Abstract
Olfactory behavioral studies have shown that when modulated through systemic injections, D1 and D2 receptors have opposing effects on odor discrimination learning. In the present study, cannulated male Sprague-Dawley rats were used to investigate how modulation of these two types of dopaminergic receptors though direct infusion of D1/D2 agonists and antagonists into the olfactory bulb affect olfactory perception. Dopaminergic modulation was locally altered by manipulations of D1 (agonist SKF 82958, 14.6mM, 43.8mM, 143.6mM; antagonist SCH-23390, 13.4mM, 40.1mM, 60.1mM) and D2 (agonists quinpirole, 78.2mM, 117.3mM, 156.4mM; antagonist sulpiride, 0.3mM, 0.9mM, 2.9mM) receptors during a simultaneous odor discrimination task. We found that modulation of D2, but not D1 receptors significantly affected rats’ odor discrimination performance. A significant positive correlation between blockade of D2 receptors and discrimination performance, as well as a significant negative correlation between D2 receptor activation and discrimination performance was observed.
Keywords: Olfactory perception, olfactory bulb, dopamine, discrimination learning, neuromodulation
Introduction
The olfactory bulb is innervated by multiple neuromodulatory fibers, notably cholinergic fibers from the horizontal limb of the diagonal band of Broca (Le Jeune & Jourdan, 1993; Macrides, Davis, Youngs, Nadi, & Margolis, 1981; Zaborszky, Carlsen, Brashear, & Heimer, 1986; Zaborszky, Cullinan, & Braun, 1991), noradrenergic fibers from the locus coeruleus (McLean & Shipley, 1991; Perez, Hernandez, & Almli, 1987; Shipley, Halloran, & de la Torre, 1985), and serotonergic fibers from the raphe nucleus (Mamounas, Mullen, O'Hearn, & Molliver, 1991; Moore, Halaris, & Jones, 1978). Receptors for these neuromodulators are found on specific cell types within the olfactory bulb, and experimental manipulation of these neuromodulatory inputs to the olfactory bulb has been shown to modulate bulbar processing, underlying phenomena such as odor discrimination (Linster & Cleland, 2002; Linster, Garcia, Hasselmo, & Baxter, 2001; Mandairon, Ferretti et al., 2006), olfactory associative learning (Doucette, Milder, & Restrepo, 2007; Mandairon et al., 2008; McLean & Harley, 2004; Yuan, Harley, & McLean, 2003), and olfactory short-term memory (Guerin, Peace, Didier, Linster, & Cleland, 2008; Ravel, Elaagouby, & Gervais, 1994).
In contrast, whereas dopamine is a bulbar neuromodulator, there are no centrifugal dopaminergic projections into the olfactory bulb (McLean & Shipley, 1988; Shipley & Ennis, 1996). Dopaminergic interactions with olfactory bulb physiology have been demonstrated in several studies. Unilateral olfactory deprivation greatly reduces the levels of both dopamine (Brunjes, Smith-Crafts, & McCarty, 1985) and tyrosine hydroxylase in the rat and mouse olfactory bulbs (Baker, 1990; Baker, Morel, Stone, & Maruniak, 1993; Brunjes et al., 1985; Cho, Min, Franzen, & Baker, 1996; Stone, Wessel, Joh, & Baker, 1990), and alters mitral/tufted cell responsiveness to odors (Guthrie, Pullara, Marshall, & Leon, 1991; Guthrie, Wilson, & Leon, 1990; West & Doty, 1995).
