS-RRMS as compared to CIS-CIS (p = 0.04), as was a positive AI for rubella (p = 0.03), OCB in CSF (p = 0.001) and MRI ≥2 lesions (p = 0.02) (Table 1). Intrathecal synthesis of IgG antibodies (AI ≥1.5) against measles virus was found in 42% of CIS patients, followed by antibody synthesis against rubella virus (36%) and varicella zoster virus (36%). MRZR was observed to be positive in 37% of all patients with CIS (Table 1). It was positive in 25% of CIS-CIS and in 47% of CIS-RRMS (Table 1). OCB were detected in 68% of CIS-CIS and in 96% of CIS-RRMS. Barkhof criteria were fulfilled in 28% of all patients with CIS and in 35% of CIS-RRMS, while two or more T2 hyperintense lesions in MRI were observed in 53% of CIS-CIS and in 78% of CIS-RRMS (Table 1).
MRZS
A score of the effects of measles (M), rubella (R) and varicella zoster (Z) antibodies was defined based on logistic regression analysis. Weights for the AI included in MRZR were calculated using the regression coefficients of M (β = 0.8922, p = 0.059), R (β = 0.8472, p = 0.090) and Z (β = 0.1127, p = 0.821) and rounded to 9 points for M, 8 points for R and 1 point for Z. This resulted in a MRZ-score (MRZS) between 0 and 18 points with possible results of 0, 1, 8, 9, 10, 17, 18 points. In the analysis the cutpoint of more than 10 points for MRZS was used to achieve the best PPV. A positive MRZS >10 points was significantly more frequent in CIS-RRMS as compared to CIS-CIS (p = 0.018, Table 1).
Predictive Value of MRZR and MRZS in CIS
Of all markers investigated, OCB showed the highest sensitivity for conversion of CIS to MS (96%), which could not be improved by adding any of the other parameters (Table 2). MRZS (90%) and Barkhof criteria (80%) [4] showed the highest specificity of all single parameters. Barkhof criteria showed a higher specificity and PPV than MRI ≥2 lesions. MRZS showed the best PPV (79%) for predicting conversion from CIS to MS of all single parameters and was superior to MRZR (70%) and Barkhof criteria (68%). PPV could be increased to 91% by combination of MRZS with MRI (≥2 lesions) or by combination of MRZS with MRI (≥2 lesions) and OCB (Table 2).
Table 2. Sensitivity, specificity, positive (PPV) and negative (NPV) predictive value in percent (exact 95% confidence interval in brackets) for CSF and MRI parameters regarding conversion of clinically isolated syndrome to definite multiple sclerosis.
| Sensitivity | Specificity | PPV | NPV | |
| OCB | 96 (86.0–99.5) | 33 (18.6–49.1) | 64 (51.5–74.4) | 87 (59.5–98.3) |
| MRI | 78 (63.4–88.2) | 48 (31.5–63.9) | 64 (50.9–76.5) | 63 (43.9–80.0) |
| Barkhof criteria | 35 (21.7–49.6) | 80 (64.4–91.0) | 68 (46.5–85.1) | 50 (37.2–62.8) |
| MRZR | 47 (32.5–61.7) | 75 (58.8–87.3) | 70 (51.3–84.4) | 54 (39.7–67.0) |
| MRZS | 31 (18.3–45.4) | 90 (76.3–97.2) | 79 (54.4–94.0) | 51 (39.2–63.6) |
| OCB+MRI | 73 (58.9–85.1) | 60 (43.3–75.1) | 69 (54.9–81.3) | 65 (47.5–79.8) |
| OCB+Barkhof | 33 (20.0–47.5) | 83 (67.2–92.7) | 70 (47.1–86.8) | 50 (37.4–62.6) |
| OCB+MRZR | 47 (32.5–61.7) | 78 (61.6–89.2) | 72 (53.3–86.3) | 54 (40.7–67.6) |
| OCB+MRZS | 31 (18.3–45.4) | 90 (76.3–97.2) | 79 (54.4–94.0) | 51 (39.2–63.6) |
| MRI+MRZR | 33 (20.0–47.5) | 90 (76.3–97.2) | 80 (56.3–94.3) | 52 (39.8–64.4) |
| Barkhof+MRZR | 14 (6.0–27.2) | 95 (83.1–99.4) | 78 (40.0–97.2) | 48 (36.2–59.0) |
| MRI+MRZS | 20 (10.2–34.3) | 98 (86.8–99.9) | 91 (58.7–99.8) | 50 (38.5–61.5) |
| Barkhof+MRZS | 10 (3.4–22.2) | 98 (86.8–99.9) | 83 (35.9–99.6) | 47 (35.9–58.3) |
| OCB+MRI+MRZR | 33 (20.0–47.5) | 93 (79.6–98.4) | 84 (60.4–96.6) | 53 (40.6–64.9) |
| OCB+Barkhof+MRZR | 14 (5.9–27.2) | 98 (86.8–99.9) | 88 (47.4–99.7) | 48 (36.9–59.5) |
| OCB+MRI+MRZS | 20 (10.2–34.3) | 98 (86.8–99.9) | 91 (58.7–99.8) | 50 (38.5–61.5) |
| OCB+Barkhof+MRZS | 10 (3.4–22.2) | 98 (86.8–99.9) | 83 (35.9–99.6) | 47 (35.9–58.3) |
MRZR = AI for measles, rubella, zoster, two or more AI ≥1.5, MRZS = MRZ score >10, OCB = oligoclonal bands in cerebrospinal fluid, MRI = two or more lesions in T2-weighted magnetic resonance imaging of the brain.
