Abstract
Best vitelliform macular dystrophy is an inherited autosomal dominant, juvenile onset form of macular degeneration caused by mutations in a chloride ion channel, human bestrophin-1 (hBest1). Mutations in Best1 have also been linked to several other forms of retinopathy. In addition to mutations, hBest1 dysfunction might come about by disruption of other processes that regulate Best1 function. Here we show that hBest1 chloride channel activity is regulated by ceramide and phosphorylation. We have identified a protein kinase C (PKC) phosphorylation site (serine 358) in hBest1 that is important for sustained channel function. Channel activity is maintained by PKC activators, protein phosphatase inhibitors, or pseudo-phosphorylation by substitution of glutamic acid for serine 358. When ceramide levels are elevated by exogenous addition of ceramide to the bath, by addition of bacterial sphingomyelinase, or by hypertonic stress, S358 is rapidly dephosphorylated. The dephosphorylation is mediated by protein phosphatase 2A. Hypertonic stress-induced dephosphorylation is blocked by a dihydroceramide, an inactive form of ceramide, and manumycin, an inhibitor of neutral sphingomyelinase. Our results support a model in which ceramide accumulation during early stages of retinopathy inhibits hBest1 function, leading to abnormal fluid transport across the retina, and enhanced inflammation.
Age-related macular degeneration (AMD) accounts for more than half of all vision loss in the white population (Congdon et al. 2004). Although the mechanisms underlying the disease remain unknown, oxidative stress and chronic inflammation contribute significantly to its pathogenesis (Boekhoorn et al. 2007). There are several kinds of inherited macular dystrophies that promise to provide insights into pathogenic processes contributing to macular degeneration (Patel et al. 2008). Best vitelliform macular dystrophy (BVMD) is an inherited autosomal dominant, juvenile onset form of macular degeneration caused by mutations in an anion channel, human bestrophin-1 (hBest1) (Marquardt et al. 1998; Petrukhin et al. 1998). Mutations in Best1 have also been linked to several other forms of retinopathy including adult onset macular dystrophy (Seddon et al. 2001), autosomal dominant vitreochoidopathy (Yardley et al. 2004), autosomal recessive bestrophinopathy (Burgess et al. 2008), and canine multifocal retinopathy (Guziewicz et al. 2007). BVMD is clinically defined by an abnormal light peak (LP) to dark trough ratio (Arden ratio < 1.5) in the electrooculogram (Arden, 1962; Cross & Bard, 1974). The diminished LP is believed to be caused by a defective Ca2+ activated Cl− channel in the basolateral membrane of the retinal pigment epithelium (RPE) (Gallemore et al. 1997, 1998; Hughes et al. 1998), where hBest1 is expressed (Petrukhin et al. 1998; Marmorstein et al. 2000).
The mechanisms of hBest1 regulation are poorly understood. hBest1 is activated by intracellular Ca2+ (Sun et al. 2002; Tsunenari et al. 2003; Yu et al. 2006, 2007; Xiao et al. 2008), which binds to an EF hand-like structure in the C-terminus (Xiao et al. 2008). In addition to being activated by Ca2+, bestrophins can be regulated by cell volume (Fischmeister & Hartzell, 2005; Chien & Hartzell, 2007, 2008). The volume regulated chloride current in Drosophila S2 cells is knocked down by dBest1 siRNA, and rescued by transfection with both wild-type dBest1 and a mutant dBest1 with altered ion permeability (Chien & Hartzell, 2007, 2008). This strongly supports the idea that the native volume-regulated anion channel in S2 cells is dBest1. In addition, hBest1, as well as mBest2, currents expressed in HEK, HeLa and ARPE-19 cell lines are inhibited by hyperosmotic solutions (Fischmeister & Hartzell, 2005). The mechanisms underlying regulation of ion channels by the cell volume are complex; multiple signalling pathways including phosphorylation of ion channels and transporters have been implicated (Lang et al. 1998).
Though hBest1 has been reported to incorporate 32P and coimmunoprecipitate with protein phosphatase 2A (PP2A) (Marmorstein et al. 2002), the location of the potential phosphorylation site(s), the kinase(s) that phosphorylate hBest1, and the role phosphorylation plays in hBest1 channel regulation are not known. Recently, we identified a Ca2+ binding site and a region in the hBest1 C-terminus that is critical for regulation of channel rundown (Xiao et al. 2008). Here we have identified a protein kinase C (PKC) phosphorylation site (serine 358) in this region of the hBest1 C-terminus that is important for sustained channel function. We show that hBest1 chloride channel activity is regulated by phosphorylation of this site by ceramide, a lipid signalling molecule that is implicated in photoreceptor apoptosis during several kinds of retinopathy including macular degeneration, diabetic retinopathy, and retinal detachment (Fox et al. 2006; Acharya et al. 2008; Yamada et al. 2008; Ranty et al. 2009). Hypertonic stress-induced dephosphorylation is blocked by a dihydroceramide, an inactive form of ceramide, and manumycin, an inhibitor of neutral sphingomyelinase. Our results support a model in which ceramide accumulation during early stages of retinopathy inhibits hBest1 function leading to abnormal fluid transport across the retina.
Methods
Constructs and molecular biology
hBest1 tagged with the myc epitope at the C-terminus in pRK5 was obtained from Dr Jeremy Nathans (Johns Hopkins University) (Sun et al. 2002). Site-specific mutations were generated using PCR-based mutagenesis (Quickchanger; Stratagene) (Xiao et al. 2008). All constructs were confirmed by sequencing.
Cell culture and transfection
HEK cells were cultured as described previously (Yu et al. 2007; Xiao et al. 2008). For electrophysiology experiments, low-passage HEK-293 cells were transiently transfected using Fugene-6 (Roche) with hBest1 (1 μg per 35 mm dish). Cells were also transfected with pEGFP (0.2 μg) for fluorescence detection of transfected cells. Transfected cells were plated at low density and investigated 24–72 h after transfection.