Behaviorally, dopaminergic modulation appears to play a role in altering odor detection thresholds as well as odor discrimination and learning capabilities. When administered the dopamine D2 receptor agonist quinpirole, for example, rat odor detection performance decreased; this effect was eliminated when pre- or posttreated with the D2 antagonist spiperone, further illustrating the specificity of the effect (Doty & Risser, 1989). In contrast, the administration of the D1-selective agonist SKF 38393 enhanced odor detection performance, whereas the D1 receptor antagonist SCH 23390 eliminated this effect (Doty et al., 1998). Similarly, activation of D1 receptors or blockade of D2 receptors each have been shown to improve the ability of adult rats to discriminate structurally and perceptually similar odorant pairs, whereas D1 blockade or D2 activation impaired odor discrimination (Yue, Cleland, Pavlis, & Linster, 2004). Modulation of D2 receptor activation specifically has been show to affect odor discrimination performance in a manner comparable to changes in odor concentration (Wei, Linster, & Cleland, 2006).
Although these experiments provide abundant evidence of dopaminergic effects on olfactory processing. Here, we investigate the specific role of bulbar dopaminergic receptors in olfactory discrimination learning through the use of intracranial cannulations. We find a dose-dependent modulation of olfactory discrimination capabilities when D2, but not D1, receptors are modulated via direct administration of specific agonists and antagonists directly into the olfactory bulb during performance of the behavioral task. In agreement with previous experiments using global injections, we show that blockade of D2 receptors in the OB via the specific antagonist sulpiride improves rats’ discrimination performance whereas activation of bulbar D2 receptors via the specific agonist quinpirole reduces discrimination performance. In contrast, we do not show an effect of D1 receptor modulation in the OB on discrimination performance, suggesting that previous results were due to modulation of D1 receptors elsewhere in the rats’ brain.
Methods
Subjects
Seven male Sprague-Dawley rats (250-300 g; Charles River Laboratories, Wilmington, MA) were housed individually in standard laboratory cages on a 12 hr light/dark cycle at a constant temperature. During the testing of spontaneous discriminations (cross-habituation task), rats were allowed access to food and water ad libitum. During the reinforced olfactory discrimination task, rats were maintained in a food-deprivation schedule designed to keep them between 85 and 95% of their body weight over the behavioral testing period. Behavioral experiments were conducted in the afternoon (1400-1800) towards the end of the light cycle. All procedures were performed under the auspices of a protocol approved by the Cornell University Institutional Animal Care and Use Committee.
Cannulation
Rats were anesthetized with an intramuscular cocktail injection of 80 mg/kg ketamine and 12 mg/kg Xylazine (in a volume of 1 ml/kg) and secured in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA, USA). Cannulae (22-gauge; Plastics One, Roanoke, VA) were inserted bilaterally for infusions into both OBs at the following coordinates with respect to bregma: AP +8.0 mm, ML ±1.5 mm, DV –4.5 mm. The tips of the guide cannulae were positioned 1 mm dorsal to the target infusion site; consequently, infusion cannulae extended 1 mm from the end of the guide cannulae which were then positioned to be in the middle of the olfactory bulb (Figure 1A). Five screws were drilled into the skull, and dental cement was used to secure the guide cannulae and cover the incision area. Dummy infusion cannulae were then placed into the guide cannulae to prevent blockage or infection. Following surgery, rats were allowed to recover for 10 days.
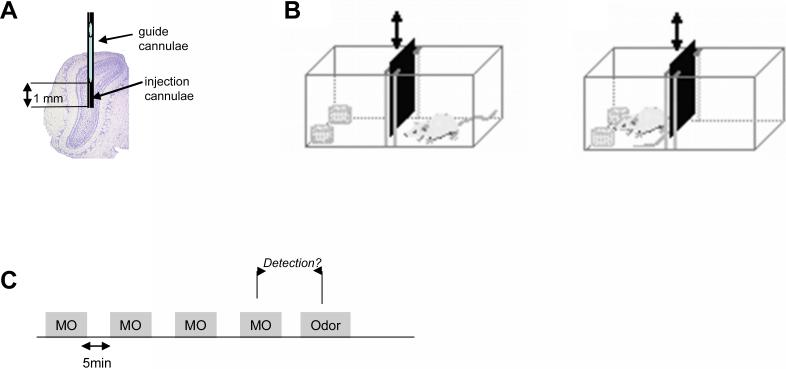
Figure1.
