Creating a versatile set of highly stable fluorophores capable of high emission rates is crucial to advancing studies of individual biomolecule function. While most biosystems exist in a multitude of states, single molecule studies remove the need for external synchronization, thereby allowing dynamics and pathways to be directly resolved.1-4 Through direct measurements of individual molecules, one can study dynamics on a variety of time scales,5 determine transient intermediates,6 and unravel complex dynamics.7 However, biological studies are limited by existing fluorophores which suffer from inherent deficiencies; most notably a lack of photostability and insufficient emission intensities,8-10 limiting study of long and short time dynamics, respectively.
As water-soluble quantum dots have low photobleaching quantum yields and very strong absorption, they hold promise over organic dyes as single molecule probes.11-13 Still, they present a number of issues when applied to biological systems, including large physical size, strong fluorescence intermittency on all timescales, and cell toxicity.14-16 Additionally, due to penetration and background issues, the development of bright emitters not only in the visible, but also in the near IR is needed to eventually enable intracellular single molecule studies with minimal perturbation.
We report on five distinct Ag nanocluster emitters encapsulated in single stranded DNA. Using oligo-cytosine scaffolds we have previously reported the creation of Ag nanocluster emitters with very promising photophysical parameters,17,18 but only in highly heterogeneous mixtures containing at least four different inseparable species. A biocompatible complex with optimizable strand length and base sequence, oligonucleotides offer a convenient scaffold to tune emission based on cluster size. As we report herein, this flexibility has enabled the creation of five distinct and essentially spectrally pure emitters with fluorescence tunable throughout the visible and near IR.
Using DNA microarrays (Combimatrix) for high throughput analysis of 12-mer strands (attached to the substrate by a T12 linker which does not contribute to Ag nanocluster formation), various oligonucleotides consisting of combinations of cytosine, thymine, and adenine were analyzed to identify optimized sequences for Ag cluster encapsulation. Guanine was excluded due to its propensity for self binding.19 Arrays were then imaged at various excitation wavelengths. Emission intensities vs. pH were used to determine the best sequences for stabilizing Ag nanocluster fluorescence (e.g. Figure 1).
Figure 1.
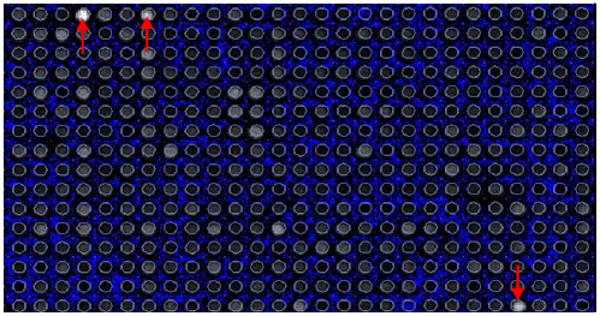
Emission from a DNA microarray excited with 543 nm light showing the specificity of particular sequences for the formation of the Red emitting Ag nanocluster. These sequences were then used in bulk synthesis to identify the oligonucleotide with the highest affinity for the formation of the target cluster.
The few most promising sequences stabilizing each emitter were obtained and utilized for Ag nanocluster synthesis. Using 50 μM solutions of each oligonucleotide, a 6:1 molar ratio of AgNO3 (Aldrich) was added. After fifteen minutes this mixture was reduced with NaBH4 (Aldrich). Employing sequences identified through these array experiments, we produced the five distinct emitters shown in Figure 2. The two highest energy emitters (Fig. 2A,B) are only created in unbuffered solutions and appear to be partially oxidized – further chemical reduction transforms them into red-shifted emitters with clear isosbestic points.20
Figure 2.
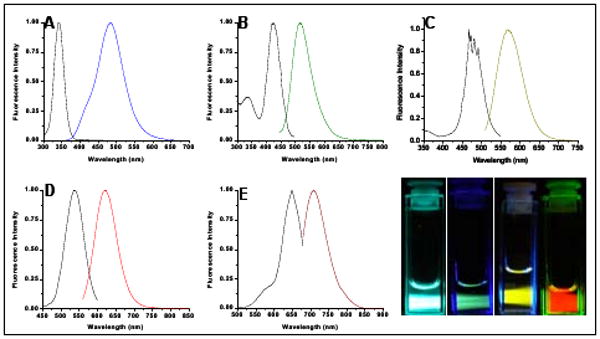
Steady-state excitation and emission spectra for five distinct ss-DNA-encapsulated Ag nanoclusters. (A) Blue emitters created in 5′-CCCTTTAACCCC-3′, (B) green emitters created in 5′-CCCTCTTAACCC-3′, (C) yellow emitters created in 5′-CCCTTAATCCCC-3′, (D) red emitters created in 5′-CCTCCTTCCTCC-3′, and (E) Near IR emitters created in 5′-CCCTAACTCCCC-3′. (F) Pictures of emissive solutions in A-D.