Electrophysiology
Within 48–72 h after transfection, single cells identified by enhanced green fluorescent protein (EGFP) fluorescence were used for whole-cell patch clamp recording with an Axopatch 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA, USA). The pipettete solution contained (mm): 146 CsCl, 2 MgCl2, 5 (Ca2+)-EGTA, 2 ATP, 10 sucrose, 8 Hepes, pH 7.3, adjusted with N-methyl-d-glucamine (NMDG). This solution supplied free Ca2+ of 11 μm, and was verified by Fura-6F measurements (Invitrogen). Two millimolar adenosine 5′-(β,γ-imido)triphosphate (2 mm AMP-PNP) replaced 2 mm ATP as indicated at Fig. 1. The standard external solution contained (mm): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 15 sucrose, 10 Hepes, pH 7.4, adjusted with NaOH. Osmolarity was adjusted with sucrose to 305 mosmol l−1. Hyperosmotic solution (365 mosmol l−1, 1.2 T, 20% hyperosmotic) was made by adding mannitol. Electrode resistances were typically 2–3 MΩ in the bath solution. Voltage ramps from −100 mV to +100 mV with a duration of 750 ms were given from a holding potential of 0 mV at 10 s interval. Data analyses were done with Clampfit 9 (Molecular Devices) and Origin 7 (OriginLab Corp., Northampton, MA, USA) software. All averaged data are presented as the mean ±s.e.m. Statistical significance of differences (defined as P < 0.05) between groups was determined by one-way ANOVA as indicated.
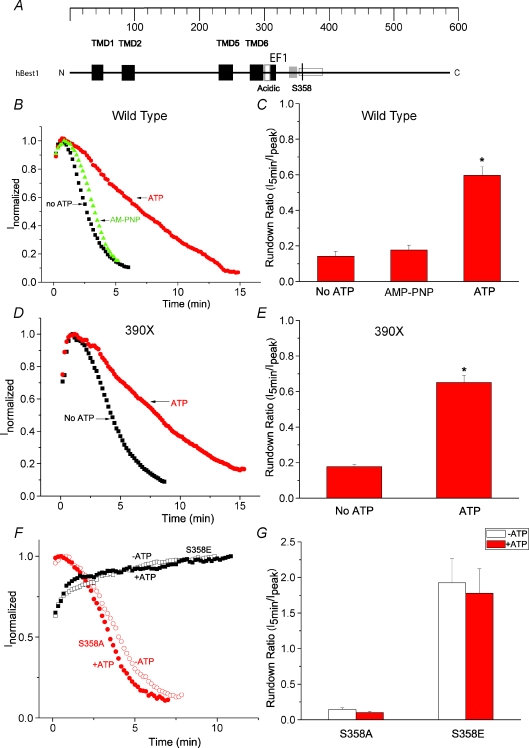
Figure 1. Identification of S358 as a phosphorylation site in hBest1.
A, model of functional domains in hBest1. Black (TMD): transmembrane domains (Milenkovic et al. 2007). Open (acidic): acidic domain. Black (EF1): EF1 domain, critical for Ca2+ binding. Open: a region identified to be responsible for channel rundown (amino acids 350–390) (Xiao et al. 2008). S358 is indicated by a black line. An SH3-binding domain between EF1 and S358 was identified by Yu et al. 2008 (Yu et al. 2008). B, D and F, time course of typical currents of wild-type (B), 390X mutant (D) and S358E and S358A mutants (F). Voltage ramps from −100 mV to +100 mV were given from a holding potential of 0 mV at 10 s intervals. The currents at +100 mV (filled symbols) were normalized to the peak current. B, wild-type hBest1 currents recorded in the presence of ATP (circles), no ATP (squares), and AMP-PNP (triangles). D, 390X mutant currents recorded in the presence of ATP (circles), and no ATP (squares). The 390X mutant was made by introducing stop codon at amino acid 390. F, S358E mutant (squares) and S358A mutant (circles) currents recorded in the presence of ATP (filled symbols) and no ATP (open symbols). C, E and G, average values of effects of ATP on channel rundown of wild-type hBest1 (C, n= 8–12), 390X mutant (E, n= 5–6) and S358E and S358A mutants (G, n= 4–7). The rundown was measured by the ratio of amplitudes of the current 5 min after peak current to the amplitudes at the peak. *P < 0.05 versus no ATP by one-way ANOVA.
In vitro phosphorylation
An hBest1 fusion protein was constructed by amplifying the nucleotides encoding amino acids 289–585 of hBest1 by PCR with Pfu polymerase and subcloning the resulting PCR product into the XmaI–SacI sites of pET-52b (Novagen). The resulting fusion protein contained a Strep-tag on the N-terminal end and a His(10) tag on the C-terminus. The fusion protein was purified by affinity chromatography on a Ni-column (Novagen). The in vitro phosphorylation reaction mixture contained 10 μl of protein kinase Cδ (10 ng μl−1, Cell Signaling Technology, Inc., Danvers, MA, USA), 0.625–5 μg of wild-type or S358E mutant fusion protein, 5 mm Mops pH 7.2, 2.5 mmβ-glycerophosphate, 1 mm EGTA, 0.4 mm EDTA, 5 mm MgCl2, 0.1 mm CaCl2, 0.05 mm DTT, 0.05 mg ml−1 phosphatidylserine, 0.005 mg ml−1 diacylglycerol, 50 mm ATP, 0.32 μCi μl−1[γ-32P]ATP. Reaction mixtures were incubated at 30°C for 30 min, spotted onto P81 phosphocellulose paper, washed 3 times with large volumes of 1% phosphoric acid, and then transferred to scintillation tube. 32P incorporation was determined by scintillation counting. All reagents were from Sigma-Aldrich except BIM and manumycin A, which were from Calbiochem.
Results
Phosphorylation of hBest1 by PKC slows channel rundown
We have previously shown that rundown of hBest1 current in whole cell recording is dependent on cytosolic [Ca2+] (Xiao et al. 2008). Because hBest1 is phosphorylated and co-immunoprecipitates with protein phosphatase 2A (PP2A) (Marmorstein et al. 2002), we hypothesized that hBest1 might be regulated by phosphorylation. Therefore, in the present series of experiments we used 11 μm free Ca2+ in the pipettete to maximize current rundown and tested whether rundown was affected by conditions that stimulated or suppressed phosphorylation. Figure 1B and C shows that rundown was slowed significantly when ATP was included in the intracellular solution. AMP-PNP had no effect on rundown, suggesting that ATP promoted channel activity by a mechanism involving phosphorylation. We also found that ATP slowed the rundown of the hBest1 truncated at residue 390 (390X mutant, Fig. 1D and E). The 390X mutant included the region previously identified as being responsible for channel rundown (amino acids 350–390) (Xiao et al. 2008) (Fig. 1A), suggesting that a potential phosphorylation site critical for regulation of channel rundown may be located here. There is a PKC and/or PKA consensus site (355RRAS358) (http://www.scansite.com) in this region. To test whether phosphorylation of this residue was important for rundown, we mutated S358 to E to mimic phosphorylation, and to A to mimic dephosphorylation. Current amplitudes were similar for wild-type, S358E, and S358A mutants. Figure 1F and G shows that the S358E mutant abolished rundown, whereas the S358A mutant ran down at about the same rate as wild-type in the absence of ATP. Both S358E and S358A mutants abolished the effect of ATP on channel rundown (Fig. 1F and G), suggesting the S358 is the potential phosphorylation site responsible for channel rundown. All experiments described below use ATP in the patch pipette.