Experimental setup. A. Cannulae placement. Cannulae were aimed at the middle of the olfactory bulb in such a manner that drug infusions cover a maximal bulbar area. B. Behavioral setup for discrimination experiments. Rats were trained in a modified rat cage separated into two chambers by an opaque sliding door. C. Behavioral paradigm for odor detection testing. Rats were presented with the carrier, mineral oil, during four trials separated by five minute intertrial intervals, followed by a single presentation of a test odor.
Drug administration
Four dopaminergic drugs, at three concentrations each, were used in this study: the D1 receptor agonist SKF82958 (14.6mM, 43.8mM, 143.6mM), the D1 receptor antagonist SCH2339 (13.4mM, 40.1mM, 60.1mM), the D2 receptor agonist quinpirole (78.2mM, 117.3mM, 156.4mM) and the D2 antagonist sulpiride (0.3mM, 0.9mM, 2.9mM). Drugs, obtained from Sigma-Aldrich (Natick, MA) were dissolved in 0.9% saline and prepared freshly every day; saline infusions served as a control. The infusion volume, 6 μl per OB, was determined in a previous study. Infusion of 6 μl methylene blue into the OB showed that the infusion volume was enough to cover the whole OB and accessory OB without spill over to neighboring brain areas (Mandairon, Ferretti et al., 2006; Mandairon et al., 2008; Mandairon, Stack et al., 2006). For drug administration, two infusion cannulae were fitted into the guide cannulae so that their tips protruded 1.0 mm beyond the ends of the guide cannulae into the center of each OB (Figure 1A). Two 10 μl Hamilton syringes containing either drug solutions or vehicle (plain saline) were attached to the cannulae with a polyethylene tube and driven with paired infusion pumps (YA-12 Genie pumps, Kent Scientific, Torrington, CT ). Drugs were delivered bilaterally into awake rats at a rate of 2 μl/min for 3 min (6 μl total volume delivered per side). The infusion cannulae remained in place for 1 additional min after the infusion ended in order to minimize backflow. Behavioral testing was initiated 20 min after drug administration was completed.
Odor sets
Ten pairs of odorants were used for the forced choice discrimination testing, each pair comprised of chemically and perceptually similar odorants (Cleland et al., 2002; see Table 1 for odors and dilutions). Drugs and odors were counterbalanced and randomized among rats. Each rat was run at least once under each drug condition and care was taken that a given rat was not tested on any given odor/drug combination more than once. To control for non-specific effects of accumulated training, saline control conditions were run at the beginning and at the end of the experiment; the first set of saline data was included in the analysis. Rats’ ability to smell the odorants was tested in a separate experiment not involving food reward training using a subset of the odorants at the same dilutions.
Table 1.
Odorsets and dilutions to achieve an approximate partial vapor pressure of 10 Pa.
| Odor Set | Odorant (% vol/vol dilution) | |
|---|---|---|
| Odor A | Odor B | |
| 1 | butyl butyrate (0.039) | methyl butyrate (0.002) |
| 2 | methyl valerate (0.006) | ethyl valerate (0.015) |
| 3 | octanone (0.043) | heptanone (0.016) |
| 4 | butyl acetate (0.006) | propyl acetate (0.002) |
| 5 | amylamine (0.002) | benzylamine (0.105) |
| 6 | heptanol (0.230) | hexanol (0.079) |
| 7 | heptanal (0.019) | hexanal (0.007) |
| 8 | butanoic acid (0.055) | pentanoic acid (0.165) |
| 9 | octanol (0.658) | nonanol (1.851) |
| 10 | hexanoic acid (0.473) | heptanoic acid (1.298) |
Rats were shaped to perform a digging task used in the forced-choice discrimination test prior to cannulation as previously described (Cleland, Morse, Yue, & Linster, 2002). Each rat was tested under each drug condition with a different pair of odorants; consequently. Training took place in a custom testing chamber (Figure 1B). Rats were trained to discriminate between two dishes (ceramic pots, 9 cm in diameter, 4.5 cm in height) of bedding (Bed-O-Cobs, The Andersons, Maumee, OH), each scented with one of odorants A and B in an odor pair. The dishes were evenly filled with 50 cm3 of bedding, after which 50 μl of diluted odorant was applied to the top of the bedding in the center of the dish. Another 50 cm3 of bedding was then added to bury the odorant within the bedding. Each training session comprised twenty immediately-consecutive discrimination trials using the same pair of odorants; for a given rat, one odor was repeatedly rewarded while the other was unrewarded. During each trial, the pot in which the rat dug first was recorded. Rats were allowed to self-correct after digging in the unrewarded odor. Trials were terminated after one minute if the rat failed to dig. To control for the possibility that rats might locate the sugar pellet (TestDiet® Richmond, IN) reward via its own odor rather than by learning the association with a training odorant, every fifth trial was performed without any reward present; once the rat registered a preference by digging in the pot scented with the reward-associated odorant, the reward was dropped onto the scented bedding to maintain the association of the odorant with the reward.
