Abstract
Objective
Human treatment with ezetimibe results in a moderate 50–54% decrease in cholesterol absorption and a 15–20% decrease in plasma LDL-cholesterol levels; nevertheless, the efficacy of ezetimibe therapy has been recently challenged by the ENHANCE trial. We examined the efficacy of a moderate decrease in cholesterol absorption in preventing atherosclerosis formation in the mouse.
Methods and Results
Congenic 14DKK animals, consisting of a castaneus (CASA/Rk) chromosome 14 interval introgressed onto the C57BL/6J background, displayed a moderate decrease in cholesterol absorption rates. The effect of moderately decreased absorption on atherosclerosis formation was determined in 14DKK apolipoprotein E knockouts (14DKK-apoEKO). When compared to chow diet fed control apoEKO mice, congenic 14DKK-apoEKO displayed a moderate 41% decrease in cholesterol absorption rates, 30–37% decrease in plasma cholesterol levels, and a 70% decrease in atherosclerosis formation. Studies on cholesterol efflux and reverse cholesterol transport (RCT) from 14DKK bone marrow derived macrophages rejected a 14DKK interval-dependent atheroprotective effects that operate in macrophages. In contrast, 14DKK-apoEKO congenics were characterized by a 60% increase in RCT from peripheral tissue macrophages.
Conclusions
These studies strongly suggest that moderately decreased cholesterol absorption rates result in a large atheroprotective effect due to decrease in plasma cholesterol levels and increase in RCT from peripheral tissue macrophages.
Key words as indexing terms: Cholesterol, Absorption, Atherosclerosis, Reverse cholesterol transport, Macrophage
The absorption of cholesterol from the intestine is an important determinant of plasma cholesterol levels, a well established risk factor for atherosclerotic cardiovascular diseases. Clinical studies that examined the effect of a specific and potent cholesterol absorption inhibitor, ezetimibe, on cholesterol absorption and plasma cholesterol levels reported a proportional reduction of 50–54% in absorption rates, from 50% to 23%, and a decrease of 15–20% in plasma low density lipoprotein cholesterol (LDL-cholesterol) levels 1, 2. Previous cholesterol lowering trials indicated that every 1% decrease in plasma LDL-cholesterol is expected to decrease the risk for major cardiovascular events by 1%. Based on these estimates treatment with ezetimibe is expected to decrease the risk for cardiovascular events by 15–20%, a premise that is currently tested by several ongoing clinical trials 3, 4. Interestingly, and in striking contrast with these expectations, the recently published ENHANCE trial is challenging this view 5. This trial compared the effect of treatment with simvastatin to combined therapy with simvastatin plus ezetimibe on atherosclerosis formation in heterozygous familial hypercholesterolemic patients. Interestingly, when compared to the simvastatin treated group, the addition of ezetimibe resulted in further significant decrease in plasma LDL-cholesterol levels; however, there was no effect for this decrease on the ratio of media-to-intima thickness in large arteries, a widely accepted predictor of major cardiovascular events. Although the design and data analysis of this trial were criticized, and the relevance of media-to-intima thickness to atherosclerosis measurement has been challenged, these findings sparked a controversy over the add-on preventive effect of ezetimibe and challenged the prospects of inhibition of cholesterol absorption in atherosclerosis prevention.
The absence of clinical studies that directly addressed the effect of ezetimibe on major cardiovascular events justified studies that examined the atheroprotective effect of this drug in laboratory animals. In the mouse, aggressive reduction in cholesterol absorption rates 6 7, far lower than the decrease reported in ezetimibe treated subjects 1, provided a clear evidence of atheroprotective effect. However, although these studies provided an important proof of principle, in the wake of the ENHANCE trial there is a need to clarify whether only a moderate decrease in cholesterol absorption is atheroprotective and if it is to what extent. We addressed this question in apoEKO animals that carry a specific castaneus (CASA/Rk) chromosome 14 interval that causes a proportional decrease of 41% in cholesterol absorption rates (from 79% to 38%). We found that moderately decreased cholesterol absorption rates were associated with a 30–37% decrease in plasma cholesterol levels, a 60% increase in RCT from peripheral tissue macrophages, and a 70% decrease in atherosclerosis formation. These findings strongly suggest that even a moderate decrease in cholesterol absorption rates, very similar to what has been reported in ezetimibe treated patients1, results in a large atheroprotective effect due to decreased plasma cholesterol levels and increased RCT from peripheral tissues macrophages. These findings are encouraging and warrant further testing of cholesterol absorption inhibitors in prevention of major cardiovascular events.
Methods
Generation of 14DKK-apoEKO animals
Mapping of the chromosome 14 locus and generation of the chromosome 14DKK congenic animals have been described before 8, 9. In brief, 14DKK animals where generated through introgression of a castaneus (CASA/Rk) chromosome 14 interval (from 19.5–60cM) onto the C57BL/6J background. 14DKK-apoEKO mice were generated by intercrossing 14DKK congenics with C57BL/6J apoEKO.
Animal studies
All animals were bred and housed in a single temperature controlled room with a 12h light-dark cycle (6am–6pm light) at the Cleveland Clinic Biological Resources Unit. Animals were fed Teklad Rodent Chow pellets (catalog # 2918). All experiments were approved by the Institutional Animal Care and Use Committee.