Discussion
OCBs indicating a polyspecific IgG response persist in the CSF of MS patients for many years [21], and MRZR was shown to be positive in 80–100% of patients with MS [13], [14]. However this finding was rarely evaluated systematically because of lack of CSF approved assays, that would allow a routine application which is not restricted to special laboratories [17]. The mechanisms underlying this “errant” intrathecal B-cell response in MS so far remain a matter of speculation [22]. A simultaneous co-infection of MS patients with different neurotropic viruses seems unlikely, and PCR for measles, rubella and varicella zoster virus was shown to be negative in MRZR positive patients with MS [13]. Measles, rubella, and varicella zoster infections are very common during childhood and may affect the brain more frequently than clinically observed [15]. They could induce the recruitment of long-lived memory B-cells, which CSF analyses have shown to constitute the majority of B-cells in the CSF [23]. The differentiation of memory B-cells into antibody secreting cells was suggested to occur independently of the antigen in a “bystander reaction” promoted by T-cells in MS [24], [25]. Consequently, the polyspecific intrathecal IgG response in MS is likely to mirror an enhanced B-cell-promoting environment, and may also reflect the individual's history of previous infections or immunizations [22]. This assumption is supported by the finding that in MS patients from Cuba a lower fraction of rubella-AI is observed, which may reflect a different immunization scheme in childhood [26].
Our data showed MRZR to be present in nearly half of CIS patients that later developed MS (Table 1, Figure 1). This finding supports the notion that immunological changes related to B-cell activation and intrathecal polyspecific IgG synthesis occur early on in the development of MS [22]. MRZR was significantly more frequent in CIS that developed MS over follow-up, indicating a prognostic relevance of MRZR in CIS. The occurrence of OCB in our patients was comparable to data in literature [9], [13], [15], [27], [28]. As expected, the sensitivity of MRI (≥2 T2-hyperintense lesions) was higher than observed for Barkhof criteria [4], though it showed a lower specificity for conversion to MS. In counting spinal MRI lesions as infratentorial lesions we went beyond the original brain-based Barkhof criteria [4], [33], but are in line with the current application of MRI criteria for diagnostics of MS [2].
The most relevant statistical parameters to predict conversion from CIS to MS are PPV and NPV, as the patient's disease status (CIS-CIS or CIS-RRMS) is unknown and the clinician needs to determine whether a positive/negative test result (for example the presence/absence of OCB in CSF) indicates that the patient really has/does not have MS. Our data showed MRZR to have the best PPV of all single parameters investigated and to be superior to Barkhof criteria [4], OCB and MRI (≥2 lesions in T2-weighted MRI).
So far studies investigating the relevance of MRZR in inflammatory diseases of the CNS did not weight the single AI included in MRZR [15], [18], [19]. Using logistic regression we found the prognostic relevance of varicella zoster to be much lower than the relevance of measles or rubella antibodies, which was found to be comparable. Consequently, we proceeded to define a score that would allow for the different prognostic relevance of the three AI included in MRZR. The PPV of the newly defined MRZS balancing the AI included in MRZ according to their predictive value was superior to MRZR and to all other single parameters (Table 2). Consequently, our data indicate the importance of weighting the different AIs included in MRZR. Our data suggest that maximum PPV can be achieved by combining MRZS with MRI (≥2 lesions), which showed the same PPV as combination of MRZS with MRI and OCB (Table 2).
