Abstract
The binding of interleukin-6 (IL-6) cytokine family ligands to the gp130 receptor complex activates the Janus kinase (JAK)/ signal transducer and activator of transcription 3 (STAT3) signal transduction pathway, where STAT3 plays an important role in cell survival and tumorigenesis. Constitutive activation of STAT3 has been frequently observed in many cancer tissues, and thus, blocking of the gp130 signaling pathway, at the JAK level, might be a useful therapeutic approach for the suppression of STAT3 activity, as anticancer therapy. AG490 is a tyrphostin tyrosine kinase inhibitor that has been extensively used for inhibiting JAK2 in vitro and in vivo. In this study, we demonstrate a novel mechanism associated with AG490 that inhibits the JAK/STAT3 pathway. AG490 induced downregulation of gp130, a common receptor for the IL-6 cytokine family compounds, but not JAK2 or STAT3, within three hours of exposure. The downregulation of gp130 was not caused by enhanced degradation of gp130 or by inhibition of mRNA transcription. It most likely occurred by translation inhibition of gp130 in association with phosphorylation of the eukaryotic initiation factor-2α . The inhibition of protein synthesis of gp130 by AG490 led to immediate loss of mature gp130 in cell membranes, due to its short half-life, thereby resulting in reduction in the STAT3 response to IL-6. Taken together, these results suggest that AG490 blocks the STAT3 activation pathway via a novel pathway.
Keywords: Interleukin-6, STAT3, AG490, Endoplasmic reticulum stress, gp130
INTRODUCTION
The interleukin-6 (IL-6) cytokine family consists of IL-6, ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), oncostatin M and cardiotrophin-1. These cytokines control many biological processes such as inflammation, differentiation of immune cells and regeneration (Heinrich et al., 2003). IL-6 binds first to the IL-6 receptor alpha (IL-6R) and then this complex binds to the signal-transducing gp130 receptor (gp130), forming a heteromeric receptor complex. The signal transducing function of gp130 is shared by other IL-6 cytokine family members such as CNTF and LIF. The engagement of gp130 by these ligands then activates the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) pathway. JAK-induced tyrosine phosphorylation of STAT3 leads to dimerization and activation of STAT3 (Battle and Frank, 2002).
Recent studies have demonstrated that IL-6 is involved in the development of several tumors including multiple myeloma, stomach cancer and gliomas (Lauta et al., 2001; Tebbutt et al., 2002; Weissenberger et al., 2004). Tumorigenesis associated by IL-6 has been attributed to constitutive or aberrant activation of STAT3 (Aggarwal et al., 2006; Germain and Frank, 2007). Given the importance of constitutively active STAT3 in tumorigenesis, targeting JAK2 has been considered a potentially good therapeutic strategy for anticancer therapy (Opdam et al., 2004; Samanta et al., 2006). Tyrphostin AG490 and its derivatives have been widely used for inhibiting JAK2, as a method of blocking STAT3 activation in vitro and in vivo (Miyamoto et al., 2001; Smanta et al., 2006).
Even though many kinase inhibitors have been widely used for targeting specific kinases, the 'target off' effects of the kinase inhibitors frequently produces non-specific effects, especially used at high concentrations. In the present study, we found that AG490 suppressed gp130 protein synthesis in a JAK2-independent manner. This resulted in immediate loss of gp130 in the membranes due to its short half-life and concomitant reduction of IL-6-induced STAT3 activation.
METHODS
Chemicals
All of the phospho-specific antibodies and recombinant IL-6 used in this work were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies to STAT3, JAK2, gp130, CHOP and beta-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). AG490 and AG1478 were purchased from Calbiochem (Gibbstown, NJ, USA). Streptavidin-conjugated agarose beads and biotin-NHS were obtained from Pierce (Rockford, IL, USA). Reverse transcriptase, RNAase inhibitor and Taq polymerase were obtained from Promega (Madison, WI, USA). All other undesignated reagents were purchased from Sigma (St. Louis, MO, USA).
Cell culture and transient transfection
Rat schwannoma RT4 cells were obtained from the American Type Culture Collection (Rockville, MD, USA) and maintained as previously described (Lee et al., 2009a).
Survival assay
The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to analyze cell survival as previously described (Lee et al., 2009a). Briefly, the cells were plated in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) at a density of 5 × 103 cells/well in a 96 well tissue culture plate overnight at 37℃. One day after plating, the cells were washed twice with PBS and grown in serum-free DMEM for 1 day in the presence or absence of the indicated reagents, followed by incubation for 1 hour in 0.5 mg/ml MTT (Sigma). The medium was then aspirated, and the cells were dissolved in isopropanol (100 µl/well). Measurement of the reaction products was performed in a microplate reader (Bio-Rad). All experiments were repeated a minimum of three times.
