Abstract
(S)-Carbamic acid 2-[4-(4-fluoro-benzoyl)-piperidin-1-yl]-1-phenyl-ethyl ester hydrochloride (YKP1447) is a novel "atypical" antipsychotic drug which selectively binds to serotonin (5-HT2A, Ki=0.61 nM, 5-HT2C, Ki=20.7 nM) and dopamine (D2, Ki=45.9 nM, D3, Ki=42.1 nM) receptors with over 10~100-fold selectivity over the various receptors which exist in the brain. In the behavioral studies using mice, YKP1447 antagonized the apomorphine-induced cage climbing (ED50=0.93 mg/kg) and DOI-induced head twitch (ED50=0.18 mg/kg) behavior. In the dextroamphetamine-induced hyperactivity and conditioned avoidance response (CAR) paradigm in rats, YKP1447 inhibited the hyperactivity induced by amphetamine (ED50=0.54 mg/kg) and the avoidance response (ED50=0.48 mg/kg); however, unlike other antipsychotic drugs, catalepsy was observed only at much higher dose (ED50=68.6 mg/kg). Based on the CAR and catalepsy results, the therapeutic index (TI) value for YKP1447 is over 100 (i.p.). These results indicate that YKP1447 has an atypical profile and less undesirable side effects than currently available drugs.
Keywords: YKP1447, Serotonin and dopamine receptors, Schizophrenia, Atypical antipsychotics, CAR, Catalepsy
INTRODUCTION
The first effective antipsychotic drugs were discovered by serendipity, and research into how such drugs acted led to the 'dopamine hypothesis' (Carlson and Lindqvist, 1963; Rossum, 1966), suggesting that the underlying abnormality in brain function in schizophrenia might be an overactivity of dopamine mechanism (for review, Mattysse, 1973; Snyder et al., 1974).
Dopamine receptor subtypes belong to the family of G-protein coupled receptors and five subtypes of dopamine receptors can be classified into two families: The D-1 like dopamine receptors include the dopamine D1 and D5 receptors and are characterized by activation of adenylyl cyclase mediated by a Gs protein, whereas the D-2 like receptor group consists of the dopamine D2, D3 and D4 receptors, which couple to Gi/o proteins and can inhibit adenylyl cyclase (Sibley et al., 1993; Seeman and Van Tol, 1994; Sokoloff and Schwartz, 1995). The dopamine D2 receptor has been the primary target for antipsychotic research, and that dopamine D3 receptor has recently been recognized as a good target for the improved drug treatment of psychosis-like schizophrenia (Sokoloff et al., 1990).
Most antipsychotic agents are dopamine D2 receptor antagonists, but many of them, notably the atypical antipsychotic agents, also block the 5-HT receptors, particularly 5-HT2A and 5-HT2C receptors. Interestingly, the ones with the strongest 5-HT receptor blocking activity tend to have the lowest extrapyramidal side effects (EPS) (Jones and Blackburn, 2002). 5-HT2A receptor antagonism may confer on antipsychotic drugs atypicality with relatively weaker D2 receptor antagonism (or partial D2 receptor agonism) because of the ability of 5-HT2A receptors to differentially modulate the activity of dopaminergic neurones depending on regions of the brain. Furthermore, some evidences suggest that the combination of 5-HT2A and 5-HT2C receptor blockade may be a more efficient means to augment antipsychotic action than either alone (Reavill et al., 1999; Meltzer et al., 2003).
Our research efforts have been focused on finding potent and selective antagonist at the serotonin 5-HT2A and 5-HT2C, and dopamine D2 and D3 receptors. We evaluated the predictive antipsychotic efficacies using animal models. We report herein, the pharmacology of a novel and preferentially orally active serotonin (5-HT2A, 5-HT2C) and dopamine (D2, D3) receptor antagonist, YKP1447 and compared it with atypical antipsychotic drugs.