Taken together, this study underlines the prognostic relevance of MRZR and MRZS to predict conversion to MS in patients with CIS. Our data indicate that CIS patients that have two or more T2-hyperintense lesions in MRI and have a positive MRZS are at highest risk to develop MS and should therefore be candidates for an early begin of an immunomodulatory therapy. Further studies are planned to confirm our findings and to evaluate a possible relevance of MRZR as a therapy response marker.
Acknowledgments
The authors thank the physicians who sent on suspect cases to our clinic.
Footnotes
Competing Interests: GR is a paid employee of Genzyme Virotech. She contributed to the manuscript by performing the ELISA assays blinded to all other data. She was not involved in study design, data analysis or the decision to publish this manuscript. All other authors have no competing interests to disclose. The authors hereby declare that this does not alter the adherence of all authors to the PLoS One policies on sharing data and materials.
Funding: This study was supported by funds from Genzyme Virotech, Germany. The funders had no role in study design and analysis, decision to publish, or preparation of the manuscript. The funder contributed to data collection by performing the ELISA assays blinded to the clinical data.
References
- 1.Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 2005;4:281–288. doi: 10.1016/S1474-4422(05)70071-5. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 3.Thrower BW. Clinically isolated syndromes: predicting and delaying multiple sclerosis. Neurology. 2007;68:S12–15. doi: 10.1212/01.wnl.0000277704.56189.85. [DOI] [PubMed] [Google Scholar]
- 4.Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120 (Pt 11):2059–2069. doi: 10.1093/brain/120.11.2059. [DOI] [PubMed] [Google Scholar]
- 5.Brettschneider J, Petzold A, Junker A, Tumani H. Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Mult Scler. 2006;12:143–148. doi: 10.1191/135248506ms1263oa. [DOI] [PubMed] [Google Scholar]
- 6.Korteweg T, Tintore M, Uitdehaag B, Rovira A, Frederiksen J, et al. MRI criteria for dissemination in space in patients with clinically isolated syndromes: a multicentre follow-up study. Lancet Neurol. 2006;5:221–227. doi: 10.1016/S1474-4422(06)70353-2. [DOI] [PubMed] [Google Scholar]
- 7.Kuhle J, Pohl C, Mehling M, Edan G, Freedman MS, et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N Engl J Med. 2007;356:371–378. doi: 10.1056/NEJMoa063602. [DOI] [PubMed] [Google Scholar]
- 8.Swanton JK, Rovira A, Tintore M, Altmann DR, Barkhof F, et al. MRI criteria for multiple sclerosis in patients presenting with clinically isolated syndromes: a multicentre retrospective study. Lancet Neurol. 2007;6:677–686. doi: 10.1016/S1474-4422(07)70176-X. [DOI] [PubMed] [Google Scholar]
- 9.Tintore M, Rovira A, Rio J, Tur C, Pelayo R, et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology. 2008;70:1079–1083. doi: 10.1212/01.wnl.0000280576.73609.c6. [DOI] [PubMed] [Google Scholar]
- 10.Whiting P, Harbord R, Main C, Deeks JJ, Filippini G, et al. Accuracy of magnetic resonance imaging for the diagnosis of multiple sclerosis: systematic review. BMJ. 2006;332:875–884. doi: 10.1136/bmj.38771.583796.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiber H. Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta. 2001;310:173–186. doi: 10.1016/s0009-8981(01)00573-3. [DOI] [PubMed] [Google Scholar]
- 12.Franciotta D, Columba-Cabezas S, Andreoni L, Ravaglia S, Jarius S, et al. Oligoclonal IgG band patterns in inflammatory demyelinating human and mouse diseases. J Neuroimmunol. 2008;200:125–128. doi: 10.1016/j.jneuroim.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Reiber H, Ungefehr S, Jacobi C. The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler. 1998;4:111–117. doi: 10.1177/135245859800400304. [DOI] [PubMed] [Google Scholar]
- 14.Felgenhauer K, Reiber H. The diagnostic significance of antibody specificity indices in multiple sclerosis and herpes virus induced diseases of the nervous system. Clin Investig. 1992;70:28–37. doi: 10.1007/BF00422934. [DOI] [PubMed] [Google Scholar]