Western blot analysis
Cells were harvested and lysed in modified radioimmune precipitation assay (RIPA) lysis buffer (150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 0.5% deoxycholic acid, 2 µg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 5 mM benzamidine, 1 mM sodium orthovanadate, and 1×protease inhibitor cocktail (Roche, Indianapolis, IN, USA)). Equivalent amounts (25~35 µg) of protein were separated by 8% SDS-PAGE, and then transferred onto a nitrocellulose membrane (Amersham Biosciences). After blocking with 0.1% Tween-20 and 5% nonfat dry milk in Tris-buffered saline (TBST; 25 mM Tris-HCl pH 7.5, 140 mM NaCl) at room temperature for 1 hour, the membrane was incubated with primary antibody in TBST containing 2% nonfat dry milk at 4℃ overnight. After three 15 min washes with TBST, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (1:3,000; Amersham Bioscience) for 1 hour at room temperature. The signals were detected using the Enhanced Chemiluminescence System (ECL Advance Kit, Amersham Biosciences). For quantification, X-ray films were then scanned using an HP scanner and analyzed with the LAS image analysis system (Fujifilm, Japan). The intensities of the gp130 bands were normalized to those of beta-tubulin.
Luciferase promoter assay
RT4 cells were transfected with luciferase reporter genes fused with the 2.5 kb glial fibrillary acidic protein (GFAP) promoter, GF1L (kindly provided by Dr. T. Taga and K. Ikenaka) using lipofectamine (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. Control transfections were performed using a lacZ gene driven by an RSV promoter. On the following day, the cells were pretreated with AG490 for 2 hrs, then stimulated with IL-6 for 6 h and solubilized with lysis buffer (Promega, Madison, WI, USA), and the luciferase activity was determined using a kit provided by Promega. β-galactosidase activity was measured using the Galactolight kit (Promega) as recommended by the manufacturer. Luciferase activity was normalized against β-galactosidase activity to correct for the variation in transfection efficiency.
Cell surface biotinylation assay
RT4 cells grown on 60 mm dishes were washed three times with ice-cold phosphate buffered saline (PBS) and then incubated with 0.5 mg/ml Biotin-X-NHS dissolved in borate buffer (10 mM boric acid, 150 mM NaCl pH 8.0) for 1 h at 4℃. The cells were extensively washed with PBS and then returned to 37℃, for the indicated time, with or without AG490. Cellular extracts were prepared with RIPA buffer and were precipitated with streptavidin-conjugated agarose beads and gel electrophoresis. Visualization of the biotinylated proteins was performed by probing the nitrocellulose membrane with an antibody against gp130.
Reverse transcription-polymerase chain reaction
The RNAeasy Protect Minikit (Qiagen, Crawley, UK) was used to extract total RNA from cells. For the cDNA synthesis, 200 ng of total RNA were used with Ready-To-Go-Your-Prime First Strand beads (Amersham Biosciences) and oligo (dT) primers, as indicated by the manufacturer. The primers were as follows: Rat gp130 (forward; 5'-CCGTCAGTGCAAGTGTTCTCA, reverse; 5'- CACTATCCACCAGCTGCAGGT) and rat GAPDH (forward; 5'- TGCCGCCTGGAGAAACCTGC-3', reverse; 5'-TGAGAGCAATGCCAGCCCCA-3'). After an initial step at 94℃ for 3 min, 28 cycles consisted of 94℃ for 30 sec, 58℃ for 1 min, and 72℃ for 30 sec before a final extension period of 5 min at 72℃. The PCR products were visualized by agarose gel electrophoresis stained with ethidium bromide.
Statistical analysis
Differences in the means between groups were statistically assessed using an analysis of variance followed by the Bonferroni post hoc test. The differences were considered to be statistically significant at a p<0.05.