METHODS
Animals and drugs
The animals used were CD-1 male mice, and Wistar and Sprague-Dawley rats (OrientBio, Kyunggido, Korea and Charles River Labs, Japan) weighing 18~26 and 200~340 g, respectively. They were housed in a conventional plastic cage in a controlled room: temperature 22±3℃, relative humidity of 50±10%, 12-h light/dark cycle (light on from 7:00 to 19:00). The standard laboratory food and tap water were given ad libitum. All experimental procedures were performed during the light cycle (e.g. from 13:00 and 17:00). All animal procedures were approved by the institutional animal care and use committee (IACUC). YKP1447 (SK Holdings Co., Ltd), clozapine (Sigma Aldrich, USA), olanzapine (Hanchem, Daejeon, Korea), quetiapine, aripiprazole and risperidone (Leadgenex, Daejeon, Korea) were dissolved in 30% polyethyleneglycol 400 (Sigma) or 0.5% methylcellulose (MC). Apomorphine and (±)-2,5-Dimethoxy-4-iodoamphetamine (DOI) hydrochloride were purchased from Sigma Aldrich and dissolved in deionized water. Dextroamphetamine sulfate (USP) was dissolved in 0.9% saline. Vehicle or drugs in a volume of 10 ml/kg for mice and 1~4 ml/kg for rats were administered intraperitoneally, subcutaneously or orally to randomly assigned animals.
Apomorphine-induced climbing in mice
The method of Protais et al. (1976) with some modifications was used. Apomorphine HCl (2 mg/kg) was injected s.c., and the animals were immediately placed inside a cylidrical wire mesh cage (12 cm in diameter, 15 cm in height). After being in the cage, the animals were observed for climbing behavior at 10-, 20- and 30-min time periods with the following scoring system: (0) no climbing behavior, (1) one paw on the cage, (2) two paws on the cage, (3) three faws on the cage, (4) all four paws on the cage. The sum of the three scores for each animal was used for statistical evaluation. YKP1447 (0.5~10 mg/kg), olanzapine (0.1~5 mg/kg), risperidone (0.03~1 mg/kg), clozapine (1~30 mg/kg), quetiapine (2.5~30 mg/kg), aripiprazole (0.1~2 mg/kg) or vehicle were administered intraperitoneally (i.p.) or orally (p.o.) before apomorphine, with 6 mice per group.
DOI-induced head twitch test in mice
The method of Darmani et al. (1990) was used with some modifications. (±)-DOI HCl (3 mg/kg) was injected s.c., and the animals were immediately placed in a conventional plastic cage. The number of head twitches was counted during 6 min, beginning 1 min after DOI administration. YKP1447, olanzapine, risperidone, clozapine, quetiapine, aripiprazole or vehicle were administered i.p. 0.5 hr prior to injection of DOI, with 6 mice per groups.
D-amphetamine-induced hyperactivity test in rats
To measure locomotion, AUTO-TRACK SYSTEM (Columbus Instruments, USA) was used. Animals (280-340 g) received i.p. injections of vehicle or drugs (n=8/groups) 30 min prior to receiving an i.p. injection of vehicle or dextroamphetamine (1.5 mg/kg). Immediately thereafter, rats were placed in the activity chambers of AUTO-TRACK SYSTEM and locomotor activities were recorded every 10 min for 1 hr.
Conditioned avoidance response in rats
Rats were tested on weekdays, but not weekends, between 0700 and 1600 hours. Rats were transported in a shoebox cage from the colony room to the CAR test room, where they were weighed. IP pretreatments were administered 30 min before rats were placed in an CAR two-way active avoidance apparatus (#EI0-16 SC, Coulboum Instruments) with two equal chamber separated by an open doorway. Upon entry into the chamber, a 30 second intertrial interval (ITI) began. At the end of the ITI, a rat's position in the chamber was noted, and sound and light (the conditioned stimuli, or CS+s) began. If the rat moved to the other side of the chamber before 10 seconds had elapsed, the response was classified as a conditioned avoidance response (CAR). If the rat did not move to the other side of the chamber during the CS+s, a 0.5 mA scrambled shock was delivered to his feet. If the rat moved to the other side of the chamber within 5 seconds of this shock, this response was classified as an escape. If the rat did not move during the 5-second shock period, his behavior was classified as an escape failure. Following avoidance, escape, or failure to escape, a new 30-second ITI was initiated. The CAR session lasted for 10 min. Some rats underwent different numbers of trials than others, because some avoided quickly, avoided slowly, escaped, or failed to escape, making the length of the trial vary (Typically, rats had anywhere from 13 to 17 trials per session). PO pretreatments were administered 60 min before rats were placed in a CAR two-way active avoidance apparatus. All other aspects of testing were identical for IP-and PO-pretreated rats.
Catalepsy
The method of Moore et al. (1992) was used with some modifications. Rats (200~270 g) were dosed i.p. and observed for a period of 2 min at 1, 2, 3 and 4 hours following dosing. At each time point, rats were placed with their forepaws on 2 water-filled jars (5 cm in diameter, 11 cm in height) taped together. The rat was timed for the number of seconds in which he remained with his forepaws on the container for maximum 120 seconds. The immobility scores (time, sec) and percentage of the 120 seconds test period from 1, 2, 3, and 4 hours post-dosing were averaged for each rat. For each dose of drug, the highest average percentage of immobility for a time period following dosing was used to determine an ED50 value.
