Abstract
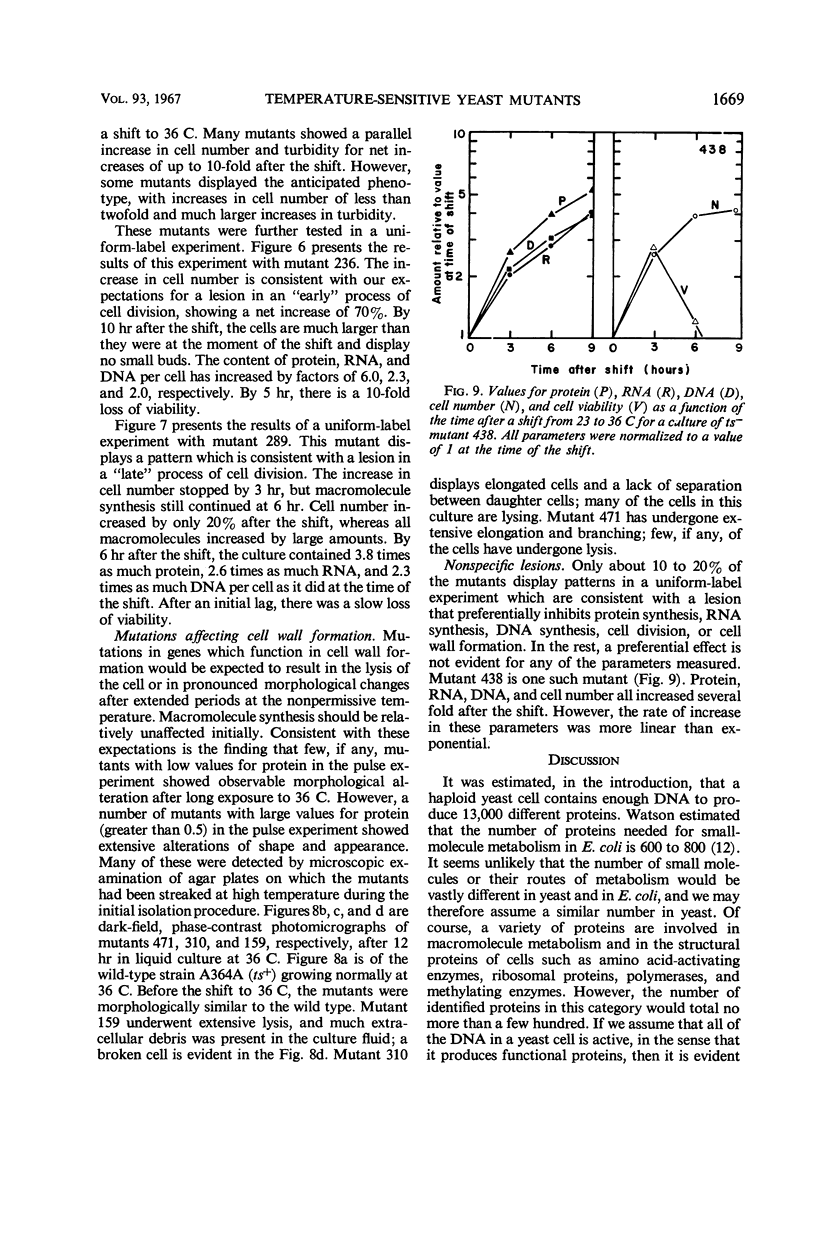
Approximately 400 temperature-sensitive mutants of Saccharomyces cerevisiae were isolated. The mutants were unable to form colonies on enriched media at 36 C, but grew normally, or nearly so, at 23 C. The mutants were tested for loss of viability, change in morphology, increase in cell number, and the ability to synthesize protein, ribonucleic acid (RNA), and deoxyribonucleic acid (DNA) after a shift from 23 to 36 C. Mutations were found which resulted in a preferential loss of ability to carry out protein synthesis, RNA synthesis, DNA synthesis, cell division, or cell-wall formation. Diploid cells heterozygous for the temperature-sensitive mutations were constructed and tested for their ability to form colonies at 36 C. Four mutations dominant to their wild-type allele were identified.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDGAR R. S., LIELAUSIS I. TEMPERATURE-SENSITIVE MUTANTS OF BACTERIOPHAGE T4D: THEIR ISOLATION AND GENETIC CHARACTERIZATION. Genetics. 1964 Apr;49:649–662. doi: 10.1093/genetics/49.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ N. H., LEUPOLD U. Some recent studies bearing on the one geneone enzyme hypothesis. Cold Spring Harb Symp Quant Biol. 1951;16:65–74. doi: 10.1101/sqb.1951.016.01.006. [DOI] [PubMed] [Google Scholar]
- Hall T. C., Cocking E. C. High-efficiency liquid-scintillation counting of 14C-labelled material in aqueous solution and determination of specific activity of labelled proteins. Biochem J. 1965 Sep;96(3):626–633. doi: 10.1042/bj0960626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohiyama M., Cousin D., Ryter A., Jacob F. Mutants thermosensibles d'Escherichia coli K 12. I. Isolement et caractérisation rapide. Ann Inst Pasteur (Paris) 1966 Apr;110(4):465–486. [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Yeast genetics. Annu Rev Microbiol. 1966;20:151–168. doi: 10.1146/annurev.mi.20.100166.001055. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. The regulation RNA synthesis in bacteria. Prog Nucleic Acid Res Mol Biol. 1964;3:145–181. doi: 10.1016/s0079-6603(08)60741-2. [DOI] [PubMed] [Google Scholar]
- OGUR M., MINCKLER S., LINDEGREN G., LINDEGREN C. C. The nucleic acids in a polyploid series of Saccharomyces. Arch Biochem Biophys. 1952 Sep;40(1):175–184. doi: 10.1016/0003-9861(52)90085-4. [DOI] [PubMed] [Google Scholar]
- Pomper S., Burkholder P. R. Studies on the Biochemical Genetics of Yeast. Proc Natl Acad Sci U S A. 1949 Aug;35(8):456–464. doi: 10.1073/pnas.35.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]