Cholesterol absorption
Cholesterol absorption was measured as we described previously 10. In brief, animals were placed in metabolic cages, received a gastric bolus of olive oil supplemented with [14C]cholesterol and [3H]β-sitostanol, feces were collected for 24h, dried in a 55°C oven, pulverized, lipids extracted by chloroform:methanol (2:1, v/v), and fecal lipids counted for labeled lipids using LS6500 Beckman counter.
Cholesterol efflux from bone marrow macrophages
Mouse bone-marrow macrophages (BMM) were derived from femurs of freshly sacrificed mice as described by Smith et al.11 and Hume et al.12 with minor modifications. Briefly, bone marrow was flushed with PBS supplemented with 50U/ml penicillin / 50ug/ml streptomycin antibiotics and 0.1% FBS. Cells were washed twice with PBS, resuspended in RPMI-1640 media supplemented with 10% FBS, 2mM glutamine, penicillin/streptomycin antibiotics, 20% murine L-cell preconditioned media (as a source of macrophage colony-stimulating factor, MCSF), 50µM β-mercaptoethanol, and were cultured overnight at 37°C in teflon bags (Welch Fluorocarbon, Dover, NH). The following day additional media was added (60ml/femur) and cells cultured for 5 additional days. Macrophages were re-plated for efflux assay or frozen and stored in liquid nitrogen until further use. Freshly isolated and thawed cells were plated in 24-well plates at a density of 1.5×106 cells per well and cultured for an additional 7 days. For efflux assay, cells were cholesterol loaded by culturing in serum free RPMI-1640 media supplemented with penicillin/streptomycin, recombinant human MCSF (10ng/ml, R&D Systems), 0.1 mg/ml of acetylated LDL protein labeled with 0.34 µCi 3[H]cholesterol/ml, and incubated overnight at 37°C. The next day cells were washed 2–3 times with pre-warmed serum free RPMI-1640 and incubated overnight in the presence of increasing concentrations of human HDL (0–200 µg/ml protein of ultracentrifugally isolated total HDL) in fresh MCSF supplemented media. Media was collected and duplicate aliquots of 200µl were counted for 3[H] label. Cellular lipids were extracted in hexane:isopropanol (3:2), the organic phase was dried under nitrogen, redissolved in EcoLite scintillation fluid (MP Biomedicals, Solon, OH), and subjected to scintillation counting. Cholesterol efflux was determined in triplicate wells and percent efflux calculated using the formula: 3[H] DPM in media / (3[H] DPM in media + 3[H] DPM in cells) × 100.
Reverse cholesterol transport
BMM were derived, cultured, loaded with acetylated LDL cholesterol, and labeled for 48h with 0.5 µCi 14[C]cholesterol per ml as described above. On the day of the experiment cells were spun down, resuspended in plain RPMI-1640 media, and directly spiked with 1µCi of 3[H]β-sitostanol. Animals were placed in metabolic cages, injected subcutaneously with 0.3 ml/animal of resuspended BMM, feces collected for 48h, and percent RCT was calculated as we described previously13.
Quantitative atherosclerosis measurements
ApoEKO and 14DKK-apoEKO animals were fed Teklad chow diet (catalog # 2918) for 13 weeks. At 16 weeks of age animals were fasted, anesthetized and blood collected via ventricular puncture into an EDTA containing syringe. The circulatory system was perfused with phosphate-buffered saline through intraventricular injection. The heart containing the aortic root was fixed in phosphate-buffered formalin and processed for aortic root quantitative atherosclerosis assay as we described previously 14.
Plasma lipids and lipoprotein cholesterol levels
Plasma cholesterol, triglyceride, non-HDL, and HDL-cholesterol levels were determined enzymatically using colorimetric assays as we described previously 15. For measurement of plasma non-HDL and HDL-cholesterol levels plasma density was increased to 1.063 gm/ml with KBr, subjected to overnight ultracentrifugation (at 4°C, 70,000 rpm, Sorvall S100-AT3 rotor, Sorvall M150 SE Micro-Ultracentrifuge), the bottom HDL containing fraction was separated from the top non-HDL fraction and washed by one additional overnight ultracentrifugation at the same density, and plasma non-HDL and HDL cholesterol levels were determined as described above. Lipoprotein cholesterol profiles were determined by analysis of Superose gel-filtered plasma as we described previously15.
Statistical analysis
Group differences in cholesterol absorption rates, plasma cholesterol and HDL-cholesterol levels, and RCT were compared using an unpaired student t-test. Genotypic effect on aortic root atherosclerosis was examined by using a nonparametric Mann-Whitney test. Finally, genotypic effect on cholesterol efflux from bone marrow macrophages was tested by using a one-way ANOVA with a Tukey posttest.