Data analysis
The drug effects were assessed by ANOVA, and the significance between individual dose conditions and the corresponding control group was analyzed by the Dunnett's test, except for the test of d-amphetamine-induced hyperactivity, which was analyzed by two tailed t-test to compare with vehicle-vehicle treated group vs. d-amp-vehicle treated group (Graphpad Prism® 4).
RESULTS
Effect on apomorphine-induced climbing behavior
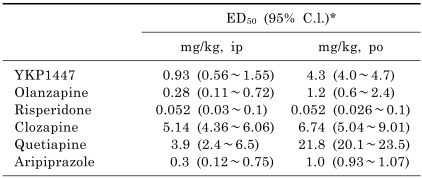
As shown in Table 1, i.p. injection of YKP1447 significantly antagonized the apomorphine-induced cage climbing behavior in mice. The calculated ED50 value, based on the percent antagonism of climbing, was 0.93 mg/kg (95% confidence limit=0.56~1.55 mg/kg). Orally administered YKP1447 also inhibited the apomorphine-induced cage climbing in mice (ED50=4.3 mg/kg; 95% confidence limit=4.0~4.7 mg/kg). In addition, other atypical antipsychotic drugs, such as olanzapine, risperidone, clozapine, quetiapine and aripiprazole, significantly blocked the apormorphine- induced climbing, showing 0.28 mg/kg (i.p.), 1.2 mg/kg (p.o.), 0.052 mg/kg (i.p. and p.o.), 5.14 mg/kg (i.p.), 6.74 mg/kg (p.o.), 3.9 mg/kg (i.p.), 21.8 mg/kg (p.o.), and 0.3 mg/kg (i.p.), 1.0 mg/kg (p.o.) of ED50 values, respectively.
Table 1.
Effects of YKP1447, olanzapine, risperidone, clozapine, quetiapine and aripiprazole on apomorphine-induced climbing in mice
*95% confidence limits.
Effect on DOI-induced head twitch behavior
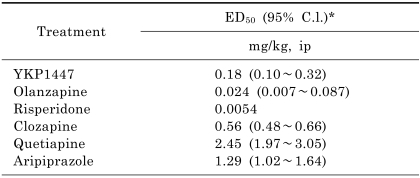
YKP1447 antagonized the DOI-induced head twitch behavior by 26%, 53% and 73%, after doses of 0.1, 0.2 and 0.3 mg/kg, respectively, compared with the control value (Table 2). The calculated ED50 value, based on the percent antagonism of head twitch behavior, was 0.18 mg/kg, i.p.. Other atypical antipsychotic drugs, including olanzapine, risperidone, clozapine, quetiapine and aripiprazole, also inhibited the head twitch behavior with calculated ED50 values of 0.024 mg/kg, 0.0054 mg/kg, 0.56 mg/kg, 2.45 mg/kg, and 1.29 mg/kg, i.p., respectively.
Table 2.
Effects of YKP1447, olanzapine, risperidone, clozapine, quetiapine and aripiprazole on DOI-induced head twitch behavior in mice
*95% confidence limits.
D-amphetamine-induced hyperactivity test in rats
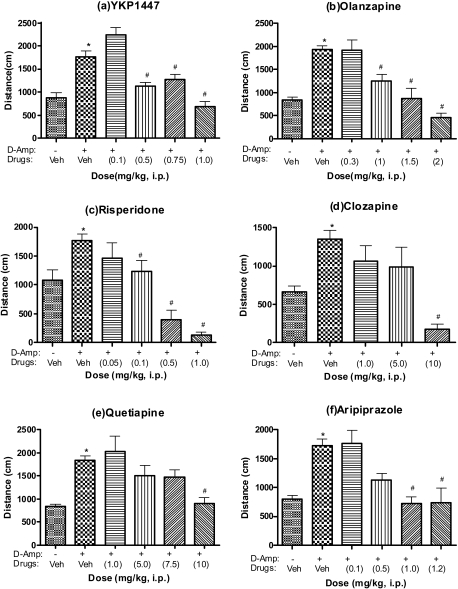
In the d-amphetamine-induced hyperactivity test (Fig. 1), dextroamphetamine (1.5 mg/kg i.p.) significantly increased locomotor activity compared with saline-injected (p<0.01). The activity increased by d-amphetamine, however, was significantly attenuated by YKP1447 (F4,40=17.90, p<0.0001), olanzapine (F4,51=21.3, p<0.0001), riperidone (F4,37=21.24, p<0.0001), clozapine (F3,31=7.251, p=0.0008), quetiapine (F4,51=5.05, p=0.0017) and aripiprazole (F4,40=8.246, p<0.0001). The calculated ED50 values were 0.54, 0.85, 0.05, 1.9, 7.0 and 0.6 mg/kg, i.p., respectively.