RESULTS
Long-term pretreatment of AG490 is required for blocking IL-6-induced STAT3 activation
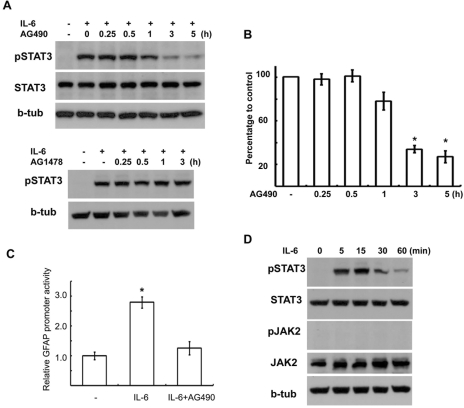
JAK2 has been implicated in the tyrosine phosphorylation of STAT3 in neuroglial cells (Satriotomo et al., 2006; Shyu et al., 2008). In order to determine the role of JAK2 in the IL-6-induced tyrosine phosphorylation of STAT3 in RT4 schwannoma cells, we first examined the effects of the specific JAK2 inhibitor, AG490, on the IL-6-induced tyrosine phosphorylation (Tyr705) of STAT3. The pretreatment of AG490 (50 µM) inhibited IL-6 (50 ng/ml)-induced tyrosine phosphorylation of STAT3 only when the cells were pretreated for more than 3 hrs before IL-6 stimulation; this did not affect protein levels of STAT3 (Fig. 1A, B). By contrast, long-term treatment with an EGFR inhibitor, AG1478, had no effect on the IL-6-induced tyrosine phosphorylation of STAT3 (Fig. 1A). We previously showed that IL-6 treatment induced GFAP expression in a STAT3- dependent manner in RT4 cells (Lee t al., 2009b). In this study we investigated whether AG490 treatment suppressed the IL-6 signaling pathway by measuring the GFAP promoter activity using a luciferase promoter assay. The pretreatment with AG490 significantly reduced IL-6-induced activation of GFAP promoter activity (Fig. 1C). These findings suggest that AG490 indeed blocks the IL-6/STAT3 signaling pathway.
Fig. 1.
AG490 inhibited IL-6-induced STAT3 activation. (A) AG490 (50 µM) suppressed tyrosine phosphorylation of STAT3 by IL-6 in a time-dependent manner without affecting STAT3 protein levels. AG490 or AG1478 (50 µM) was pretreated for the indicated times, and then stimulated with IL-6 for 15 min. (B) Quantitative results showing the change in pSTAT3 levels following AG490 treatment for the indicated times. (C) Luciferase promoter assay. RT4 cells were transfected with a GFAP promoter-luciferase reporter construct (GF1L). One day after transfection, the cells were pretreated with AG490 (50 µM) for 2 hrs, and then simulated with IL-6 for 6 hrs. The results are expressed as a relative value to the untreated controls. Data are the mean±S.E. from three independent experiments. (D) Temporal profile of tyrosine phosphorylation of STAT3 and JAK2 by IL-6. The cells were treated with IL-6 (50 ng/ml) for the indicated times, and then Western blot analysis was performed to examine tyrosine phosphorylation of STAT3 and JAK2. b-tub; beta-tubulin. *p<0.05.
The inhibition of IL-6-induced STAT3 activation by AG490 suggests the involvement of JAK2 in this pathway. Therefore, we thus investigated whether JAK2 was activated by IL-6 in the RT4 cells, and unexpectedly found that IL-6 did not activate JAK2, based on Western blot analysis with an antibody against phospho-JAK2 (Fig. 1D). With regard to inhibitor pretreatment, the requirement of AG490 treatment, for more than 3 hrs, to suppress the IL-6/STAT3 pathway was unexpected. Most kinase inhibitors, in our experience, are effective after 20~30 min of pretreatment. Thus, we questioned whether AG490 might have some property, not yet determined that inhibits STAT3 activation by IL-6. Because 50 µM of AG490 very mildly reduced cell survival as measured by the MTT assay 1 day after treatment (87.2%±5.3 of controls, data not shown), the AG490 effects on the IL-6/STAT3 pathway were likely not be a consequence of nonspecific cytotoxic effects.