To test whether phosphorylation by PKC regulates rundown, we treated cells with a PKC activator and an inhibitor. Pre-incubation with the protein kinase C activator, phorbol 12-myristate 13-acetate (PMA), greatly attenuated rundown, whereas the PKC inhibitor bisindolylmaleimide (BIM) caused a rapid decrease in current (Fig. 2A and B). Pre-incubation with a specific PKC inhibitor, myristoylated PKC peptide substrate, significantly increased channel rundown (Fig. 2B), suggesting that a PKC phosphorylation site is important for channel rundown. The S358A mutant abolished the effect of PMA (Fig. 2B) and the PKC inhibitor BIM had no effect on the current induced by the S358E mutation (Fig. 2C and D), suggesting S358 is the PKC phosphorylation site. To test whether S358 can be phosphorylated by PKA, we treated the cells with 5 μm forskolin in the bath solution and 100 μm cAMP in the patch pipette (Fig. 2E). However, neither drug had an effect on wild-type currents, suggesting S358 is not a PKA phosphorylation site. cAMP also had no effect on S358A mutant, suggesting that PKC phosphorylation of S358 is sufficient to explain channel rundown.
Figure 2. Phosphorylation of S358 by PKC reduced channel rundown.
A, time course of wild-type hBest1 currents recorded from HEK cells pretreated with 100 nm PMA for 2–5 min (circles) or untreated (squares). BIM at 1 μm was applied at the end of recording as indicated by the arrow. B, effect of PMA and myristoylated PKC peptide inhibitor on channel rundown of wild-type and S358A mutant (n= 5–12 cells; *P < 0.05 versus wild-type control; #P < 0.05 versus wild-type with PMA pretreatment by one-way ANOVA). C, time course of S358E mutant current recorded in the presence of 1 μm BIM indicated by the arrow. D, effect of BIM on wild-type and S355E mutant currents. Inhibition was measured as the percentage of the current amplitude 2 min after application of BIM to the amplitude just before BIM application (n= 5–6 cells; *P < 0.05 versus wild-type by one-way ANOVA). E, effects of forskolin and cAMP on channel rundown. Forskolin at 5 μm was applied in the bath solution and 100 μm cAMP was applied in the path pipette (n= 5–13 cells). Channel rundown (B, E and F) was measured as described in Fig. 1.
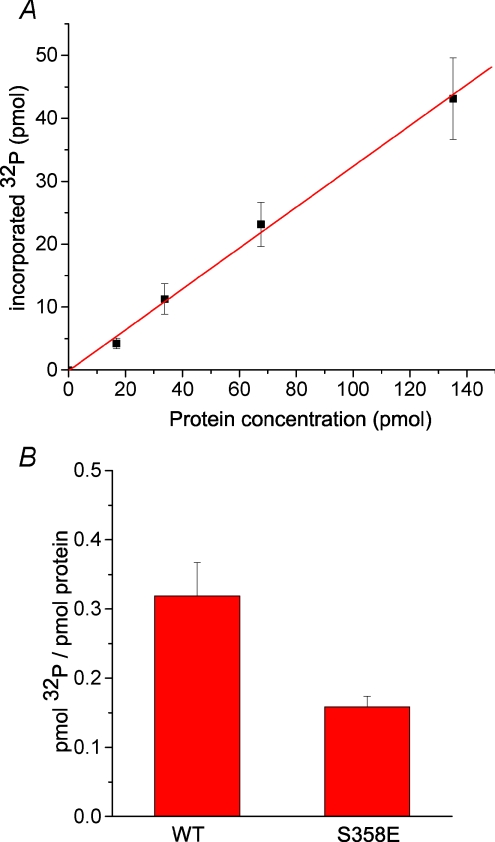
In order to further test whether PKC can directly phosphorylate S358, we examined the phosphorylation of purified wild-type and S358E mutant hBest1 C terminal fragments with PKC in vitro. Figure 3A and B shows that phosphorylation of hBest1 by PKC was linear with protein concentration, and S358E mutation blocked the phosphorylation ∼50%, suggesting that S358 can be directly phosphorylated by PKC. However, these data also suggest that there is another PKC phosphorylation site within the C terminus, but because S358 explains channel rundown, we have not tried to identify this other site.
Figure 3. In vitro phosphorylation of hBest1 C-terminus by PKC.
A, incorporation of 32P as a function of hBest1 C-terminus protein concentration. Reactions were incubated at 30°C for 30 min with 100 ng of PKC. Phosphorylation stoichiometry is estimated to be 0.32 pmol 32P/pmol protein. B, absence of phosphorylation of the S358E mutant of the hBest1 C-terminus. Incorporation of 32P by 5 μg of purified wild-type and S358E mutant fusion proteins was measured (n= 3).
Dephosphorylation of hBest1 by PP2A accelerates channel rundown
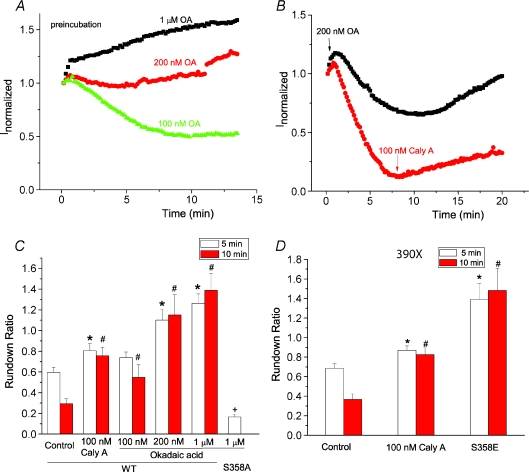
Since hBest1 has been demonstrated to bind to PP2A (Marmorstein et al. 2002), we examined the effects of protein phosphatase inhibitors on hBest1 current rundown. Pre-incubation with the protein phosphatase inhibitors okadaic acid and calyculin-A slowed rundown significantly (Fig. 4A and C). Pre-incubation with concentrations of okadaic acid > 200 nm completely abolished rundown. It was even possible to reverse rundown when okadaic acid or calyculin were applied shortly after establishing whole cell recording (Fig. 4B). Although these data establish the involvement of a protein phosphatase in channel rundown, they do not unambiguously show that this phosphatase is PP2A because the concentrations of OA and calyculin required were higher than required to inhibit PP2A. However, because, like Marmorstein et al. (2002), we can co-immunoprecipitate PP2A with hBest1 (data not shown), we believe the phosphatase is PP2A. The S358A mutation abolished the effect of OA (Fig. 4C), suggesting that PP2A dephosphorylates hBest1 at S358. To further test whether PP2A affected hBest1 channel rundown at other phosphorylation sites, we used the 390X mutation, which deleted any potential phosphorylation sites beyond position 390. Pre-incubation of calyculin-A significantly reduced channel rundown of 390X mutation (Fig. 4D). We further made a S358E/390X mutation, which completely abolished channel rundown, suggesting that S358 is sufficient for regulation of channel rundown.