- 15.Sindic CJ, Monteyne P, Laterre EC. The intrathecal synthesis of virus-specific oligoclonal IgG in multiple sclerosis. J Neuroimmunol. 1994;54:75–80. doi: 10.1016/0165-5728(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 16.Frederiksen JL, Sindic CJ. Intrathecal synthesis of virus-specific oligoclonal IgG, and of free kappa and free lambda oligoclonal bands in acute monosymptomatic optic neuritis. Comparison with brain MRI. Mult Scler. 1998;4:22–26. doi: 10.1177/135245859800400106. [DOI] [PubMed] [Google Scholar]
- 17.Jarius S, Eichhorn P, Jacobi C, Wildemann B, Wick M, et al. The intrathecal, polyspecific antiviral immune response: Specific for MS or a general marker of CNS autoimmunity? J Neurol Sci. 2008 doi: 10.1016/j.jns.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Jarius S, Franciotta D, Bergamaschi R, Rauer S, Wandinger KP, et al. Polyspecific, antiviral immune response distinguishes multiple sclerosis and neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2008;79:1134–1136. doi: 10.1136/jnnp.2007.133330. [DOI] [PubMed] [Google Scholar]
- 19.Tumani H, Tourtellotte WW, Peter JB, Felgenhauer K. Acute optic neuritis: combined immunological markers and magnetic resonance imaging predict subsequent development of multiple sclerosis. The Optic Neuritis Study Group. J Neurol Sci. 1998;155:44–49. doi: 10.1016/s0022-510x(97)00272-4. [DOI] [PubMed] [Google Scholar]
- 20.Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 21.Walsh MJ, Tourtellotte WW. Temporal invariance and clonal uniformity of brain and cerebrospinal IgG, IgA, and IgM in multiple sclerosis. J Exp Med. 1986;163:41–53. doi: 10.1084/jem.163.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol. 2006;59:880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 23.Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, et al. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 25.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 26.Robinson-Agramonte M, Reiber H, Cabrera-Gomez JA, Galvizu R. Intrathecal polyspecific immune response to neurotropic viruses in multiple sclerosis: a comparative report from Cuban patients. Acta Neurol Scand. 2007;115:312–318. doi: 10.1111/j.1600-0404.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 27.Tintore M, Rovira A, Brieva L, Grive E, Jardi R, et al. Isolated demyelinating syndromes: comparison of CSF oligoclonal bands and different MR imaging criteria to predict conversion to CDMS. Mult Scler. 2001;7:359–363. doi: 10.1177/135245850100700603. [DOI] [PubMed] [Google Scholar]
- 28.Zipoli V, Hakiki B, Portaccio E, Lolli F, Siracusa G, et al. The contribution of cerebrospinal fluid oligoclonal bands to the early diagnosis of multiple sclerosis. Mult Scler. 2009;15:472–478. doi: 10.1177/1352458508100502. [DOI] [PubMed] [Google Scholar]
- 29.Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs. J Neurol Sci. 2001;184:101–122. doi: 10.1016/s0022-510x(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 30.Reiber H, Otto M, Trendelenburg C, Wormek A. Reporting cerebrospinal fluid data: knowledge base and interpretation software. Clin Chem Lab Med. 2001;39:324–332. doi: 10.1515/CCLM.2001.051. [DOI] [PubMed] [Google Scholar]
- 31.Reiber H, Thompson EJ, Grimsley G, Bernardi G, Adam P, et al. Quality assurance for cerebrospinal fluid protein analysis: international consensus by an Internet-based group discussion. Clin Chem Lab Med. 2003;41:331–337. doi: 10.1515/CCLM.2003.053. [DOI] [PubMed] [Google Scholar]
- 32.Reiber H. Cerebrospinal fluid–physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler. 1998;4:99–107. doi: 10.1177/135245859800400302. [DOI] [PubMed] [Google Scholar]
- 33.Greenhouse JB, Bromberg JA, Fromm D. An introduction to logistic regression with an application to the analysis of language recovery following a stroke. J Commun Disord. 1995;28:229–246. doi: 10.1016/0021-9924(94)00011-n. [DOI] [PubMed] [Google Scholar]
- 34.Newcombe RG. Confidence intervals: enlightening or mystifying? BMJ. 1992;304:381–382. doi: 10.1136/bmj.304.6823.381-c. [DOI] [PMC free article] [PubMed] [Google Scholar]