Selective reduction of gp130 protein levels by AG490
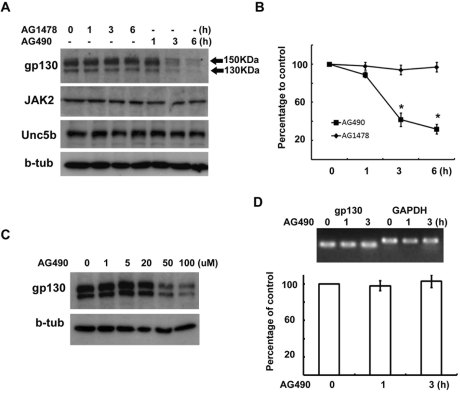
It has been previously reported that the IL-6 receptor gp130 can be degraded by various chemical stimuli with various protease systems (Hideshima et al., 2003; Graf et al., 2006 and 2008; Tanaka et al., 2008). Therefore, we investigated the protein levels of gp130 following AG490 treatment using Western blot analysis (Fig. 2A). The gp130 receptor is known to undergo posttranslational modifications. The Western blot analysis showed two apparent bands of gp130, 130 kDa and 150 kDa (Fig. 2A). Based on previous studies (Gerhartz et al., 1994), the upper band (150 kDa) was the mature form of gp130 that resides in the membrane whereas the lower band (130 kDa) was the intracellular immature gp130. AG490, but not AG1478, treatment resulted in a dose- and time-dependent reduction in the amount of intracellular and membrane gp130 proteins observed (Fig. 2A~C). The suppression of gp130 protein levels was detectable 3 hrs after AG490 treatment at 50 µM concentration. This finding was very similar to the profile of AG490 inhibition of the IL-6-induced STAT3 phosphorylation (Fig. 1A). By contrast, the proteins JAK2, beta-tubulin and the membrane protein Unc5b had stable levels. Thus, the results demonstrated that gp130 was specifically down-regulated by AG490.
Fig. 2.
AG490 downregulates gp130 expression. (A, B) RT4 cells were treated with AG490 (50 µM) or AG1478 (50 µM) for the indicated times, and then Western blot analysis was performed to examine the protein levels of gp130, JAK2, Unc5b and beta-tubulin. (B) Quantitative results showing the changes in gp130 levels following AG490 or AG1478 treatment. gp130 showed two bands with different molecular weights, and the intensity values of each of two gp130 bands were added for analysis. (C) A dose-dependent downregulation of gp130 by AG490 treatment. (D) RT4 cells were treated with AG490 (50 µM) for the indicated time, and total RNA was extracted for RT-PCR analysis. GAPDH mRNA levels were used as a control. Quantitative data represent the means±S.E of three independent experiments. *p<0.05.
The reduction of gp130 levels by AG490 may simply be caused by downregulation of gp130 transcription. Therefore, we examined the mRNA expression of gp130 following AG490 treatment. The mRNA levels of gp130 were not altered by AG490 treatment (Fig. 2D). This finding suggests that AG490 downregulates gp130 at posttranscriptional levels.
Protease-mediated degradation of gp130 was not increased by AG490
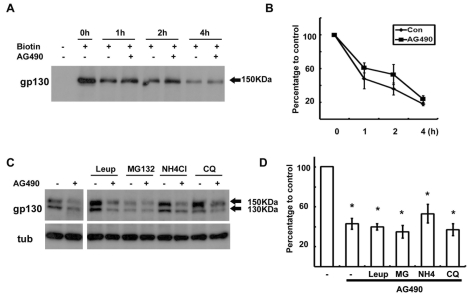
Downregulation of gp130 by AG490 at the posttranscriptional level suggests the occurrence of proteolytic degradation of gp130. In order to determine whether AG490 is involved in the degradation of gp130, we first examined whether AG490 induced degradation of the cell surface (mature form) gp130. The cell surface proteins were labeled with biotin, then precipitated and the degradation rate of biotin-labeled gp130 was assessed with or without AG490 (Fig. 3A, B). The cell surface biotinylation only labeled the 150 kDa gp130 on the cell surface. The biotin-labeled gp130 was rapidly degraded within 2~4 hrs of basal conditions without AG490, similar to the known half-life of gp130. AG490 did not enhance the degradation rate of biotin- labeled gp130. These findings indicated that the increased degradation of cell surface gp130 was not the mechanism underlying gp130 downregulation by AG490.
Fig. 3.
Degradation of gp130 was not enhanced by AG490. (A) Cell surface proteins were biotinylated at 4℃, and then cells were returned to 37℃ for the indicated time in the presence or absence of AG490 (50 µM). Protein extracts were precipitated with streptavidin-agarose beads, and analyzed by Western blotting with an antibody against gp130. (B) Quantitative results showing the change in biotinylated gp130 levels following AG490 treatment. (C) Cells were pretreated with protease inhibitors for 30 min and then cells were exposed to AG490 (50 µM) for 3 hrs. (D) Quantitative results showing the change in gp130 levels following AG490 treatment in the presence of several protease inhibitors. Leup; leupeptin, MG; MG132, NH4; NH4Cl, CQ; chloroquine. Data represent the means±S.E of three independent experiments. *p<0.05.
