Abstract
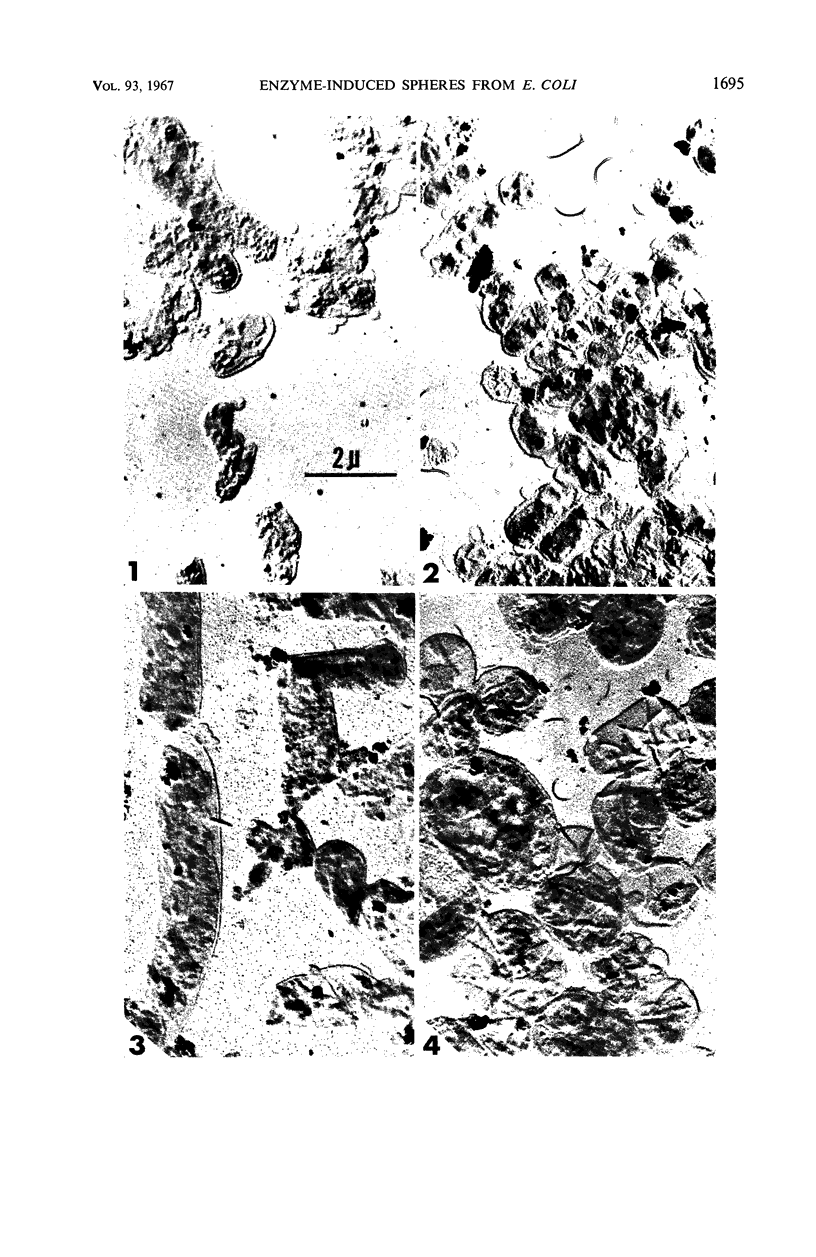
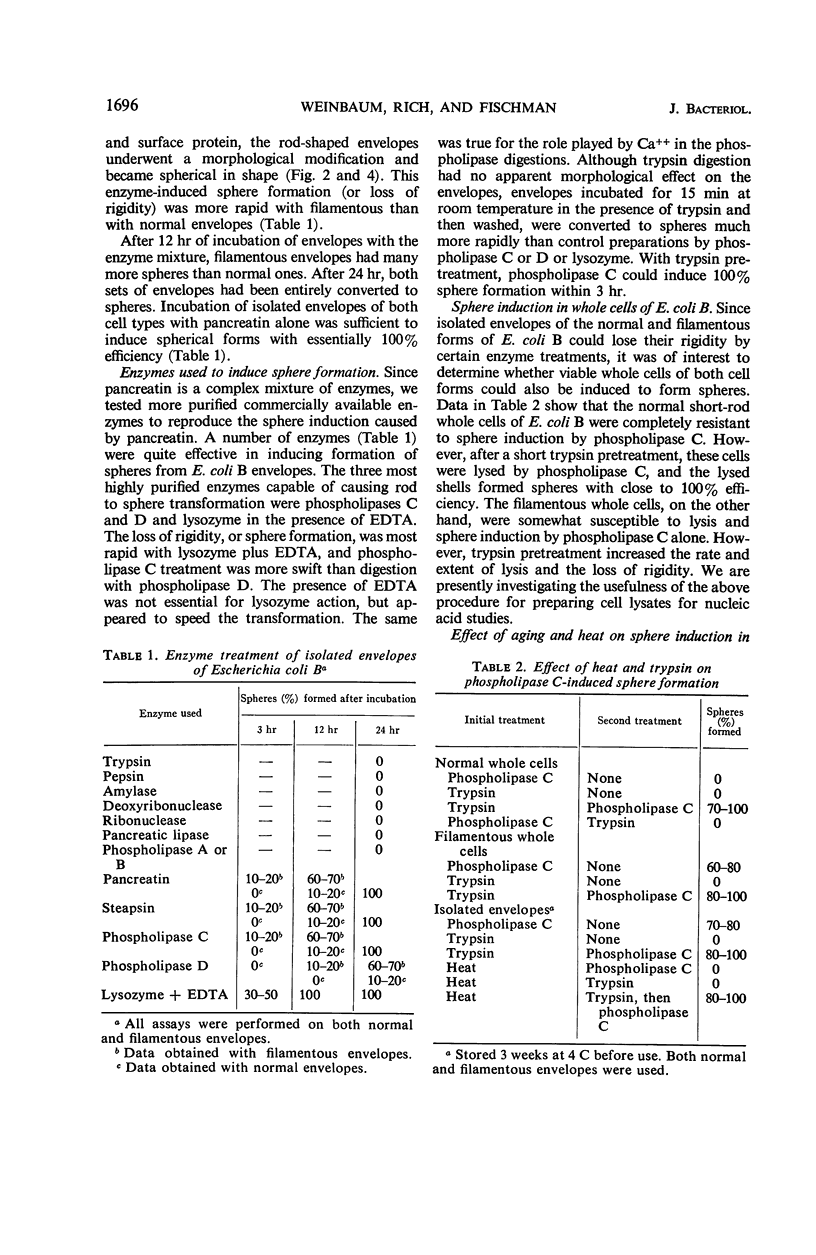
Normal and filamentous whole cells and isolated envelopes of Escherichia coli B were exposed to various enzymatic treatments to remove surface layers and to characterize the component(s) conferring rigidity in this organism. Modification of cell rigidity was determined by sphere formation in both whole cells and isolated envelopes. Enzymes capable of converting trypsinized normal or untreated filamentous whole cells and untreated envelopes to spheres included: lysozyme plus ethylenediaminetetraacetic acid, clostridial phospholipase C, and phospholipase D from cabbage. These data suggest that there are at least two components essential for maintenance of cell rigidity in E. coli B. The first is the peptidoglycan (mucopeptide), which is susceptible to lysozyme. The second is a phospholipid which is either covalently linked to the mucopeptide or in close association with it. This phospholipase C-sensitive component is protected more completely in normal than in filamentous whole cells by a protein layer which is easily modified by trypsin treatment to allow enzymatically induced sphere formation to occur.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., DAWSON R. M. Electrokinetic requirements for the reaction between Cl. perfringens alpha-toxin (phospholipase C) and phospholipid substrates. Biochim Biophys Acta. 1962 May 7;59:103–115. doi: 10.1016/0006-3002(62)90701-1. [DOI] [PubMed] [Google Scholar]