Results
We have previously reported that 14DKK congenics are characterized by a moderate decrease in cholesterol absorption rates9. Here we examined the effect of the 14DKK interval on fasting plasma lipid levels. Measurement of plasma total cholesterol, triglycerides, non-HDL, and HDL cholesterol levels revealed that 14DKK congenics are characterized by increased plasma total cholesterol (75±6 vs 88±4 mg/dl in control C57BL/6J and 14DKK congenics, respectively; P<0.03), increased plasma triglycerides (10±2 vs 22±7 in C57BL/6J and 14DKK congenics, respectively; P<0.007), increased non-HDL cholesterol (19±3 vs 27±4 mg/dl in C57BL/6J vs 14DKK congenics, respectively; P<0.05), with no differences in HDL-cholesterol levels (53±5 vs 55±4 mg/dl in C57BL/6J and 14DKK congenics, respectively). Therefore, in 14DKK animals moderately decreased cholesterol absorption rates are associated with mildly increased fasting plasma levels of cholesterol in non-HDL proatherogenic lipoproteins.
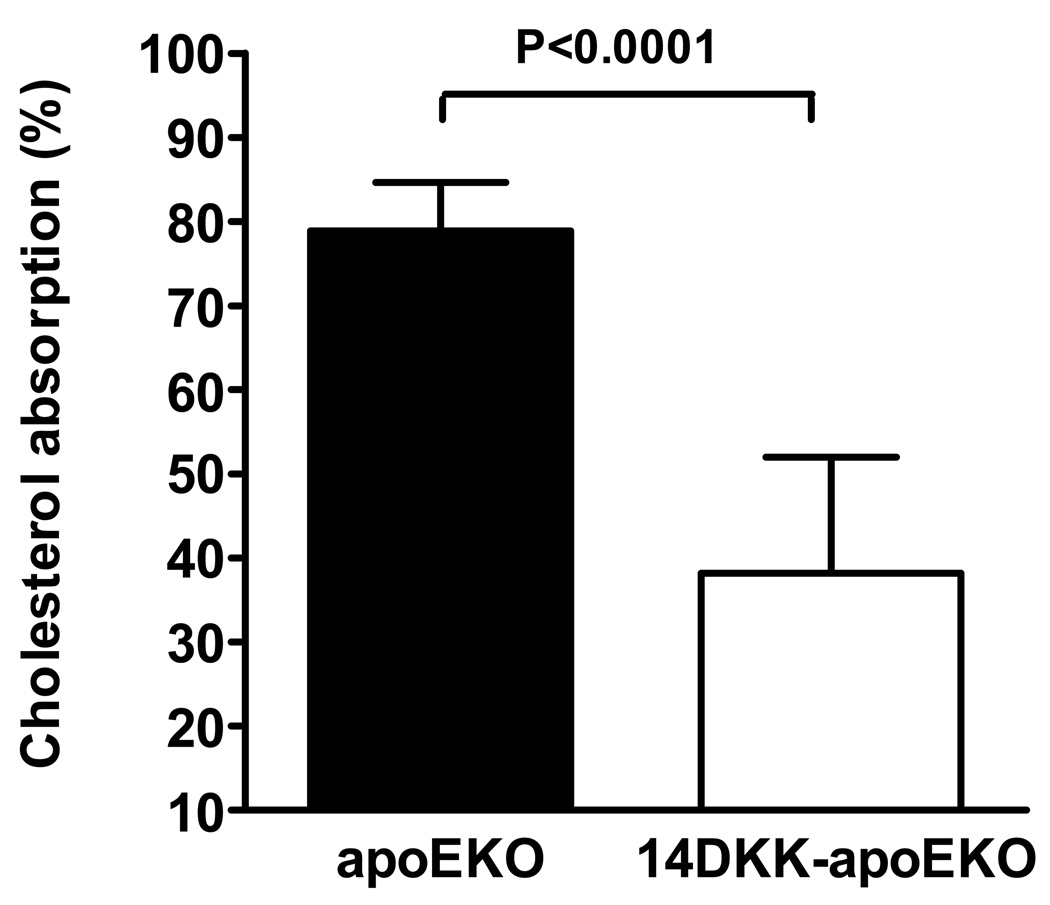
To examine the 14DKK interval effect on atherosclerosis formation we first examined the effect of this interval on cholesterol absorption in animals targeted for the apoE gene. We and others have previously reported that low cholesterol chow diet fed C57BL/6J wild type and apoEKO animals displayed indistinguishable cholesterol absorption rates 16,17. In agreement with these reports and in agreement with our original studies in 14DKK congenics, as shown in Figure 1, when compared to apoEKO, 14DKK-apoEKO animals displayed a moderate decrease of 41% in cholesterol absorption rates (79±6% vs 38±14% in C57BL/6J controls and 14DKK congenics, respectively; P<0.0001).
Figure 1.
Effect of the 14DKK interval on cholesterol absorption from the intestine. Control apoEKO and 14DKK-apoEKO males were placed in metabolic cages, received a gastric bolus of olive oil supplemented with radiolabeled cholesterol and β-sitostanol, and cholesterol absorption rates determined as described in the Methods section (N=6 per group; values shown are mean ± SD).