The remaining species formed best in buffered solutions: the yellow at a pH range of 6.5-8 (phosphate 20 mM, fig 2C), the red at pH 5 (citrate 20 mM, fig 2D, stable up to pH 7), and the near IR at a pH range of 6.5-8 (ammonium acetate 20 mM, fig 2E). Although array methods yielded our first spectrally pure yellow species, parallel work has shown that oligo length can help stabilize clusters. Consequently, a similar sequence lengthened by oligocytosine (5′-AATTCCCCCCCCCCCCAATT-3′ at pH 7) yielded further improved photophysics, with quantum yield increasing by 30% for the yellow emitter. With improved photophysics, the 5′ end similarity to the array-identified nanocluster-binding internal sequence suggests that end-binding is favorable, likely due to entropy considerations. While near complete spectral purity has been attained, detailed cluster size determinations await due to incompatibility of mass spectroscopy and buffered conditions, but all evidence indicates that all emitters are smaller than six Ag atoms,17,20 with no nanoparticle formation. Smaller than the ∼3.3nm hydrodynamic radius, RH, of Cy5-DNA, the RH, of ss-DNA encapsulated clusters shrinks to ∼2.5nm.
Both the blue and green emitting species are excited at high energy, 340 nm and 425 nm respectively, and initial fluorescence studies show a lack of photostability. However, the yellow, red, and near IR emitting species show great promise as potential single molecule biolabels, as chemical stability in buffer and photostability are drastically increased with these longer wavelength emitters. They are stable at biologically relevant pH and are excited with relatively low energy light allowing for less background fluorescence. We have previously reported near IR-emitting single nanoclusters exhibiting constant intensity trajectories lasting tens of minutes.21 These new spectrally pure species behave similarly as single molecule fluorophores, as reported herein through single molecule photostability comparisons with commonly used cyanine dyes (GE Biosciences).
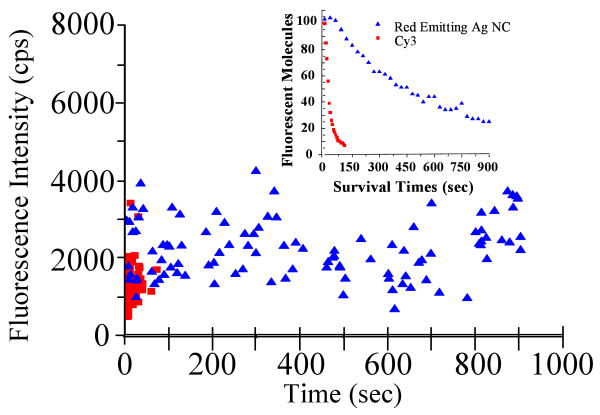
The red emitting species and Cy3-conjugated 12-mer poly-cytosine were separately isolated at low concentrations in poly vinyl alcohol (PVA). Using weak green excitation from a Hg lamp, CCD images of each were used to compare emission intensities and photobleaching rates. A 2-D plot of intensity versus failure time (Figure 3) for 100 molecules of each clearly shows that red emitting Ag nanocluster has a higher emission rate and a significantly longer emissive lifetime than Cy3. At these low excitation intensities, the Ag nanoclusters yield an average of ∼2500 detected counts/sec while the Cy3 conjugated to DNA yielded at an average detected count rate of ∼1500 per second. Similar results were obtained for the near IR emitter compared to Cy5 (not shown).
Figure 3.
Detected intensity vs failure time for 100 molecules each of red emitting Ag nanoclusters and of Cy3 conjugated 12-mer oligo-cytosine isolated in PVA under identical imaging conditions (∼30W/cm2, 510-550nm bandpass Hg-lamp excitation; MicroMax CCD detection (Princeton Instruments)). Inset: survival times for the same molecules.
To achieve an accurate comparison of photobleaching, the survival times of these same 100 molecules are plotted (Fig. 3, inset). The number of red emitting Ag nanoclusters decayed to 1/e at approximately 580 seconds, while the number of Cy3 molecules decayed to 1/e at approximately 9 seconds. These results indicate that DNA encapsulated Ag nanoclusters represent a vast improvement over the commonly used cyanine dyes. As shown in Table 1 the yellow, red and NIR emitting species show comparable or better photophysical parameters to those of cyanine dyes with large extinction coefficients and quantum yields of over 30%. For these three species, the greatly reduced bleaching and blinking21 lead to significant advantages over existing dyes for single molecule studies. Limiting our fluorescence correlation spectroscopy-based measurement of extinction coefficients, a decreased photostability and higher energy excitation precluded full photophysical characterization of the less-promising blue and green emitters.
Table 1.