Figure 4. Dephosphorylation of S358 by PP2A regulates channel rundown.
A and B, time course of wild-type hBest1 currents recorded from cells pretreated with 100 nm (triangles), 200 nm (circles) and 1 μm (squares) okadaic acid (OA) for 2–5 min (A) and from cells treated with 200 nm OA (squares) or 100 nm calyculin A (circles) after establishing whole cell recording (B). C, effects of okadaic acid and calyculin A on channel rundown. Channel rundown was measured as the ratio of current amplitude 5 min (open columns) or 10 min (filled columns) after peak current to the peak amplitude (n= 4–12 cells; *P < 0.05 versus wild-type control with rundown for 5 min; #P < 0.05 versus wild-type control with rundown for 10 min; +P < 0.05 versus wild-type treated with 1 μm okadaic acid). D, effects of calyculin A and S358E mutation on channel rundown of 390X mutations. Channel rundown was measured as the ratio of current amplitude 5 min (open columns) or 10 min (filled columns) after peak current to the peak amplitude (n= 4–5 cells; *P < 0.05 versus control with rundown for 5 min; #P < 0.05 versus control with rundown for 10 min).
Hypertonic stress inhibits hBest1 through dephosphorylation of S358
Previous studies showed that hypertonic solutions inhibited hBest1 currents expressed in HEK, HeLa, and ARPE-19 cell lines (Fischmeister & Hartzell, 2005). Consistent with previous studies, hypertonic solutions caused a 50% inhibition of hBest1 current within 2–5 min (Fig. 5A and C). The time course of current inhibition was similar to current rundown in the absence of ATP, but considerably faster than rundown in the presence of ATP, suggesting hypertonic solution may accelerate channel rundown by increasing dephosphorylation of S358. Pretreatment of cells with the PKC activator PMA and PP2A inhibitor OA significantly reduced the inhibition of hBest1 current by hypertonic solution (Fig. 5A and C). The S358E mutant completely abolished this hypertonic inhibition (Fig. 5B and C), suggesting that hypertonic solution inhibited hBest1 currents by dephosphorylating S358.
Figure 5. Hypertonic solutions inhibited hBest1 currents through dephosphorylation of S358.
A, effects of hypertonic solutions (1.2 T, 365 mosmol l−1) on wild-type hBest1 currents recorded from control cell (squares), cell pretreated with 1 μm PMA (circles) or 1 μm okadaic acid (triangles). B, hypertonic solution had no effect on the current induced by S358E mutation. Hypertonic solution was applied when current reached peak amplitude as indicated by the arrow (A and B). C, effects of OA, PMA and S358E mutation on hypertonic inhibition of hBest1 currents. The channel rundown was measured as ratio of amplitudes of the current 2 min (open columns) or 5 min (filled columns) after hypotonic solution was applied at the peak to the amplitudes at the peak (n= 5–10 cells; *P < 0.05 versus wild-type control).
Hypertonic stress activates neutral sphingomyelinase
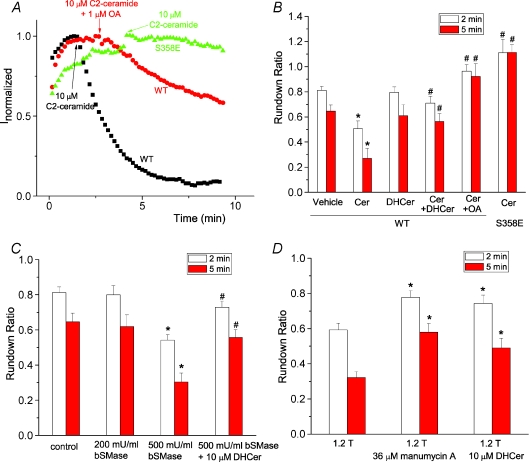
PP2A has been reported to be activated by ceramide (Dobrowsky et al. 1993), a lipid second messenger involved in regulation of many cellular responses to external stimuli (Kolesnick & Kronke, 1998; Ruvolo, 2001). We hypothesized that hypertonic inhibition of hBest1 currents is mediated by ceramide. C2-ceramide inhibited hBest1 current similarly to the hypertonic solution, and PP2A inhibitor OA and the S358E mutation blocked this effect (Fig. 6A and B). This suggested that hypertonic stress may release ceramide to activate PP2A and dephosphorylate hBest1. Dihydroceramide, an inactive form of C2 ceramide, had no effect on channel rundown, but it blocked the inhibitory effect of ceramide when added together with ceramide (Fig. 6B). The effect of hypertonic solution occurred within minutes, suggesting that the hypertonic stress may activate sphingomyelinase to release ceramide. We used a bacterial sphingomyelinase (bSMase) to treat the HEK cells to mimic the effect of hypertonic solution. bSMase (500 mU ml−1) significantly inhibited hBest1 currents, similar to the hypertonic solution. Dihydroceramide blocked this inhibitory effect of bSMase. We then used a neutral sphingomyelinase inhibitor to test whether sphingomyelinase is involved in hypertonic inhibition of hBest1 currents. Pretreatment of cells with neutral sphingomyelinase inhibitor, manumycin A, for 0.5–1 h significantly blocked the effect of hypertonic stress (Fig. 6D), suggesting that hypertonic stress activated neutral sphingomyelinase. Furthermore, dihydroceramide also blocked the effects of hypertonic stress (Fig. 6D), further confirming that ceramide mediates the hypertonic inhibition of hBest1.
Figure 6. Ceramide mediates hypertonic inhibition of hBest1 currents.