We next tested the effects of protease inhibitors implicated in gp130 degradation (lysosome, caspases, proteasomes) on the downregulation of gp130 by AG490 (Fig. 3C, D). It was found that three lysosomal inhibitors, leupeptin (50 µg/ml), NH4Cl (20 mM) and chloroquine (100 µM), and a pan-caspase inhibitor Z-VAD-FMK (50 µM, data not shown) had no or only very mild inhibitory effects on the downregulation of gp130 by AG490. It was difficult to estimate the role of the proteasomes because the proteasome inhibitors, MG132 (10 µM, Fig. 3C) and lactacystin (20 µM, data not shown), themselves each induced gp130 downregulation as previously reported (Hideshima et al., 2003). Therefore, these findings indicate that protease-dependent degradation may not be involved in the downregulation of intracellular gp130 by AG490.
Inhibition of gp130 protein synthesis by AG490
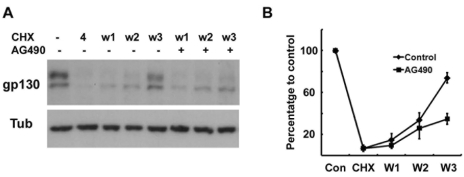
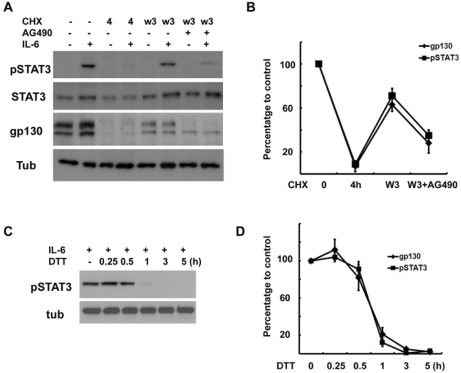
It has been reported that the inhibition of protein synthesis with cycloheximide (CHX) rapidly reduced gp130 protein levels due to its short half-life (Tanaka et al., 2008). We also found that 4 hrs of treatment with CHX completely removed the two forms of gp130 without affecting the beta- tubulin levels in the RT4 cells (Fig. 4A). The removal of CHX resulted in the reappearance of both forms of gp130 within 3 hrs, whereas AG490 significantly delayed it (Fig. 4A, B). This finding indicates that AG490 inhibited the synthesis of intracellular gp130, thereby reducing the total amount of gp130 in the cells.
Fig. 4.
Protein synthesis of gp130 is suppressed by AG490. (A) Cells were pretreated with cycloheximide (CHX, 10 µM) for 4 hrs, and then washed and reincubated in normal growth medium with or without AG490 (50 µM) for the indicated times. W1; reincubation in normal medium for 1 hr after 4 hrs of CHX treatment. (B) Quantitative results showing the change in gp130 levels following CHX treatment.
Phosphorylation of eIF-2α by AG490
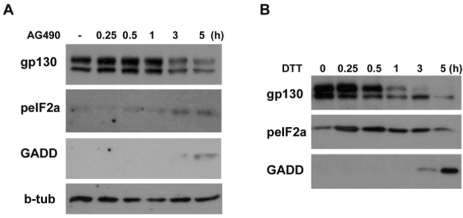
Various stressful conditions including endoplasmic reticulum (ER) stress lead to inhibition of general protein synthesis by phosphorylation of eukaryotic initiation factor-2α (eIF-2α) (Harding et al., 1999). To determine whether AG490 inhibits translational initiation by phosphorylation of eIF-2α, we examined the phosphorylation of eIF-2α by AG490. As shown in Fig. 5A, AG490 caused an increase in the phosphorylation of eIF-2α in a time-dependent manner. The phosphorylation of eIF-2α is known to initiate the expression of growth arrest- and DNA damage-inducible gene 153 (GADD153), resulting in stress-induced cell cycle arrest and apoptosis (Szegezdi et al., 2006). In this study, we found a consistent induction of GADD153 expression following 3 hrs of AG490 treatment (Fig. 5A), further supporting AG490-induced inhibition of eIF-2α activity. We next examined whether treatment of a known ER stress inducer dithiothreitol (DTT) leads to down-regulation of gp130 protein levels with phosphorylation of eIF-2α. DTT caused a dramatic concomitant induction of peIF-2α and GADD153 with significant reduction of gp130 protein levels (Fig. 5B). The downregulation of gp130 by DTT occurred within one hr and this appeared to be correlated with a strong and very early phosphorylation of eIF-2α, resulting in cessation of protein translation within one hr. Thus, it appears that AG490-induced phosphorylation of eIF-2α might be related to the downregulation of the protein synthesis of gp130.
Fig. 5.