To test if drug injections interfered with odor detection per se, rats were tested in a habituation/discrimination paradigm under the highest dosage of each drug. Briefly, rats were habituated to one the carrier, mineral oil, during four sequential 50 second presentations separated by five minute intertrial intervals, followed by a single presentation of one of the test odorants (Figure 1C). The magnitude of their novelty response to the test odor was indicative of the rats’ ability to detect the odorant and discriminate it from the carrier. Mineral oil and test odors were presented by placing 60 μl of the mixture onto a filter paper disc (Whatman #1) contained within a weighing dish that was placed on top of the wire cage lid. The amount of time that the rat spent actively investigating each presented odorant was measured using a stopwatch. Active investigation was defined as directed sniffing within 1 cm of the odor source.
Data analysis
Data analyses were performed using SPSS statistical software (SPSS, Chicago, IL) with the number of correct choices made as the dependent variable. Analysis of variance with experimental group as main effect was performed on the number of correct trials per session. Rats that dug for fewer than 15 out of 20 trials were eliminated from the analysis; actual numbers of rats used in each group are indicated in the result figure.
Histological verification
At the end of the experiments, 6 uL of methylene blue was infused bilaterally into the cannulaes and rats were perfused for subsequent histological verifications of the local infusion sites. Only rats for which cannulae were placed correctly and methylene blue spread indicated that these were not blocked were included into subsequent analysis.
Results
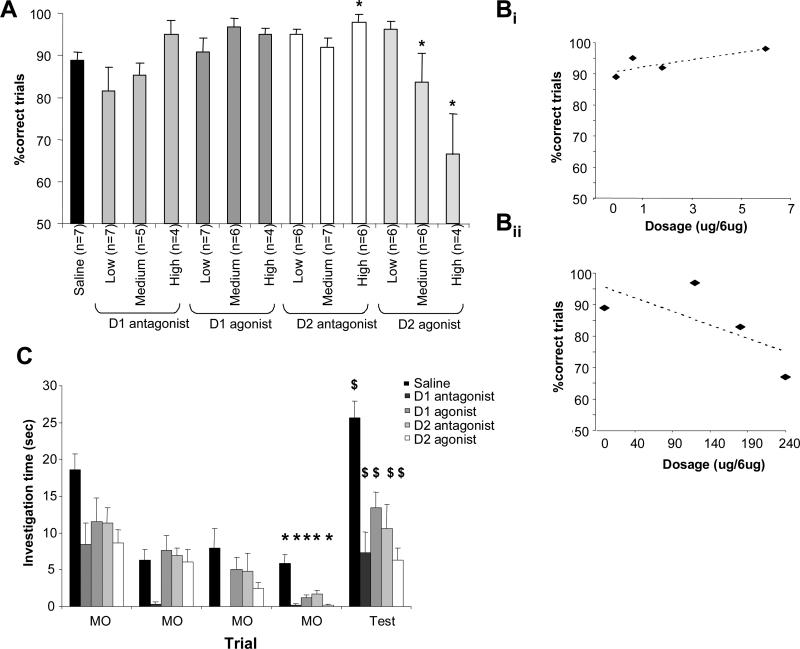
Analysis of variance with treatment (drug type and dosage) as main effect and number of correct choices as dependent variable showed a significant effect of drug treatment (F(12, 85) = 3.314, p < 0.01) indicating that rats’ performance in the two-choice discrimination task was significantly affected by the drug treatment. Posthoc analysis using Fisher's LSD test showed that rats injected with the higher dosage of the D2 agonist quinpirole as well as rats injected with the medium and high dosages of the D2 antagonist sulpiride performed significantly different from saline control rats (p < 0.05). Figure 2A shows the average number of correct choices for each drug group. These results indicate a role for D2, but not D1 receptor activation in the two-choice discrimination task performed here.