A, effects of ceramide on wild-type hBest1 currents recorded from control cells (squares), cells pretreated with 1 μm okadaic acid (circles), and cells transfected with S358E mutant (triangles). C2-ceramide at 10 μm was applied when currents reached peak as indicated by the arrow. B, ceramide inhibited hBest1 currents through dephosphorylation of S358. C2-ceramide (Cer) at 10 μm, 10 μm dihydroceramide (DHCer), 10 μm C2-ceramide plus 10 μm dihydroceramide together (Cer+DHCer), 10 μm C2-ceramide and 1 μm OA together, or vehicle control (0.2% dimethyl sulphoxide (DMSO)) was applied when currents reached peak amplitudes as in A. The channel rundown was measured as ratio of amplitudes of the current 2 min (open columns) or 5 min (filled columns) after drugs were applied at the peak to the amplitudes at the peak (n= 5–9 cells; *P < 0.05 versus vehicle control; #P < 0.05 versus wild-type currents treated with ceramide only). C, effects of bacterial sphingomyelinase (bSMase) on wild-type hBest1 channel rundown. bSMase at 200 mU ml−1, 500 mU ml−1 bSMase, and 500 mU ml−1 bSMase and 10 μm dihydroceramide together were applied when currents reached peak amplitudes as described in A. The channel rundown was measured as described in B (n= 6–9 cells. *P < 0.05 versus control; #P < 0.05 versus 500 mU ml−1 bSMase only). D, blockade of hypertonic inhibition of hBest1 currents by manumycin A and dihydroceramide. Cells were pretreated with 36 μm manumycin for 0.5–1 h, or with 10 μm dihydroceramide for 5–10 min. Hypertonic solutions containing 36 μm manumycin or 10 μm dihydroceramide were applied when currents reached peak amplitudes as described in A. The channel rundown was measured as described in B. n= 6–10. *P < 0.05 versus control.
Discussion
We have identified a PKC phosphorylation site (S358) located in the C terminal region of hBest1 critical for channel rundown (Xiao et al. 2008). Phosphorylation of this site by PKC activators and PP2A inhibitors reduces channel rundown. In addition, hBest1 channel rundown is accelerated by hypertonic stress, which can be blocked by a PKC activator and PP2A inhibitor, as well as the phosphorylation-mimicking S358E mutation. Ceramide, a PP2A activator, as well as a bacterial sphingomyelinase, mimics the effect of hypertonic stress, and a neutral sphingomyelinase inhibitor blocks the effect of hypertonic stress, suggesting that hypertonic stress activates neutral sphingomyelinase to release ceramide.
Phosphorylation at S358 regulates channel rundown
hBest1 can be phosphorylated when transfected into RPE-J cells, and the phosphorylation can be enhanced by a PP2A inhibitor (Marmorstein et al. 2002). Coimmunoprecipitation of PP2A with hBest1 from human RPE cells further suggests that phosphorylation may play an important role in regulation of hBest1 (Marmorstein et al. 2002). However, the location of the potential phosphorylation site(s) and the role of phosphorylation on channel regulation are unknown. There are phosphorylation sites in the C-terminus (http://www.scansite.com) for PKA/PKC (for example S358 and T536; Hartzell et al. 2008). S358 attracted our attention because it is located in a region (amino acids 350–390) previously identified as being responsible for channel rundown (Xiao et al. 2008). The amino acid sequence (355RRASFMGST363) is a PKC phosphorylation consensus motif (R/K1-3-X-S/T-X+1, where X is an uncharged amino acid and X+1 is a hydrophobic amino acid) (Aitken, 1999). The S358E mutation reduces 32P incorporation into the C-terminus by 50%, suggesting that another PKC phosphorylation site exists, at least in vitro. However, we see no evidence for a functional effect of this phosphorylation site because deletion of the C terminus beyond amino acid 390 does not have any effect on channel activity (Fig. 1D and E) and the S358E/390X mutation completely abolishes channel rundown (Fig. 4D). Thus, we have not further pursued the identity of this phosphorylation site, though we speculate the T536 may be the candidate. Since forskolin and cAMP do not alter hBest1 rundown, S358 does not appear to be a PKA phosphorylation site. This is curious because S358 does have the necessary determinants to be a PKA site. Thus, phosphorylation at S358 by PKC is critical in regulation of channel rundown.
S358 is located in a highly conserved region, which was previously identified as an important domain regulating bestrophin channel function (Qu et al. 2007; Xiao et al. 2008). Although S358 is conserved, the PKC recognition site is not conserved among bestrophins. For example, the arginines (R355 and R356 in hBest1), which are important for PKC recognition (Aitken, 1999), are missing in other bestrophins. Thus, this PKC phosphorylation site in hBest1 may not exist in other bestrophins. However, it is possible that this serine in other bestrophins can be phosphorylated by other kinases, which have not been identified yet.
Physiological functions of phosphorylation
The characteristic feature of BVMD is the diminished light peak in the electrooculogram (Arden, 1962; Cross & Bard, 1974). The light peak is thought to be caused by depolarization of basolateral membrane of RPE cells produced by a diffusible ‘light peak substance’ (LPS) released from photoreceptors when stimulated by light (Linsenmeier & Steinberg, 1982; Gallemore et al. 1988). The depolarization is generated by activation of a Ca2+-activated Cl− channel (Gallemore et al. 1997, 1998; Hughes et al. 1998). Though the identity of the LPS remains unknown, ATP is a favoured candidate because it mimics the LP (Peterson WM et al. 1997; Strauss, 2005; Hartzell et al. 2008). It is thought that ATP released from photoreceptors acts on a Gq protein coupled receptor on the RPE cells to increase intracellular Ca2+, which activates a Ca2+-activated Cl− channel at the basolateral membrane (Hartzell et al. 2008). Since hBest1is located in the RPE basolateral membrane (Petrukhin et al. 1998; Marmorstein et al. 2000), it has been suggested that bestrophin is the Ca2+-activated Cl− channel (Hartzell et al. 2008). In such a scenario, activation of Gq-coupled receptors would activate PLC. The resulting second messengers, IP3, which mobilizes Ca2+ stores, and DAG, which activates PKC, could both serve to regulate hBest1 and the LP. The light peak signal could be terminated by dephosphorylation of hBest1 by PP2A. When PKC activity is decreased due to less released LPS in the dark, PP2A could dephosphorylate hBest1 to terminate the LP signal.