Fig. 1.
Effects of YKP1447, olanzapine, risperidone, clozapine, quetiapine and aripiprazole on dextroamphetamine-induced hyperactivity in rats. The locomotor activity of dextroamphetamine was measured every 10 min for 1 h immediately after injection of amphetamine (1.5 mg/kg, i.p.). YKP1447 (a, top left panel), olnazapine (b, top right panel), risperidone (c, middle left), clozapine (d, middle right), quetiapine (e, bottom left) and aripiprazole (f, bottom right) or vehicle (Veh), administered (i.p.) 30 min before the injection of dextroamphetamine. The locomotor activities for 1 h are expressed as the total counts. Each value is mean±S.E.M. of seven to eight rats. *p<0.01, compared with vehicle-vehicle group (t-test), #p<0.05, when compared with amphetamine control group (the post-hoc test was Dunnett's multiple comparisons test).
Conditioned avoidance response in rats
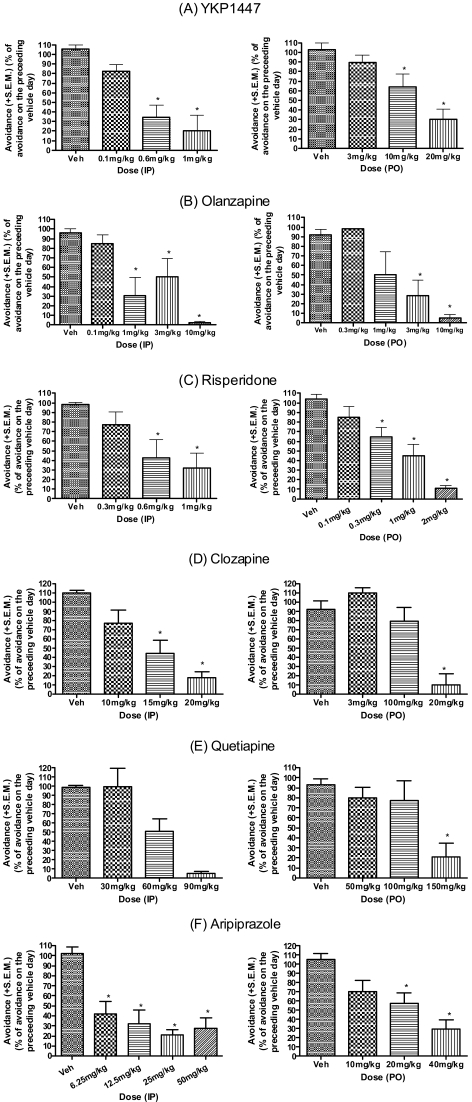
Fig. 2 presents the mean percentages of avoidances compared to the preceding vehicle day with YKP1447, olanzapine, risperidone, clozapine, quetiapine and aripiprazole. All compounds effectively inhibited avoidance response in a dose-dependent manner. A significant effect of YKP1447 was found in i.p. (F3,20=11.05, p=0.0002) or p.o. (F3,20=10.29, p=0.0003) injection, evidenced by one-way ANOVA. The calculated ED50 values were 0.54 mg/kg (i.p.), and 14.46 mg/kg (p.o.). Olanzapine showed a significant effect in i.p. (F4,29=13.51, p=0.0001) or p.o. injection (F4,25=8.247, p=0.0002), shown by one-way ANOVA. The calculated ED50 values were 3.15 mg/kg (i.p.) and 3.44 mg/kg (p.o.). Risperidone showed a significant effect in CAR [F3,26=10.83, p=0.0007 (i.p.), and F4,35=29.27, p=0.0001 (p.o.)]. The calculated ED50 values were 0.65 mg/kg (i.p.) and 0.92 mg/kg (p.o.). Both i.p. or p.o. injection of Clozapine showed a significant effect [F3,20=11.15, p=0.0001 (i.p.), and F3,20=15.81, p=0.0001 (p.o.)] with ED50 values 13.8 mg/kg (i.p.) and 13.4 mg/kg (p.o.). A significant effects of quetiapine and aripiprazole also were found in i.p. (F3,20=14.15, p=0.0001, F4,31=18.08, p<0.0001) or p.o. (F3,24=9.757, p=0.0002, F4,29=13.51, p=0.0001) administration. The calculated ED50 values were 56.3 mg/kg (i.p.) and 118.6 mg/kg (p.o.), and 14.3 mg/kg (i.p.) and 26.6 mg/kg (p.o.), respectively.