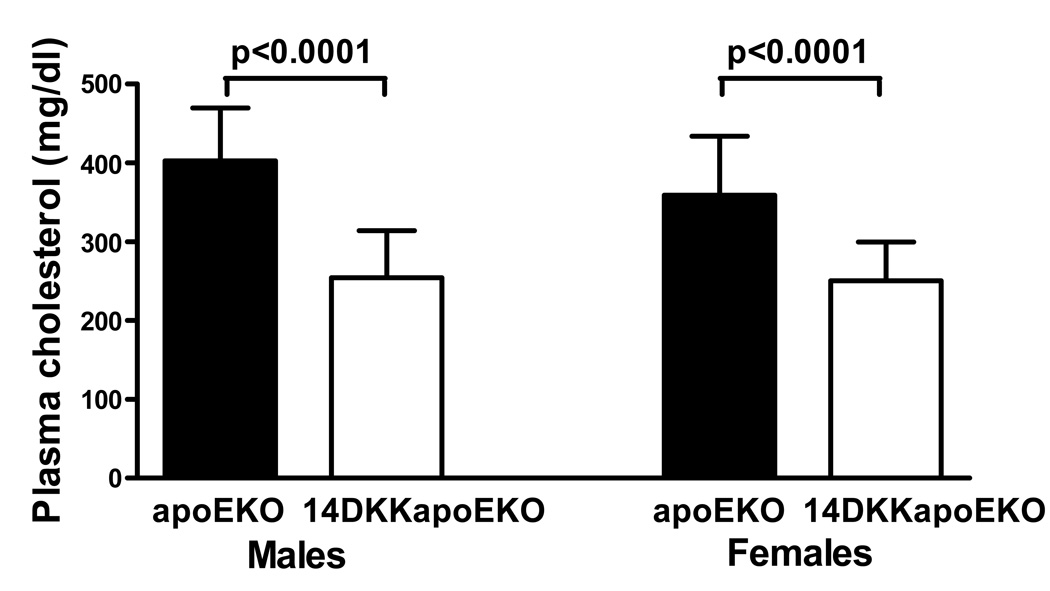
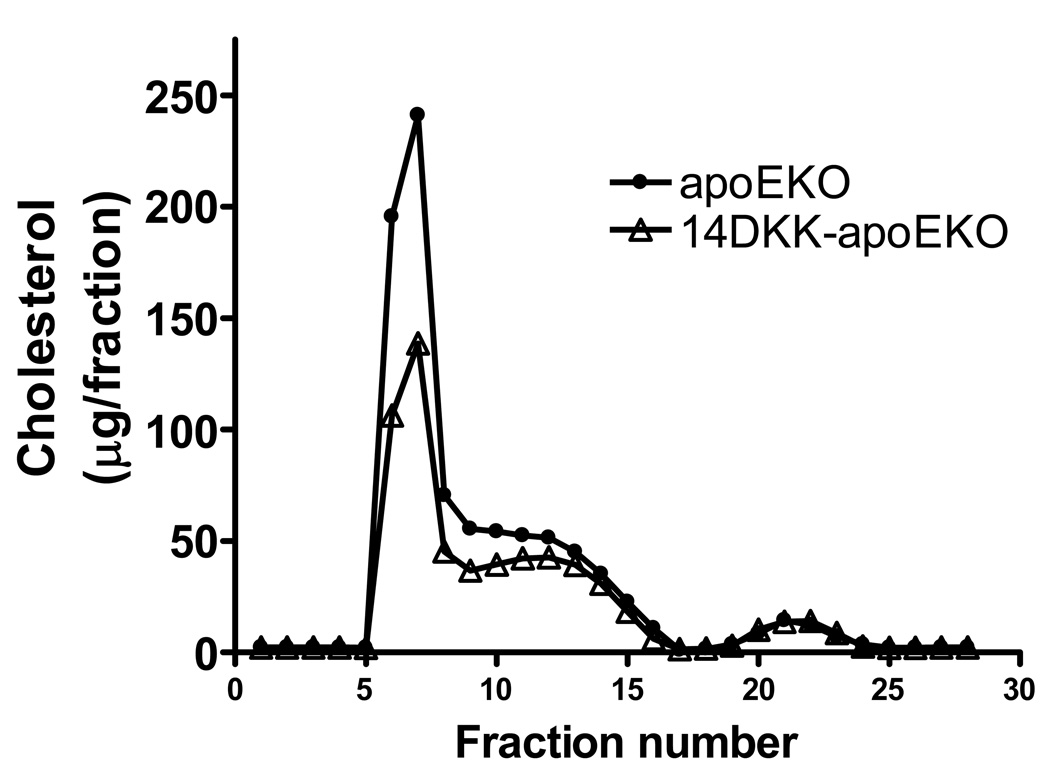
Next, the effect of moderately decreased cholesterol absorption rates on plasma cholesterol levels and atherosclerosis formation was examined. In agreement with previous studies in apoEKO animals 6, and as shown in Figure 2, when compared to control apoEKO mice, 14DKK-apoEKO congenic males and females displayed a 37% and 30% decrease in fasting plasma cholesterol levels, respectively (403±67 vs 254±60 mg/dl in males and 359±75 vs 251±49 mg/dl in females apoEKO and 14DKK-apoEKO congenics, respectively; P<0.0001). Moreover, as shown in Figure 3, Superose gel chromatography revealed that in 14DKK-apoEKO the decrease in plasma cholesterol is largely attributable to decrease in VLDL and IDL with no differences in HDL-cholesterol levels (further confirmed by ultracentrifugation and direct measurement of HDL cholesterol levels: 26±7 vs 27±5 mg/dl in males and 22±8 vs 24±6 mg/dl in females apoEKO and14DKK-apoEKO congenics, respectively). Interestingly, as shown in Figures 4A and 4B, in 14DKK-apoEKO congenics a 30–37% decrease in plasma cholesterol levels were associated with a 68% and 71% decrease in atherosclerosis lesion size in the aortic root of males and females, respectively (17,590±11,533 vs 5,662±4,279 µm2, P<0.001, and 73,231±34,491 vs 21,131±12,256 µm2, P<0.0001, in males and females apoEKO and 14DKK-apoEKO congenics, respectively). These results strongly suggest that a moderate decrease in cholesterol absorption rates displays a large atheroprotective effect.