This scheme is hypothetical and there are a number of questions that need to be answered. First, the relationship of hBest1 current rundown in a heterologous expression system to the LP and physiology of the retina remains to be established. Furthermore, the identity of the channel responsible for the LP has been brought into question. This challenge largely comes from mBest1 knockout mice, which have a normal LP and Ca2+-activated Cl− current (Marmorstein et al. 2006). However, mBest1 knockout mice may not be a good model for BVMD because they have no obvious ocular pathology (Marmorstein et al. 2006). It seems there is another Ca2+-activated Cl− channel in mouse RPE cells which may be responsible for the LP. A newly discovered Ca2+-activated Cl− channel (TMEM16A) (Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008; Hartzell et al. 2009) seems a candidate. Furthermore, the LP is abolished in mice with the voltage-gated Ca2+ channel subunits β4 (Marmorstein et al. 2006) and α1.3 (Wu et al. 2007) knocked out, suggesting that voltage gated Ca2+ channels are important for the LP. The finding that hBest1 but not mBest1 can regulate Ca2+ channels further suggests that mBest1 does not function like hBest1 (Yu et al. 2008). Furthermore, it is unclear how activation of Ca2+ channels is responsible for activation of Ca2+-activated chloride currents in mouse RPE cells.
Regulation of hBest1 by hypertonic stress
We have previously found that hBest1 can be inhibited by hypertonic solution (Fischmeister & Hartzell, 2005). However, the mechanisms underlying this inhibition are unknown. The fact that rundown is accelerated by hypertonic solution as well as dephosphorylation suggests that hypertonic inhibition of hBest1 current is mediated by dephosphorylation of S358. In agreement with this idea, ceramide, a PP2A activator, mimics, and an inactive form of ceramide, dihydroceramide, blocks, the inhibition of hBest1 by hypertonic solution. Therefore, ceramide mediates the hypertonic inhibition of hBest1 currents.
Ceramide has been recognized as a second messenger mediating several cellular responses to stress stimuli including cytokines, oxidative stress, radiation, chemotherapeutic agents and hypertonic stress (Obeid et al. 1993; Hannun, 1996; Kolesnick & Kronke, 1998; Hannun & Luberto, 2000; Ruvolo, 2001). Ceramide formation in response to stress stimuli is variable, ranging from seconds or minutes to hours or days (Hannun, 1996; Levade & Jaffrezou, 1999). Since the effects of ceramide and hypertonic solution occurs within minutes, hypertonic solution most likely increases ceramide production through activation of sphingomyelinase (SMase), rather than through the de novo synthesis of ceramide, which would be slow (Kolesnick & Kronke, 1998). This rapid response also indicates that ceramide formation and subsequent inhibition of hBest1 currents is the early response to hypertonic stress (Hannun, 1996; Kolesnick & Kronke, 1998). Cells usually undergo a process of regulatory volume increase (RVI) to resume their original cell volumes after being placed in hypertonic solution (Lang et al. 1998). Thus, hBest1 may play a key role in RVI in retinal pigment epithelial cells through decrease in Cl− efflux by ceramide.
Ceramide, a very hydrophobic molecule, tends to remain within the cell membrane, and thus may function at the subcellular site of production (Kolesnick & Kronke, 1998). Hypertonic stress is likely to activate a plasma membrane-bound SMase to inhibit hBest1 currents. Two major SMases determine the production of ceramide: the lysosomal acid SMase and the plasma membrane-bound neutral SMase (Liu et al. 1997; Kolesnick & Kronke, 1998). Hypertonic solution is likely to activate the neutral SMase since it is located in the plasma membrane and has a pH optimum of 7.4. This is further supported by evidence that manumycin A, a neutral SMase inhibitor which competes with sphingomyelin for binding of neutral SMase (nSMase) (Arenz et al. 2001), blocks the hypertonic inhibition of hBest1 current. In addition, the nSMase activity increases in rat neurohypophysis following hypertonic stress (Guy et al. 1983). It is interesting that caveolar nSMase is activated by mechanostimulation in vascular endothelium as a mechanosensor (Czarny et al. 2003; Czarny & Schnitzer, 2004). It is likely that mechanical force from hypertonic cell shrinkage activates nSMase to release ceramide, which inhibits hBest1 currents through activation of PP2A. However, acid sphingomyelinase has been observed in the plasma membrane (Liu & Anderson, 1995; Grassme et al. 2001, 2003). Hypertonic stress has been reported to increase ceramide production through activation of acid SMase in hepatocytes (Reinehr et al. 2006) and erythrocytes (Lang et al. 2004) with different kinetics of ceramide formation and signalling pathway. Thus, different SMase can be activated by the same stimuli (hypertonic stress) to trigger distinct signalling pathways, which may lead to cell-specific responses (Levade & Jaffrezou, 1999).
Several bestrophins including hBest1, mBest2 and dBest1 are regulated by both Ca2+ and cell volume (Fischmeister & Hartzell, 2005; Chien & Hartzell, 2007, 2008; Hartzell et al. 2008). Regulation of bestrophins by Ca2+ and cell volume can be independent of one another (Chien & Hartzell, 2007), but the two pathways also can interact with each other in that Ca2+ potentiates the dBest1 current amplitude activated by increases in cell volume (Chien & Hartzell, 2007; Chien et al. 2006), and increases in cell volume often result in increases in intracellular Ca2+ (McCarty & O’Neil, 1992). Studies of hBest1 activation by hyposmolarity are thwarted by findings that endogenous volume regulated anion channels (VRACs) are ubiquitously expressed in cell lines such as HEK (Fischmeister & Hartzell, 2005). However, hBest1 could be activated by hyposmotic swelling through Ca2+, since hBest1 currents are activated by intracellular Ca2+ with EC50 of 140 nm (Hartzell et al. 2008; Xiao et al. 2008), which can be reached following hyposmotic treatment.
Acknowledgments
This work was supported by NIH grants EY014852 and GM60448. Q.X. is supported by an American Heart Association postdoctoral fellowship.
Glossary
Abbreviations
- BVMD
Best vitelliform macular dystrophy
- hBest1
human bestrophin-1
- LP
light peak
- LPS
light peak substance
- PKC
protein kinase C
- PP2A
protein phosphatase 2A
- RPE
retinal pigment epithelium
- RVI
regulatory volume increase
- SMase
sphingomyelinase.
Author contributions
All authors contributed to conception, design, analysis and interpretation of data. Q.X. drafted the article, and H.C.H. and K.Y. revised it critically for intellectual content. All authors approved the version to be published.