Phosphorylation of eIF-2α by AG490. (A) Temporal profile of phosphorylation of eIF-2α and induction of GADD153 (GADD) by AG490 (50 µM). The cells were treated with AG490 for the indicated times, and then Western blot analysis was performed. (B) Cells were treated with an ER stress inducer dithiothreitol (DTT, 3 mM) for the indicated times, and protein levels of gp130, peIF-2α and GADD153 were analyzed by Western blotting.
Correlation between IL-6 response and gp130 expression
In order to further substantiate that IL-6 responsiveness was related to protein levels of gp130, the cells were stimulated with IL-6 for 4 hrs after CHX treatment. The CHX treatment significantly removed gp130 expression with complete inhibition of the IL-6-induced STAT3 phosphorylation. The IL-6 response returned to ~70% of normal levels 3 hrs after removal of CHX and the gp130 levels increased. The inclusion of AG490 during the recovery period significantly delayed not only the reappearance of gp130 but also IL-6 responses (Fig. 6A, B). These results indicate that downregulation of gp130 by AG490 might be causally related to the loss of IL-6 responses.
Fig. 6.
Correlation between gp130 levels and IL-6 response. (A) Cells were pretreated with CHX (10 µM) for 4 hrs, and then washed and reincubated in normal growth medium with or without AG490 (50 µM) for 3 hrs, and then IL-6 (50 ng/ml) was added for 15 min. The protein extracts were analyzed by Western blotting. (B) Quantitative results showing the changes in gp130 and pSTAT3 levels. Data represent the means±S.E of three independent experiments. (C) The cells were pretreated with dithiothreitol (DTT, 3 mM) for the indicated times and then stimulated with IL-6 for 15 min. Protein extracts were analyzed by Western blotting for pSTAT3. (D) Quantitative results showing the changes in gp130 and pSTAT3 levels in a time-dependent manner. Data represent the means±S.E of three independent experiments.
Finally, we examined whether the downregulation of gp130 by DTT also inhibits IL-6-induced STAT3 phosphorylation. DTT significantly blocked the tyrosine phosphorylation of STAT3 by IL-6 (Fig. 6C, D) in a time-dependent manner. When we quantitatively compared STAT3 activation by IL-6 (Fig. 6C) to gp130 levels (150 kDa upperband, Fig. 5B) in the DTT-treated samples, the results showed a significant correlation between the two biochemical events (Fig. 6D). Taken together, these results indicate that AG490-induced downregulation of gp130 appears to be causally related to the loss of IL-6 responses.
DISCUSSION
AG490 has been shown to block JAK2 in patients with acute lymphoblastic leukemia and in genetically active variants of JAK2 at relatively low concentrations (Meydan et al., 1996; Verstovsek et al., 2008). In addition, AG490 variants such as WP1066 have been successfully used for treating cancers with active JAK2 and STAT3 (Ferrajoli et al., 2007; Verstovsek et al., 2008). While many kinase inhibitors often have off-target effects in the enzymes they target, AG490 is relatively specific to JAK2. It does not inhibit other tyrosine kinases such as Lck, Src, JAK1, or Tyk2 (Ferrajoli et al., 2007; Verstovsek et al., 2008). Herein, we have provided evidence that AG490 effectively reduced the protein levels of gp130 in the cell membrane by suppressing synthesis of gp130 at high concentrations. This finding suggests that AG490 desensitizes the cell's response to IL-6 by reducing the total amount of the signal transducing receptor for IL-6. Indeed, the protein levels of gp130 were significantly correlated with the levels of IL-6-induced tyrosine phosphorylation of STAT3. Thus, it is plausible to speculate that AG490 has two different ways to inhibit the STAT3 pathway, one at the gp130 receptor level and the other at JAK2 level. It is also possible that AG490 might ablate or diminish STAT3 signaling caused by other gp130-related cytokines such as CNTF and LIF in addition to IL-6, because gp130 is a shared receptor for the gp130-related cytokines (Heinrich et al., 2003).