Figure 2.
Effect of the 14DKK interval on plasma cholesterol levels. Plasma cholesterol levels were determined in fasted animals as described in the Methods section. (N=14–19 animals per group; values shown are mean ± SD).
Figure 3.
Plasma lipoprotein cholesterol profiles in apoEKO and 14DKK-apoEKO animals: ApoEKo and 14DKK-apoEKO animals were fasted, plasma samples isolated, plasma of animals in each group pooled, and plasma lipoprotein cholesterol profiles were analyzed as described in the Methods section (N=5 males in each group).
Figure 4.
Effect of the 14DKK interval on aortic root atherosclerotic lesions. Chow diet fed control apoEKO and 14DKK-apoEKO congenics males (A) and females (B) were fed with rodent chow diet, sacrificed at 16 weeks of age, and atherosclerosis lesion area at the aortic root was determined as described in the Methods section (N=14–19 animals per group).
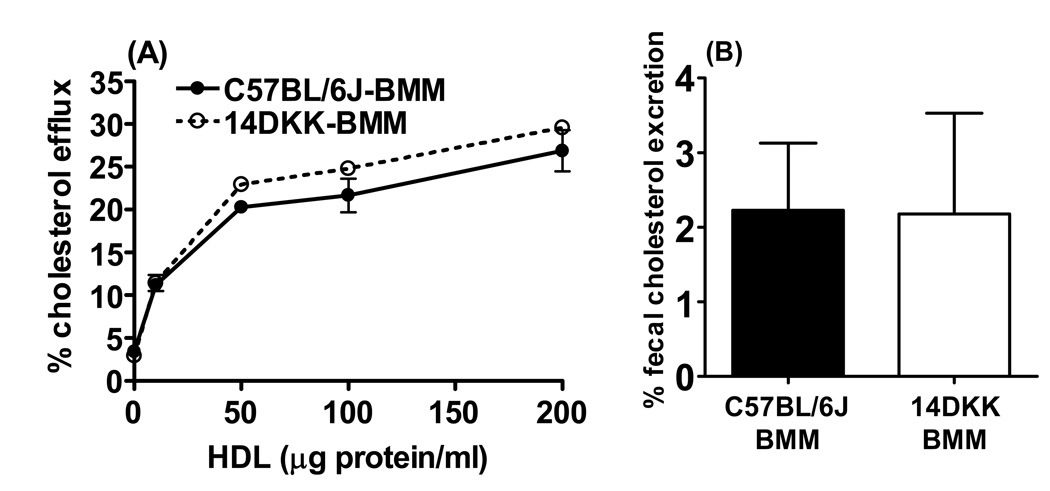
To determine whether the 14DKK interval atheroprotective effect can be attributed, at least in part, to mechanisms that operate in macrophages, we first examined the in-vitro efflux of cholesterol from cholesterol loaded bone marrow macrophages of control C57BL/6J and 14DKK congenics. As shown in Figure 5A, regardless the concentrations of HDL as a cholesterol acceptor in the incubation media, 14DKK macrophages displayed cholesterol efflux rates that were indistinguishable from efflux displayed by C57BL/6J control macrophages. Next, we examined whether cholesterol loaded 14DKK congenic bone marrow macrophages are characterized by increased in-vivo RCT levels. In these experiments, donor C57BL/6J control and 14DKK congenic bone marrow macrophages were loaded with cholesterol, injected subcutaneously into two different groups of recipient C57BL/6J control animals, and RCT was determined as we described previously13. As shown in Figure 5B, there was no difference in RCT from control C57BL/6J and 14DKK congenic macrophages (2.2±0.9% vs 2.2±1.4% RCT from C57BL/6J and 14DKK macrophages, respectively). Similar RCT rates were observed in experiments where donor apoEKO and 14DKK-apoEKO congenic macrophages were injected into recipient apoEKO animals (3.4±1.2% vs 2.9±1.4% from apoEKO and 14DKK-apoE macrophages, respectively). When combined, these experiments strongly suggested that the 14DKK interval atheroprotective effect can not be attributed to mechanisms that modify the efflux of cholesterol or RCT from peripheral tissue macrophages.
Figure 5.
Effect of the 14DKK interval on in-vitro cholesterol efflux and in-vivo RCT from peripheral tissue macrophages. (A) Bone marrow macrophages (BMM) from control C57BL/6J and 14DKK congenic animals were loaded with cholesterol and labeled with 3[H]cholesterol. Cholesterol efflux was determined in macrophages incubated in absence or presence of the indicated concentrations of human HDL protein. Percent cholesterol efflux was determined as described in the Methods section. Data is a representative one experiment out of a total of three experiments. (B) Donor bone marrow macrophages of control C57BL/6J and 14DKK congenics were loaded with cholesterol and labeled with radiolabeled cholesterol and β-sitostanol. Labeled donor macrophages were injected into recipient C57BL/6J control animals and RCT determined as described in the Methods section (N=5 per group; values shown are mean ± SD).