References
- Acharya JK, Dasgupta U, Rawat SS, Yuan C, Sanxaridis PD, Yonamine I, Karim P, Nagashima K, Brodsky MH, Tsunoda S, Acharya U. Cell-nonautonomous function of ceramidase in photoreceptor homeostasis. Neuron. 2008;57:69–79. doi: 10.1016/j.neuron.2007.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken A. Protein consensus sequence motifs. Mol Biotechnol. 1999;12:241–253. doi: 10.1385/MB:12:3:241. [DOI] [PubMed] [Google Scholar]
- Arden GB. Alterations in the standing potential of the eye associated with retinal disease. Trans Ophthalmol Soc U K. 1962;82:63–72. [PubMed] [Google Scholar]
- Arenz C, Thutewohl M, Block O, Waldmann H, Altenbach HJ, Giannis A. Manumycin A and its analogues are irreversible inhibitors of neutral sphingomyelinase. Chembiochem. 2001;2:141–143. doi: 10.1002/1439-7633(20010202)2:2<141::AID-CBIC141>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Boekhoorn SS, Vingerling JR, Witteman JCM, Hofman A, de Jong PTVM. C-reactive protein level and risk of aging macula disorder: The Rotterdam Study. Arch Ophthalmol. 2007;125:1396–1401. doi: 10.1001/archopht.125.10.1396. [DOI] [PubMed] [Google Scholar]
- Burgess R, Millar ID, Leroy BP, Urquhart JE, Fearon IM, De Baere E, Brown PD, Robson AG, Wright GA, Kestelyn P, Holder GE, Webster AR, Manson FD, Black GC. Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am J Hum Genet. 2008;82:19–31. doi: 10.1016/j.ajhg.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Chien LT, Hartzell HC. Drosophila bestrophin-1 chloride current is dually regulated by calcium and cell volume. J Gen Physiol. 2007;130:513–524. doi: 10.1085/jgp.200709795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien LT, Hartzell HC. Rescue of volume-regulated anion current by bestrophin mutants with altered charge selectivity. J Gen Physiol. 2008;132:537–546. doi: 10.1085/jgp.200810065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien LT, Zhang ZR, Hartzell HC. Single Cl− channels activated by Ca2+ in Drosophila S2 cells are mediated by bestrophins. J Gen Physiol. 2006;128:247–259. doi: 10.1085/jgp.200609581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Cross HE, Bard L. Electro-oculography in Best's macular dystrophy. Am J Ophthalmol. 1974;77:46–50. doi: 10.1016/0002-9394(74)90603-5. [DOI] [PubMed] [Google Scholar]
- Czarny M, Liu J, Oh P, Schnitzer JE. Transient mechanoactivation of neutral sphingomyelinase in caveolae to generate ceramide. J Biol Chem. 2003;278:4424–4430. doi: 10.1074/jbc.M210375200. [DOI] [PubMed] [Google Scholar]
- Czarny M, Schnitzer JE. Neutral sphingomyelinase inhibitor scyphostatin prevents and ceramide mimics mechanotransduction in vascular endothelium. Am J Physiol Heart Circ Physiol. 2004;287:H1344–H1352. doi: 10.1152/ajpheart.00222.2004. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- Fischmeister R, Hartzell HC. Volume sensitivity of the bestrophin family of chloride channels. J Physiol. 2005;562:477–491. doi: 10.1113/jphysiol.2004.075622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TE, Han X, Kelly S, Merrill AH, Martin RE, Anderson RE, Gardner TW, Kester M. Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes. 2006;55:3573–3580. doi: 10.2337/db06-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallemore RP, Griff ER, Steinberg RH. Evidence in support of a photoreceptoral origin for the ‘light-peak substance’. Invest Ophthalmol Vis Sci. 1988;29:566–571. [PubMed] [Google Scholar]
- Gallemore RP, Hughes BA, Miller SS. Retinal pigment epithelial transport mechanisms and their contributions to the electroretinogram. Prog Retinal Eye Res. 1997;16:509–566. [Google Scholar]
- Gallemore RP, Hughes BA, Miller SS. Light-induced responses of the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. Oxford: Oxford University Press; 1998. pp. 175–198. [Google Scholar]
- Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signalling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- Guy NC, Clarke JT, Spence MW, Cook HW. Stimulation of neutral, magnesium-stimulated sphingomyelinase activity in the neurohypophysis of the rat by hypertonic saline ingestion. Brain Res Bull. 1983;10:603–606. doi: 10.1016/0361-9230(83)90028-x. [DOI] [PubMed] [Google Scholar]
- Guziewicz KE, Zangerl B, Lindauer SJ, Mullins RF, Sandmeyer LS, Grahn BH, Stone EM, Acland GM, Aguirre GD. Bestrophin gene mutations cause canine multifocal retinopathy: a novel animal model for best disease. Invest Ophthalmol Vis Sci. 2007;48:1959–1967. doi: 10.1167/iovs.06-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Qu Z, Yu K, Xiao Q, Chien LT. Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol Rev. 2008;88:639–672. doi: 10.1152/physrev.00022.2007. [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol. 2009;587:2127–2139. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes BA, Gallemore RP, Miller SS. Transport mechanisms in the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ, editors. The Retinal Pigment Epithelium. Oxford: Oxford University Press; 1998. pp. 103–134. [Google Scholar]
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lang KS, Myssina S, Brand V, Sandu C, Lang PA, Berchtold S, Huber SM, Lang F, Wieder T. Involvement of ceramide in hyperosmotic shock-induced death of erythrocytes. Cell Death Differ. 2004;11:231–243. doi: 10.1038/sj.cdd.4401311. [DOI] [PubMed] [Google Scholar]
- Levade T, Jaffrezou JP. Signalling sphingomyelinases: which, where, how and why? Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- Linsenmeier RA, Steinberg RH. Origin and sensitivity of the light peak in the intact cat eye. J Physiol. 1982;331:653–673. doi: 10.1113/jphysiol.1982.sp014396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Obeid LM, Hannun YA. Sphingomyelinases in cell regulation. Semin Cell Dev Biol. 1997;8:311–322. doi: 10.1006/scdb.1997.0153. [DOI] [PubMed] [Google Scholar]