To investigate the mechanism by which AG490 induces gp130 downregulation, we first suspected enhanced degradation of gp130 by unknown toxic effects of AG490. Using specific inhibitors of several protease systems, we found no evidence of involvement of enhanced proteolytic degradation of gp130 by AG490. This was an unexpected finding because gp130 is known to be susceptible to proteases activated by several stimuli (Hideshima et al., 2003; Graf et al., 2006 and 2008; Tanaka et al., 2008). In a previous study, we found that constitutive endocytotic degradation was enhanced by methylglyoxal, a reactive aldehyde (Lee et al., 2009a). Notably, in contrast to this, we recently reported that capsaicin treatment resulted in downregulation of gp130 by inducing ER stress (Lee et al., 2009c). In this study, we observed that ER stress might also be involved in AG490-induced down-regulation of gp130. First, AG490 did not enhance degradation of gp130 in the membrane as demonstrated by the cell surface biotinylation assay. Instead, it reduced the net amount of gp130 in the membrane by inhibiting the protein synthesis of gp130, which consequently led to depletion of the total gp130 levels due to its short half-life. In addition, an ER stress inducer, DTT, led to down-regulation of gp130 and concomitant inhibition of IL-6/STAT3 signaling. Moreover, the downregulation of gp130 by AG490 was specific because STAT3, JAK2 and Unc5b levels were not significantly altered by AG490 treatment. These findings might be explained by the relative short half-life of gp130 compared to that of STAT3, JAK2 and Unc5b or to different mechanisms of mRNA translation of these molecules. Whatever the mechanism is, our findings illustrate that the specificity of this novel mechanism, AG490 inhibition on the STAT3 pathway, might be mediated by gp130 down-regulation.
It should be also mentioned that the downregulation of both bands of gp130 by DTT occurred within one hr of each other with a more significant reduction of the 150 kDa band than the 130 kDa band. The reduction of the 130 kDa band might have been related to the down-regulation of the protein synthesis of gp130 by AG490-induced phosphorylation of eIF-2α. The profound down-regulation of the 150 kDa band compared to the 130 kDa band might indicate that another mechanism, such as protease-mediated degradation of cell surface gp130, is implicated in the down-regulation of the 150 kDa band by DTT.
The results of this study showed that the JAK2 specific inhibitor, AG490, induced phosphorylation of eIF-2a. Phosphorylation of eIF-2a is caused by cell stress through multiple kinases such as RNA-dependent protein kinase-like ER kinase, heme-regulator inhibitor kinase and positive general control of transcription 2 (Bertolotti et al., 2000). Phosphorylated eIF-2a cannot initiate the protein translation; that would allow cells to alleviate loads of ER under stressful conditions. However, certain mRNAs such as mRNA for activating transcription factor 4 are efficiently translated under these conditions. The increase of activating transcription factor 4 in turn induces expression of GADD153 that negatively regulates cell cycle progression and induces apoptosis under stressful conditions (Harding et al., 1999; Szegezdi et al., 2006). We found that AG490 induced GADD153 expression, further supporting activation of the eIF-2a/GADD153 pathway by AG490. However, we do not know whether AG490 actually induces ER stress or non-specifically activates one of the upstream kinases of eIF-2a. Identification of the kinases responsible for eIF-2a phosphorylation by AG490 may help in understanding the molecular mechanism associated with AG490-induced inhibition of protein synthesis.
It has long been suggested that inhibition of STAT3 activity sensitizes cells to the effects of several anticancer drugs (Rahaman et al., 2002; Scholz et al., 2003; Aggarwal et al., 2006; Germain and Frank, 2007). Our findings suggest that inhibition of general protein synthesis may reduce cell STAT3 activity by the mechanisms explained above, thereby increasing the cytotoxic effects of anticancer drugs. Thus, our findings suggest a novel mechanism involved in the downregulation of membrane gp130 levels. These findings might help inform new anti-cancer strategies.
ACKNOWLEDGEMENT
This study was supported by research funds from Dong-A University.