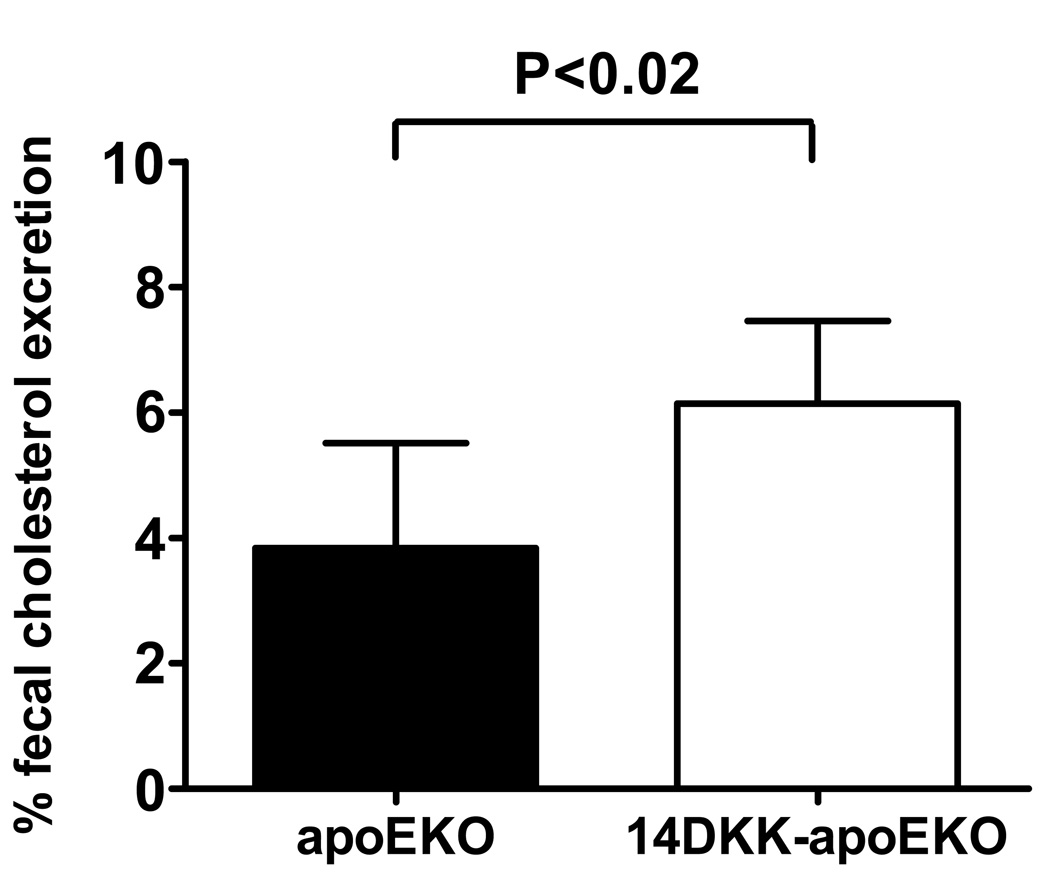
We have previously reported that suppression of cholesterol absorption from the intestine increases the RCT from peripheral tissue macrophages13. Specifically, we have shown that in 14DKK congenics decreased cholesterol absorption rates are associated with a 67% increase in RCT from subcutaneously injected murine 264.7 RAW macrophages 13. Here we turned to validate these findings by examining the 14DKK interval effect on RCT from cholesterol loaded apoEKO bone marrow macrophages. In these experiments donor control apoEKO bone marrow macrophages were injected subcutaneously into recipient control apoEKO and 14DKK-apoEKO congenics, and RCT determined as we described previously13. As shown in Figure 6, and in agreement with our previous studies, moderately decreased cholesterol absorption rate in 14DKK congenics was associated with a 60% increase in RCT from subcutaneously injected control apoEKO bone marrow macrophages (3.8±1.7% vs 6.1±1.7% in apoEKO and 14DKK-apoEKO congenics, respectively; P<0.02). Similar differences in RCT were observed when donor C57BL/6J wild type bone marrow macrophages were injected into recipient C57BL/6J control and 14DKK congenics (3.2.±1.2% vs 5.9±1.9% in C57BL/6J controls and 14DKK congenics, respectively; P<0.03). These experiments strongly suggest that in 14DKK congenics decreased cholesterol absorption rates leads to an increase in RCT from peripheral tissue macrophages, which is likely to explain at least part of the atheroprotective effect of this interval.
Figure 6.
Effect of the 14DKK interval on RCT from apoEKO bone marrow macrophages: Donor bone marrow macrophages of control apoEKO were loaded with cholesterol, injected subcutaneously into recipient apoEKO and 14DKK-apoEKO congenic animals and RCT was determined as described in the Methods section (N=6 nimals per group; values shown are mean ± SD).