Figure 2.
Experimental results. A. Average discrimination performance of rats under each drug. The graph shows the average number of correct choices made during sessions of 20 trials for each of the treatments. *indicate a significant difference from the saline injected control group (p < 0.05 with Fisher LSD). B. Dose-response curves of discrimination perforance as a function of drug dosage. The graphs show that average numbers of correct trials for each treatment plotted as a function of the dosage of the D2 antagonist sulpiride (Bi) and the D2 agonist quinpirole (Bii). C. Odor detection tests. The graph shows the average investigation times of rats treated with the highest dosage of each drug during four habituation trials (MO) and one test trial (Test). * indicate a significant decrease in investigation time between the first and last presentation of MO (p < 0.05 with Fisher LSD) and $ indicates a significant increase in investigation time between the last presentation of MO and the test odor presentation (p < 0.05 using Fisher LSD).
Further analysis on the D2 agonists and antagonists treatments yielded a significant positive correlation between dosage and the number of correct choices for rats treated with the D2 antagonist sulpiride (Pearson's R = −0.348, p < 0.05, Figure 2Bi) as well as a significant negative correlation between drug dosage and the number of correct choices for rats treated with the D2 agonist quinpirole (Pearson's R = 0.369, p < 0.02, Figure 2Bii).
In summary, rats’ performance on a two choice discrimination task is significantly affected by modulation of the D2 receptors in the OB in a dose-dependent manner.
To ensure that odor detection per se was not affected by the drug treatments at the odor concentrations used here, we subsequently tested the rats in an odor detection task under the influence of the highest drug dosages used in this experiment. ANOVA with drug and trial as main effects showed a significant effect of drug (F(4, 125) = 20.252; p < 0.001) and trial (F(4, 125) = 30.881; p < 0.001) as well as a significant interaction (F(4, 125) = 2.477; p < 0.001). All drug groups investigated the odors significantly less than the saline control group (p < 0.005 in all pairwise comparisons). Figure 2C shows that all drug treatment groups habituated to the mineral oil (as shown by the significant decrease in investigation time during the last compared to the first habituation trial, p < 0.05 in all cases using Fisher LSD) and detected the novel test odorants (as shown by the significant increase in investigation time between the last habituation trial and the test trial, p < 0.05 in all cases using Fisher LSD).
Discussion
The results presented here show that the modulation of olfactory function reported in previous experiments using global injections of dopaminergic agents can at least partially be ascribed to modulation of olfactory bulb dopaminergic function. More specifically, local injections of D2 dopaminergic receptor agonist and antagonists resulted in behavioral performance changes similar to those obtained in experiments using global injections of these agents (Pavlis, Feretti, Levy, Gupta, & Linster, 2006; Wei et al., 2006; Yue et al., 2004). However, at the dosages used here, local manipulations of D1 dopaminergic receptor function in the olfactory bulb did not result in significant changes in olfactory discrimination performance. This may suggest that modulation of olfactory discrimination performance observed in previous experiments using global injections of D1 receptor agents (Wei et al., 2006; Yue et al., 2004) may have been due to the role of the dopaminergic system in mediating reward-guided behaviors (Berridge & Robinson, 1998; Dayan & Balleine, 2002; Robbins & Everitt, 1996; Schultz, 2002).