- Carson K. J., Eagon R. G. Lysozyme sensitivity of the cell wall of Pseudomonas aeruginosa. Further evidence for the role of the non-peptidoglycan components in cell wall rigidity. Can J Microbiol. 1966 Feb;12(1):105–108. doi: 10.1139/m66-015. [DOI] [PubMed] [Google Scholar]
- Fischman D. A., Weinbaum G. The formation of multiple layers of membrane-like structures in Escherichia coli B. J Cell Biol. 1967 Feb;32(2):524–528. doi: 10.1083/jcb.32.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUTGEB W., MAASS D., WEIDEL W. HERSTELLUNG UND QUANTITATIVE ZUSAMMENSETZUNG PROTEIN-UND GLUCOSEFREIER STUETZMEMBRANEN (R-LAYERS) AUS COLI-ZELLWAENDEN. Z Naturforsch B. 1963 Dec;18:1062–1064. [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. A high speed shaker for the disruption of cells at low temperatures. Biochim Biophys Acta. 1957 Apr;24(1):203–204. doi: 10.1016/0006-3002(57)90168-3. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- Weinbaum G. Characteristics of cell walls from morphological variants of Escherichia coli. J Gen Microbiol. 1966 Jan;42(1):83–92. doi: 10.1099/00221287-42-1-83. [DOI] [PubMed] [Google Scholar]
- Weinbaum G., Markman R. A rapid technique for distinguishing enzymatically active proteins in the cell"envelope" of Escherichia coli B. Biochim Biophys Acta. 1966 Jul 27;124(1):207–209. doi: 10.1016/0304-4165(66)90335-7. [DOI] [PubMed] [Google Scholar]