Discussion
The present study addressed the effect of moderately decreased cholesterol absorption rates on atherosclerosis in the mouse. We found that in 14DKK congenics a 41% decrease in cholesterol absorption rates resulted in a 30–37% decrease in plasma cholesterol levels, a 60% increase in RCT from peripheral tissue macrophages, and a 70% decrease in aortic root atherosclerosis lesion size. These findings strongly suggest that a moderate decrease in cholesterol absorption is associated with a large atheroprotective effect.
Previous mouse studies examined the effect of potent inhibition of cholesterol absorption on atherosclerosis formation and have consistently shown a strong atheroprotective effect. For example, Davis et al. examined the effect of treatment with high dose ezetimibe on cholesterol absorption, plasma cholesterol levels, and atherosclerosis formation in the carotid artery of apoEKO mice 6. In these studies treatment with high dose ezetimibe (0.005% w/w in the diet) resulted in a dramatic 90% decrease in cholesterol absorption rates and a 91–97% decrease in atherosclerosis formation. Furthermore, the same investigators demonstrated that in apoEKO mice knocking out of Npc1l1, the target of ezetimibe, resulted in a 77% decrease in cholesterol absorption rates and an absolute protection of atherosclerosis 18. In the present study we examined the genetic effect of only a moderate decrease in cholesterol absorption rates on atherosclerosis formation. We found that a decrease of 41% in cholesterol absorption rates and 30–37% decrease in plasma cholesterol levels were associated with 70% decrease in aortic root atherosclerosis lesion size. Our current results together with the studies of Davis et al may suggest a non-linear relationship between cholesterol absorption and atherosclerosis formation. Such a relationship is supported by our prior study in which we demonstrated that cholesterol absorption is a non-linear saturable process 10. Nevertheless, we are not aware of prior studies that systematically examined the relationship of cholesterol absorption rates to atherogenesis in mice.
In humans, the expectation that inhibition of cholesterol absorption is atheroprotective primarily relies on the concordance between the decrease in cholesterol absorption and plasma cholesterol levels. This expectation is supported by a recent study that found a modest but consistent correlation of plasma surrogate measures of cholesterol absorption and angiographic severity of coronary heart disease 19. Here, we found that a moderate 41% decrease in cholesterol absorption rates proportionally decreased the plasma level of cholesterol by 30–37% in apoEKO animals. These findings are in agreement with the notion that in apoEKO animals increased plasma cholesterol levels are due to plasma retention of intestinally derived cholesterol in the form of chylomicron remnants (Figure 3). It is therefore interesting that in 14DKK-apoEKO congenics a decrease of 30–37% in plasma cholesterol levels resulted in a 70% decrease in atherosclerosis formation. These findings suggest that additional mechanisms, independent from plasma cholesterol levels, may play a role in protecting the 14DKK congenic animals from atherosclerosis. To determine the nature of these mechanisms we examined the efflux of cholesterol and RCT from cholesterol loaded 14DKK-congenics bone marrow macrophages (Figures 5A and 5B). Our results clearly indicate that 14DKK bone marrow macrophages display in-vitro cholesterol efflux and in-vivo RCT that are indistinguishable from the corresponding rates in control C57BL/6J bone marrow macrophages. These findings reject the possibility that the 14DKK-interval atheroprotective effect can be ascribed to mechanisms that operate in macrophages. In contrast, subcutaneous injection of cholesterol loaded control apoEKO bone marrow macrophages into recipient 14DKK-apoEKO animals resulted in 60% increase in RCT (Figure 6). These findings strongly suggest that the 14DKK interval increases RCT from peripheral tissue macrophages through inhibition of cholesterol absorption from the intestine. Jointly, our findings favor a scenario where the 14DKK interval is atheroprotective through dual cholesterol-absorption modifiable effects: (i) a decrease in plasma cholesterol levels, and (ii) an increase in RCT from atherosclerotic lesion macrophages. It should be noted however that the 14DKK 40.5cM congenic interval harbors a large number of genes, and we can not exclude the possibility that CASA/Rk variants in this interval may modify atherosclerosis formation through mechanisms independent of cholesterol absorption effects. Finally, we have previously shown that treatment with 0.005% ezetimibe is associated with a 6 fold increase in RCT from peripheral tissue macrophages 13. These findings agree with a nearly absolute protection of atherogenesis in high ezetimibe dose treated and Npc1l1 targeted apoEKO mice 6, 18.
Clinical trials almost invariably find that a decrease in plasma cholesterol levels decreases the risk for major atherosclerotic cardiovascular events. Therefore, a consistently reported decrease in plasma cholesterol levels in response to treatment with ezetimibe strongly suggested that this therapy is likely to result in protection from these events. However, contrary to these expectations, the ENHANCE trial, that examined the add-on effect of ezetimibe in heterozygous familial hypercholesterolemic patients, found no additive effect for this drug on carotid intima-to-media thickness ratio and sparked a controversy regarding the capacity of this therapy to fulfill its potential atheroprotective effect 5. Furthermore, the results of this trial strongly contrasted with mouse studies that consistently find a strong atheroprotective effect in the treated mice with decreased cholesterol absorption rates. It should be noted, however, that although these studies provide a solid proof of concept, extrapolation to humans must be regarded cautiously. This caution is particularly needed for mouse studies where cholesterol absorption rates are far lower than levels reported in human studies 1. In the current study, we examined the effect of moderately decreased cholesterol absorption rates that are proportionally very similar to what has been reported in humans (a decrease of 50–54% and 41% in ezetimibe treated humans and 14DKK-apoEKO congenics, respectively). Our mouse findings clearly indicate that a moderate decrease in cholesterol absorption exerts a large atheroprotective effect and further support the targeting of the cholesterol absorption process for prevention of atherosclerosis cardiovascular events. It remains to be seen whether ongoing large clinical trials will confirm the atheroprotective premise in ezetimibe therapy using endpoints more relevant than the carotid intima-media thickness3, 4.