Multiple studies have reported that D2 receptor activation presynaptically inhibits olfactory nerve terminals (Berkowicz & Trombley, 2000; Davila, Blakemore, & Trombley, 2003; Ennis et al., 2001; Hsia, Vincent, & Lledo, 1999), thus depressing or even blocking synaptic transmission between olfactory receptor neurons and their synaptic targets, including mitral cells and some subclasses of tufted and periglomerular cells (Berkowicz & Trombley, 2000; Davila et al., 2003; Ennis et al., 2001; Hsia et al., 1999; Sallaz & Jourdan, 1992). This weakened synaptic transmission, mimicking the response to lower-concentration stimuli, might account for the reduced olfactory discrimination performance observed upon injection of the D2 agonist quinpirole. In agreement with this idea, previous experiments have demonstrated that odor discrimination performance in rats was modulated by activation of D2 receptors in a manner similar to the modulation obtained by decreasing odor concentration (Wei et al., 2006) Likewise, enhanced transmission at OSN output synapses via blockade of baseline D2 receptor activation by spontaneous dopaminergic PG cell activity (Puopolo, Bean, & Raviola, 2005) may yield synaptic input to mitral cells resembling that generated by higher stimulus concentrations, which would explain the heightened discrimination capabilities observed in rats injected with the dopamine D2 receptor antagonist spiperone. Indeed, in vivo administration of the D2 antagonist spiperone increased the responsiveness of rat olfactory bulb mitral cells to odors (Wilson & Sullivan, 1995), and modulated odor discrimination performance in a manner similar to that obtained with higher odor concentrations (Wei et al., 2006). Improved discrimination performance may be a simple consequence of higher intensity OSN output to mitral cells, whether resulting from increased odor stimulus concentrations or from reduced D2 inhibition of this OSN output. Specifically, the direct innervation of dopaminergic PG cells by OSNs and the inhibition of OSN terminals by PG cells form a negative feedback loop that can normalize the degree of excitation provided to mitral/tufted cells (Ennis et al., 2001; McGann et al., 2005; Wachowiak & Cohen, 1999). Dopaminergic inhibition of OSN output within a given glomerulus will depend on the activation level of that convergent OSN population, such that increased OSN activity presynaptically inhibits its own output. In the present experiments, this presynaptic inhibition was enhanced with a D2 agonist, hence reducing the odor intensity perceived by the rats and their discrimination performance. Blockade of D2 receptors on the other hand would decrease presynaptic inhibition, increasing the perceived stimulus intensity and accompanying discrimination performance.
In summary, bulbar D2-type dopamine receptors see to be important regulators of olfactory processing and perception. Modulation of D2 receptor activation can enhance or impair the discrimination of odors, presumably by altering the perceived intensity of a given odorant through changes in the effective sensitivity of bulbar neurons to OSN activity. Perceptually, D2 receptor-mediated changes in gain would be similar to genuine changes in odor concentration, with corresponding effects on detection thresholds, discrimination performance, and olfactory associative learning.