Photophysical Parameters of Ag Nanoclusters
| Species | Emission | Lifetime(ns) | Φ (%) | ε (105M-1cm-1) |
|---|---|---|---|---|
| Blue | 485 nm | 2.98 +/- .01 | x | x |
| Green | 520 nm | 0.22 +/- .01 | 16 +/- 3 | x |
| Yellow | 572 nm | 4.35 +/- .01 | 38 +/- 2 | 2.0 +/- 0.4 |
| Red | 620 nm | 2.23 +/- .01 | 32 +/- 4 | 1.2 +/- 0.3 |
| NIR | 705 nm | 3.46 +/-.01 | 34 +/- 5 | 3.5 +/- 0.7 |
Through the use of DNA microarrays, we have created new Ag nanocluster fluorophores with outstanding spectral and photophysical properties. The biodiversity inherent in ss-DNA via modification of base sequence and strand length should enable further optimization as well as identification of new Ag nanocluster based emitters for single molecule spectroscopy and imaging. Comparisons to commonly used cyanine dyes show this new class of fluorophores offers great promise in single molecule and bulk fluorescence studies.
Acknowledgments
The authors gratefully acknowledge NIH grants R01-GM072021, U54-CA119338, and P20-GM068732, and NSF 0323453. CIR acknowledges NIH R. Kirchenstein NRSA F31EB008324.
References
- 1.Liu SX, Bokinsky G, Walter NG, Zhuang XW. Proc Nat Acad Sci, USA. 2007;104:12634–12639. doi: 10.1073/pnas.0610597104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myong S, Bruno MM, Pyle AM, Ha T. Science. 2007;317:513–516. doi: 10.1126/science.1144130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elf J, Li GW, Xie XS. Science. 2007;316:1191–1194. doi: 10.1126/science.1141967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roeffaers MBJ, De Cremer G, Uji-i H, Muls B, Sels BF, Jacobs PA, De Schryver FC, De Vos DE, Hofkens J. Proc Nat Acad Sci, USA. 2007;104:12603–12609. doi: 10.1073/pnas.0610755104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwille P, Kummer S, Heikal AA, Moerner WE, Webb WW. Proc Nat Acad Sci, USA. 2000;97:151–156. doi: 10.1073/pnas.97.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipman EA, Schuler B, Bakajin O, Eaton WA. Science. 2003;301:1233–1235. doi: 10.1126/science.1085399. [DOI] [PubMed] [Google Scholar]
- 7.Hanson JA, Duclerstacit K, Watkins LP, Bhattacharyya S, Brokaw J, Chu JW, Yang H. Proc Nat Acad Sci, USA. 2007;104:18055–18060. doi: 10.1073/pnas.0708600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggeling C, Widengren J, Rigler R, Seidel CAM. Anal Chem. 1998;70:2651–2659. doi: 10.1021/ac980027p. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt T, Kubitscheck U, Rohler D, Nienhaus U. Single Mol. 2002;3:327. [Google Scholar]
- 10.Lu HP, Xun LY, Xie XS. Science. 1998;282:1877–1882. doi: 10.1126/science.282.5395.1877. [DOI] [PubMed] [Google Scholar]
- 11.Chan WCW, Nie SM. Science. 1998;281:2016–2018. doi: 10.1126/science.281.5385.2016. [DOI] [PubMed] [Google Scholar]
- 12.Zhang CY, Ma H, Nie SM, Ding Y, Jin L, Chen DY. Analyst. 2000;125:1029–1031. [Google Scholar]
- 13.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 14.Kuno M, Fromm DP, Hamann HF, Gallagher A, Nesbitt DJJ. Chem Phys. 2000;112:3117–3120. [Google Scholar]
- 15.Derfus AM, Chan WCW, Bhatia SN. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SF, Osborne MAJ. Amer Chem Soc. 2007;129:8936–8937. doi: 10.1021/ja071876+. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie CM, Johnsen KR, Kiser JR, Antoku Y, Dickson RM, Petty JTJ. Phys Chem C. 2007;111:175–181. doi: 10.1021/jp0648487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petty JT, Zheng J, Hud NV, Dickson RMJ. Amer Chem Soc. 2004;126:5207–5212. doi: 10.1021/ja031931o. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Yuan G, Liu JJ, Zhan CG. Chemistry-a European Journal. 2007;13:945–949. doi: 10.1002/chem.200600424. [DOI] [PubMed] [Google Scholar]
- 20.Antoku Y. Ph D Thesis. Georgia Institute of Technology; 2007. [Google Scholar]
- 21.Vosch T, Antoku Y, Hsiang JC, Richards CI, Gonzalez JI, Dickson RM. Proc Nat Acad Sci, USA. 2007;104:12616–12621. doi: 10.1073/pnas.0610677104. [DOI] [PMC free article] [PubMed] [Google Scholar]