- Liu P, Anderson RG. Compartmentalized production of ceramide at the cell surface. J Biol Chem. 1995;270:27179–27185. doi: 10.1074/jbc.270.45.27179. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD, Marmorstein LY, Rayborn M, Wang X, Hollyfield JG, Petrukhin K. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral membrane of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2000;97:12758–12763. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein LY, McLaughlin PJ, Stanton JB, Yan L, Crabb JW, Marmorstein AD. Bestrophin interacts physically and functionally with protein phosphatase 2A. J Biol Chem. 2002;277:30591–30597. doi: 10.1074/jbc.M204269200. [DOI] [PubMed] [Google Scholar]
- Marmorstein LY, Wu J, McLaughlin P, Yocom J, Karl MO, Neussert R, Wimmers S, Stanton JB, Gregg RG, Strauss O, Peachey NS, Marmorstein AD. The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1) J Gen Physiol. 2006;127:577–589. doi: 10.1085/jgp.200509473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt A, Stohr H, Passmore LA, Kramer F, Rivera A, Weber BH. Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease) Hum Mol Genet. 1998;7:1517–1525. doi: 10.1093/hmg/7.9.1517. [DOI] [PubMed] [Google Scholar]
- McCarty NA, O’Neil RG. Calcium signalling in cell volume regulation. Physiol Rev. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Milenkovic VM, Rivera A, Horling F, Weber BHF. Insertion and topology of normal and mutant bestrophin-1 in the endoplasmic reticulum membrane. J Biol Chem. 2007;282:1313–1321. doi: 10.1074/jbc.M607383200. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Patel N, Adewoyin T, Chong NV. Age-related macular degeneration: a perspective on genetic studies. Eye. 2008;22:768–776. doi: 10.1038/sj.eye.6702844. [DOI] [PubMed] [Google Scholar]
- Peterson WM, Meggyesy CF, Yu KF, Miller SS. Extracellular ATP activates calcium signalling, ion, and fluid transport in retinal pigment epithelium. J Neurosci. 1997;17:2324–2337. doi: 10.1523/JNEUROSCI.17-07-02324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrukhin K, Koisti MJ, Bakall B, Li W, Xie G, Marknell T, Sandgren O, Forsman K, Holmgren G, Andreasson S, Vujic M, Bergen AAB, McGarty-Dugan V, Figueroa D, Austin CP, Metzker ML, Caskey CT, Wadelius C. Identification of the gene responsible for Best macular dystrophy. Nat Genet. 1998;19:241–247. doi: 10.1038/915. [DOI] [PubMed] [Google Scholar]
- Qu ZQ, Yu K, Cui YY, Ying C, Hartzell C. Activation of bestrophin Cl− channels is regulated by C-terminal domains. J Biol Chem. 2007;282:17460–17467. doi: 10.1074/jbc.M701043200. [DOI] [PubMed] [Google Scholar]
- Ranty ML, Carpentier S, Cournot M, Rico-Lattes I, Malecaze F, Levade T, Delisle MB, Quintyn JC. Ceramide production associated with retinal apoptosis after retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2009;247:215–224. doi: 10.1007/s00417-008-0957-6. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Becker S, Braun J, Eberle A, Grether-Beck S, Haussinger D. Endosomal acidification and activation of NADPH oxidase isoforms are upstream events in hyperosmolarity-induced hepatocyte apoptosis. J Biol Chem. 2006;281:23150–23166. doi: 10.1074/jbc.M601451200. [DOI] [PubMed] [Google Scholar]
- Ruvolo PP. Ceramide regulates cellular homeostasis via diverse stress signalling pathways. Leukemia. 2001;15:1153–1160. doi: 10.1038/sj.leu.2402197. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon JM, Afshari MA, Sharma S, Bernstein PS, Chong S, Hutchinson A, Petrukhin K, Allikmets R. Assessment of mutations in the best macular dystrophy (VMD2) gene in patients with adult-onset foveomacular vitelliform dystrophy, age-related maculopathy, and bull's-eye maculopathy. Ophthalmol. 2001;108:2060–2067. doi: 10.1016/s0161-6420(01)00777-1. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Sun H, Tsunenari T, Yau K-W, Nathans J. The vitelliform macular dystrophy protein defines a new family of chloride channels. Proc Natl Acad Sci U S A. 2002;99:4008–4013. doi: 10.1073/pnas.052692999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunenari T, Sun H, Williams J, Cahill H, Smallwood P, Yau K-W, Nathans J. Structure-function analysis of the bestrophin family of anion channels. J Biol Chem. 2003;278:41114–41125. doi: 10.1074/jbc.M306150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Marmorstein AD, Striessnig J, Peachey NS. Voltage-dependent calcium channel CaV1.3 subunits regulate the light peak of the electroretinogram. J Neurophysiol. 2007;97:3731–3735. doi: 10.1152/jn.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Prussia A, Yu K, Cui YY, Hartzell HC. Regulation of bestrophin Cl channels by calcium: role of the C terminus. J Gen Physiol. 2008;132:681–692. doi: 10.1085/jgp.200810056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Tian J, Yang Y, Cutler RG, Wu T, Telljohann RS, Mattson MP, Handa JT. Oxidized low density lipoproteins induce a pathologic response by retinal pigmented epithelial cells. J Neurochem. 2008;105:1187–1197. doi: 10.1111/j.1471-4159.2008.05211.x. [DOI] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- Yardley J, Leroy BP, Hart-Holden N, Lafaut BA, Loeys B, Messiaen LM, Perveen R, Reddy MA, Bhattacharya SS, Traboulsi E, Baralle D, De Laey JJ, Puech B, Kestelyn P, Moore AT, Manson FD, Black GC. Mutations of VMD2 splicing regulators cause nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC) Invest Ophthalmol Vis Sci. 2004;45:3683–3689. doi: 10.1167/iovs.04-0550. [DOI] [PubMed] [Google Scholar]
- Yu K, Cui Y, Hartzell HC. The bestrophin mutation A243V, linked to adult-onset vitelliform macular dystrophy, impairs its chloride channel function. Invest Ophthalmol Vis Sci. 2006;47:4956–4961. doi: 10.1167/iovs.06-0524. [DOI] [PubMed] [Google Scholar]
- Yu K, Qu Z, Cui Y, Hartzell HC. Chloride channel activity of bestrophin mutants associated with mild or late-onset macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:4694–4705. doi: 10.1167/iovs.07-0301. [DOI] [PubMed] [Google Scholar]
- Yu K, Xiao Q, Cui G, Lee A, Hartzell HC. The best disease-linked Cl− channel hBest1 regulates CaV1 (L-type) Ca2+ channels via src-homology-binding domains. J Neurosci. 2008;28:5660–5670. doi: 10.1523/JNEUROSCI.0065-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]