Acknowledgments
Supported by CRCNS DC008702 (NIDCD, CL) and the Leadership Alliance (DM)
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
References
- Baker H. Unilateral, neonatal olfactory deprivation alters tyrosine hydroxylase expression but not aromatic amino acid decarboxylase or GABA immunoreactivity. Neuroscience. 1990;36(3):761–771. doi: 10.1016/0306-4522(90)90018-y. [DOI] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614(1-2):109–116. doi: 10.1016/0006-8993(93)91023-l. [DOI] [PubMed] [Google Scholar]
- Berkowicz DA, Trombley PQ. Dopaminergic modulation at the olfactory nerve synapse. Brain Res. 2000;855(1):90–99. doi: 10.1016/s0006-8993(99)02342-2. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Smith-Crafts LK, McCarty R. Unilateral odor deprivation: effects on the development of olfactory bulb catecholamines and behavior. Brain Res. 1985;354(1):1–6. doi: 10.1016/0165-3806(85)90063-x. [DOI] [PubMed] [Google Scholar]
- Cho JY, Min N, Franzen L, Baker H. Rapid down-regulation of tyrosine hydroxylase expression in the olfactory bulb of naris-occluded adult rats. J Comp Neurol. 1996;369(2):264–276. doi: 10.1002/(SICI)1096-9861(19960527)369:2<264::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116(2):222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Davila NG, Blakemore LJ, Trombley PQ. Dopamine modulates synaptic transmission between rat olfactory bulb neurons in culture. J Neurophysiol. 2003 doi: 10.1152/jn.01058.2002. (in press) [DOI] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36(2):285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Doty RL, Li C, Bagla R, Huang W, Pfeiffer C, Brosvic GM, Risser JM. SKF 38393 enhances odor detection performance. Psychopharmacology (Berl) 1998;136(1):75–82. doi: 10.1007/s002130050541. [DOI] [PubMed] [Google Scholar]
- Doty RL, Risser JM. Influence of the D-2 dopamine receptor agonist quinpirole on the odor detection performance of rats before and after spiperone administration. Psychopharmacology. 1989;98(3):310–315. doi: 10.1007/BF00451680. [DOI] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem. 2007;14(8):539–547. doi: 10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol. 2001;86(6):2986–2997. doi: 10.1152/jn.2001.86.6.2986. [DOI] [PubMed] [Google Scholar]
- Guerin D, Peace ST, Didier A, Linster C, Cleland TA. Noradrenergic neuromodulation in the olfactory bulb modulates odor habituation and spontaneous discrimination. Behav Neurosci. 2008;122(4):816–826. doi: 10.1037/a0012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Pullara JM, Marshall JF, Leon M. Olfactory deprivation increases dopamine D2 receptor density in the rat olfactory bulb. Synapse. 1991;8(1):61–70. doi: 10.1002/syn.890080109. [DOI] [PubMed] [Google Scholar]
- Guthrie KM, Wilson DA, Leon M. Early unilateral deprivation modifies olfactory bulb function. J Neurosci. 1990;10(10):3402–3412. doi: 10.1523/JNEUROSCI.10-10-03402.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol. 1999;82(2):1082–1085. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]
- Le Jeune H, Jourdan F. Cholinergic innervation of olfactory glomeruli in the rat: an ultrastructural immunocytochemical study. J Comp Neurol. 1993;336(2):279–292. doi: 10.1002/cne.903360209. [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw. 2002;15(4-6):709–717. doi: 10.1016/s0893-6080(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci. 2001;115(4):826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- Macrides F, Davis BJ, Youngs WM, Nadi NS, Margolis FL. Cholinergic and catecholaminergic afferents to the olfactory bulb in the hamster: a neuroanatomical, biochemical, and histochemical investigation. J Comp Neurol. 1981;203(3):495–514. doi: 10.1002/cne.902030311. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006;24(11):3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Peace S, Karnow A, Kim J, Ennis M, Linster C. Noradrenergic modulation in the olfactory bulb influences spontaneous and reward-motivated discrimination, but not the formation of habituation memory. Eur J Neurosci. 2008;27(5):1210–1219. doi: 10.1111/j.1460-9568.2008.06101.