Acknowledgments
Sources of Funding
This work was supported by a Grant-in-Aid 0755295B from the American Heart Association (to E.S.) and by the National Institute of Health Grant P50 HL077107 (to J.D.S.).
References
- 1.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 2.Brown WV. Cholesterol absorption inhibitors: defining new options in lipid management. Clin Cardiol. 2003;26:259–264. doi: 10.1002/clc.4950260604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannon CP, Giugliano RP, Blazing MA, Harrington RA, Peterson JL, Sisk CM, Strony J, Musliner TA, McCabe CH, Veltri E, Braunwald E, Califf RM. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008;156:826–832. doi: 10.1016/j.ahj.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Peto R, Emberson J, Landray M, Baigent C, Collins R, Clare R, Califf R. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359:1357–1366. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 5.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 6.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–2038. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 7.Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7:508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Sehayek E, Duncan EM, Lutjohann D, Von Bergmann K, Ono JG, Batta AK, Salen G, Breslow JL. Loci on chromosomes 14 and 2, distinct from ABCG5/ABCG8, regulate plasma plant sterol levels in a C57BL/6J × CASA/Rk intercross. Proc Natl Acad Sci U S A. 2002;99:16215–16219. doi: 10.1073/pnas.212640599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sehayek E, Fung YY, Yu HJ, Lembcke J, Ceglarek U, Teupser D, Thiery J, Lutjohann D, von Bergmann K, Breslow JL. A complex plasma plant sterol locus on mouse chromosome 14 has at least two genes regulating intestinal sterol absorption. J Lipid Res. 2006;47:2291–2296. doi: 10.1194/jlr.M600202-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Sehayek E, Ono JG, Shefer S, Nguyen LB, Wang N, Batta AK, Salen G, Smith JD, Tall AR, Breslow JL. Biliary cholesterol excretion: A novel mechanism that regulates dietary cholesterol absorption. Proc Natl Acad Sci U S A. 1998;95:10194–10199. doi: 10.1073/pnas.95.17.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JD, Peng DQ, Dansky HM, Settle M, Baglione J, Le Goff W, Chakrabarti E, Xu Y, Peng X. Transcriptome profile of macrophages from atherosclerosis-sensitive and atherosclerosis-resistant mice. Mamm Genome. 2006;17:220–229. doi: 10.1007/s00335-005-0099-7. [DOI] [PubMed] [Google Scholar]
- 12.Hume DA, Allan W, Golder J, Stephens RW, Doe WF, Warren HS. Preparation and characterization of human bone marrow-derived macrophages. J Leukoc Biol. 1985;38:541–552. doi: 10.1002/jlb.38.4.541. [DOI] [PubMed] [Google Scholar]
- 13.Sehayek E, Hazen SL. Cholesterol Absorption From the Intestine Is a Major Determinant of Reverse Cholesterol Transport From Peripheral Tissue Macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1296–1297. doi: 10.1161/ATVBAHA.108.165803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dansky HM, Charlton SA, Sikes JL, Heath SC, Simantov R, Levin LF, Shu P, Moore KJ, Breslow JL, Smith JD. Genetic background determines the extent of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:1960–1968. doi: 10.1161/01.atv.19.8.1960. [DOI] [PubMed] [Google Scholar]
- 15.Sehayek E, Ono JG, Duncan EM, Batta AK, Salen G, Shefer S, Neguyen LB, Yang K, Lipkin M, Breslow JL. Hyodeoxycholic acid efficiently suppresses atherosclerosis formation and plasma cholesterol levels in mice. J Lipid Res. 2001;42:1250–1256. [PubMed] [Google Scholar]
- 16.Sehayek E, Shefer S, Nguyen LB, Ono JG, Merkel M, Breslow JL. Apolipoprotein E regulates dietary cholesterol absorption and biliary cholesterol excretion: Studies in C57BL/6 apolipoprotein E knockout mice. Proc Natl Acad Sci U S A. 2000;97:3433–3437. doi: 10.1073/pnas.050016197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woollett LA, Osono Y, Herz J, Dietschy JM. Apolipoprotein E competitively inhibits receptor-dependent low density lipoprotein uptake by the liver but has no effect on cholesterol absorption or synthesis in the mouse. Proc Natl Acad Sci U S A. 1995;92:12500–12504. doi: 10.1073/pnas.92.26.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis HR, Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, Graziano MP, Altmann SW. Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:841–849. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 19.Silbernagel G, Fauler G, Renner W, Landl EM, Hoffmann MM, Winkelmann BR, Boehm BO, Marz W. The relationships of cholesterol metabolism and plasma plant sterols with the severity of coronary artery disease. J Lipid Res. 2009;50:334–341. doi: 10.1194/jlr.P800013-JLR200. [DOI] [PubMed] [Google Scholar]