ABBREVIATIONS
- IL-6
interleukin-6
- JAK
janus kinase
- STAT3
signal transducer and activator of transcription 3
- CNTF
ciliary neurotrophic factor
- LIF
leukemia inhibitory factor
- eIF-2α
eukaryotic initiation factor-2α
- GADD153
growth arrest- and DNA damage-inducible gene 153
- CHX
cycloheximide
- ER stress
endoplasmic reticulum stress
References
- 1.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer- and-activator-of-transcription (STAT)-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 2.Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2002;2:381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- 3.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 4.Ferrajoli A, Faderl S, Van Q, Koch P, Harris D, Liu Z, Hazan-Halevy I, Wang Y, Kantarjian HM, Priebe W, Estrov Z. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 2007;67:11291–11299. doi: 10.1158/0008-5472.CAN-07-0593. [DOI] [PubMed] [Google Scholar]
- 5.Gerhartz C, Dittrich E, Stoyan T, Rose-John S, Yasukawa K, Heinrich PC, Graeve L. Biosynthesis and half-life of the interleukin-6 receptor and its signal transducer gp130. Eur J Biochem. 1994;223:265–274. doi: 10.1111/j.1432-1033.1994.tb18991.x. [DOI] [PubMed] [Google Scholar]
- 6.Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13:5665–5669. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- 7.Graf D, Haselow K, Münks I, Bode JG, Häussinger D. Caspase-mediated cleavage of the signal-transducing IL-6 receptor subunit gp130. Arch Biochem Biophys. 2008;477:330–338. doi: 10.1016/j.abb.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Graf D, Kohlmann C, Haselow K, Gehrmann T, Bode JG, Häussinger D. Bile acids inhibit interleukin-6 signaling via gp130 receptor-dependent and -independent pathways in rat liver. Hepatology. 2006;44:1206–1217. doi: 10.1002/hep.21368. [DOI] [PubMed] [Google Scholar]
- 9.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hideshima T, Chauhan D, Hayashi T, Akiyama M, Mitsiades N, Mitsiades C, Podar K, Munshi NC, Richardson PG, Anderson KC. Proteasome inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene. 2003;22:8386–8393. doi: 10.1038/sj.onc.1207170. [DOI] [PubMed] [Google Scholar]
- 12.Lauta VM. Interleukin-6 and the network of several cytokines in multiple myeloma: an overview of clinical and experimental data. Cytokine. 2001;16:79–86. doi: 10.1006/cyto.2001.0982. [DOI] [PubMed] [Google Scholar]
- 13.Lee HK, Seo IA, Suh DJ, Lee HJ, Park HT. A novel mechanism of methylglyoxal cytotoxicity in neuroglial cells. J Neurochem. 2009a;108:273–284. doi: 10.1111/j.1471-4159.2008.05764.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee HK, Seo IA, Shin YK, Park JW, Suh DJ, Park HT. Capsaicin inhibits the IL-6/STAT3 pathway by depleting intracellular gp130 pools through endoplasmic reticulum stress. Biochem Biophys Res Commun. 2009c;382:445–450. doi: 10.1016/j.bbrc.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 15.Lee HK, Seo IA, Suh DJ, Hong JI, Yoo YH, Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 2009b;108:776–786. doi: 10.1111/j.1471-4159.2008.05826.x. [DOI] [PubMed] [Google Scholar]
- 16.Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman CM. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto N, Sugita K, Goi K, Inukai T, Lijima K, Tezuka T, Kojika S, Nakamura M, Kagami K, Nakazawa S. The JAK2 inhibitor AG490 predominantly abrogates the growth of human B-precursor leukemic cells with 11q23 translocation or Philadelphia chromosome. Leukemia. 2001;15:1758–1768. doi: 10.1038/sj.leu.2402260. [DOI] [PubMed] [Google Scholar]
- 18.Opdam FJ, Kamp M, de Bruijn R, Roos E. Jak kinase activity is required for lymphoma invasion and metastasis. Oncogene. 2004;23:6647–6653. doi: 10.1038/sj.onc.1207887. [DOI] [PubMed] [Google Scholar]
- 19.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 20.Samanta AK, Lin H, Sun T, Kantarjian H, Arlinghaus RB. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 2006;66:6468–6472. doi: 10.1158/0008-5472.CAN-06-0025. [DOI] [PubMed] [Google Scholar]
- 21.Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 2006;98:1353–1368. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- 22.Scholz A, Heinze S, Detjen KM, Peters M, Welzel M, Hauff P, Schirner M, Wiedenmann B, Rosewicz S. Activated signal transducer and activator of transcription 3 (STAT3) supports the malignant phenotype of human pancreatic cancer. Gastroenterology. 2003;125:891–905. doi: 10.1016/s0016-5085(03)01064-3. [DOI] [PubMed] [Google Scholar]
- 23.Shyu WC, Lin SZ, Chiang MF, Chen DC, Su CY, Wang HJ, Liu RS, Tsai CH, Li H. Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J Clin Invest. 2008;118:133–148. doi: 10.1172/JCI32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka Y, Tanaka N, Saeki Y, Tanaka K, Murakami M, Hirano T, Ishii N, Sugamura K. c-Cbl-dependent monoubiquitination and lysosomal degradation of gp130. Mol Cell Biol. 2008;28:4805–4818. doi: 10.1128/MCB.01784-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, Heath JK, Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–1097. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- 27.Verstovsek S, Manshouri T, Quintás-Cardama A, Harris D, Cortes J, Giles FJ, Kantarjian H, Priebe W, Estrov Z. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin Cancer Res. 2008;14:788–796. doi: 10.1158/1078-0432.CCR-07-0524. [DOI] [PubMed] [Google Scholar]
- 28.Weissenberger J, Loeffler S, Kappeler A, Kopf M, Lukes A, Afanasieva TA, Aguzzi A, Weis J. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23:3308–3316. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]