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Stack C, Kiselycznyk C, Linster C. Broad activation of the olfactory bulb produces long-lasting changes in odor perception. Proc Natl Acad Sci U S A. 2006;103(36):13543–13548. doi: 10.1073/pnas.0602750103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron. 2005;48(6):1039–1053. doi: 10.1016/j.neuron.2005.10.031. [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW. Olfactory learning in the rat pup: a model that may permit visualization of a mammalian memory trace. Neuroreport. 2004;15(11):1691–1697. doi: 10.1097/01.wnr.0000134988.51310.c3. [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Postmitotic, postmigrational expression of tyrosine hydroxylase in olfactory bulb dopaminergic neurons. J Neurosci. 1988;8(10):3658–3669. doi: 10.1523/JNEUROSCI.08-10-03658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Postnatal development of the noradrenergic projection from locus coeruleus to the olfactory bulb in the rat. J Comp Neurol. 1991;304(3):467–477. doi: 10.1002/cne.903040310. [DOI] [PubMed] [Google Scholar]
- Pavlis M, Feretti C, Levy A, Gupta N, Linster C. l-DOPA improves odor discrimination learning in rats. Physiol Behav. 2006;87(1):109–113. doi: 10.1016/j.physbeh.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Perez H, Hernandez A, Almli CR. Locus coeruleus stimulation modulates olfactory bulb evoked potentials. Brain Res Bull. 1987;18(6):767–770. doi: 10.1016/0361-9230(87)90213-9. [DOI] [PubMed] [Google Scholar]
- Puopolo M, Bean BP, Raviola E. Spontaneous activity of isolated dopaminergic periglomerular cells of the main olfactory bulb. J Neurophysiol. 2005 doi: 10.1152/jn.00225.2005. [DOI] [PubMed] [Google Scholar]
- Ravel N, Elaagouby A, Gervais R. Scopolamine injection into the olfactory bulb impairs short-term olfactory memory in rats. Behav Neurosci. 1994;108(2):317–324. doi: 10.1037//0735-7044.108.2.317. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Sallaz M, Jourdan F. Apomorphine disrupts odour-induced patterns of glomerular activation in the olfactory bulb. Neuroreport. 1992;3(10):833–836. doi: 10.1097/00001756-199210000-00003. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30(1):123–176. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res. 1985;329(1-2):294–299. doi: 10.1016/0006-8993(85)90537-2. [DOI] [PubMed] [Google Scholar]
- Stone DM, Wessel T, Joh TH, Baker H. Decrease in tyrosine hydroxylase, but not aromatic L-amino acid decarboxylase, messenger RNA in rat olfactory bulb following neonatal, unilateral odor deprivation. Brain Res Mol Brain Res. 1990;8(4):291–300. doi: 10.1016/0169-328x(90)90042-c. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Presynaptic inhibition of primary olfactory afferents mediated by different mechanisms in lobster and turtle. J Neurosci. 1999;19(20):8808–8817. doi: 10.1523/JNEUROSCI.19-20-08808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CJ, Linster C, Cleland TA. Dopamine D(2) receptor activation modulates perceived odor intensity. Behav Neurosci. 2006;120(2):393–400. doi: 10.1037/0735-7044.120.2.393. [DOI] [PubMed] [Google Scholar]
- West SE, Doty RL. Influence of epilepsy and temporal lobe resection on olfactory function. Epilepsia. 1995;36(6):531–542. doi: 10.1111/j.1528-1157.1995.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J Neurosci. 1995;15(8):5574–5581. doi: 10.1523/JNEUROSCI.15-08-05574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH. Mitral cell beta1 and 5-HT2A receptor colocalization and cAMP coregulation: a new model of norepinephrine-induced learning in the olfactory bulb. Learn Mem. 2003;10(1):5–15. doi: 10.1101/lm.54803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue EL, Cleland TA, Pavlis M, Linster C. Opposing effects of D1 and D2 receptor activation on odor discrimination learning. Behav Neurosci. 2004;118(1):184–190. doi: 10.1037/0735-7044.118.1.184. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986;243(4):488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Cullinan WE, Braun A. Afferents to basal forebrain cholinergic projection neurons: an update. Adv Exp Med Biol. 1991;295:43–100. doi: 10.1007/978-1-4757-0145-6_2. [DOI] [PubMed] [Google Scholar]