Fig. 2.
Dose-response of YKP1447 (A), olanzapine (B), risperidone (C), clozapine (D), quetiapine (E) and aripiprazole (F) on CAR paradigm in rats. All drugs were administered i.p. (left panels) 30 min or p.o. (right panels) 60 min prior to test. Values are mean±S.E.M. (n=6~10) percentage avoidances compared to the preceding vehicle-day (e.g. pre-test), with each animal serving as their own control. Statistical analyses were performed by a one-way ANOVA with post hoc Dunnett's test. *p<0.05.
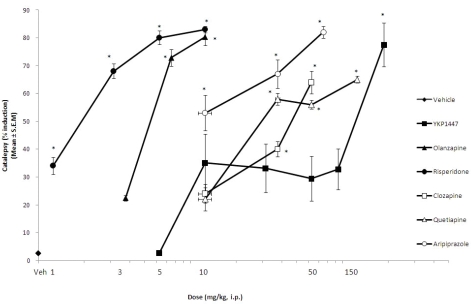
Catalepsy
As shown in Fig. 3, the ED50 values for YKP1447 were 3.7 mg/kg, olanzapine was 5.3 mg/kg, risperidone 0.98 mg/kg, clozapine 37.3 mg/kg, quetiapine 46.7 mg/kg and aripiprazole 24.3 mg/kg, i.p.. Significant effects of YKP1447 (F6,34=4.429, p=0.0021), olanzapine (F3,20=32.32, p<0.0001), risperidone (F4,25=14.03, p<0.0001), clozapine (F3,20=7.968, p=0.0011), quetiapine (F4,25=12.47, p<0.0001) and aripiprazole (F3,20=28.27, p<0.0001) were found, by using a one-way ANOVA. However, post hoc analysis using Dunnett's test revealed that the effect of YKP1447 was significant only at the dose of 150 mg/kg. On the other hand, olanzapine, risperidone, clozapine, quetiapine and aripiprazole, were significant at all or median to high doses in the present experimental condition.
Fig. 3.
Effect of YKP1447, olanzapine, risperidone, clozapine, quetiapine and aripiprazole on induction of catalepsy in rats. Data are expressed as mean±S.E.M. *p<0.05 vs. vehicle (Veh)-treated group (the post hoc test was Dunnett's).
DISCUSSION
YKP1447 is a compound which highly selectively binds to 5-HT2A, 5-HT2C, D2 and D3 receptors (Table 3), however, shows the least binding affinities to histamine (H1), muscarinic (M1) and adrenergic (α1) receptors. It is postulated that the high affinity for α1-adrenoceptors, histamine and muscarinic receptors results in adverse side effects such as weight gain, drowsiness, dry mouth, blurred vision, constipation and decreases in blood pressure (Stahl, 1997). The histamine H1 receptor is suggested to be related with serious side effect, weight gain in human (Peroutka et al., 1980; Kroeze et al., 2003). The atypical antipsychotic drugs, such as clozapine, olanzapine and quetiapine, that are reported to induce substantial weight gain in short-term studies have high affinity for the histamine 1 receptor (Ki=1.2 nM, 2 nM and 11 nM, respectively) (Kroeze et al., 2003). Compared to those drugs, YKP1447 (Ki=2100 nM) is predicted to have low risk to induce body weight gain in patients. The results from binding affinities, therefore, indicate that YKP1447 is much safer than currently available antipsychotic drugs.
Table 3.
Binding affinity (Ki to nM or % inhibition at 100 nM except M1 which is % inhibition at 1 µM) of YKP1447 for the various receptors. YKP1447 showed highly selective binding affinities to the 5-HT2A, 2C, D2 and 3 receptor subtypes
All data generated from MDS pharma service.
Apomorphine-induced climbing behavior is due to the stimulation of dopamine receptors and has been used as a convenient means to in vivo screen dopamine agonists or antagonists (neuroleptics) and to assess striatal dopamine activity (Protais et al., 1975; Costentin et al., 1976; Park et al., 2003). YKP1447 blocked apomorphine-induced cage climbing behavior of mice when treated both intraperitoneally and orally, with no any hypoactivity. It is likely due to selective blockade of dopaminergic receptors, and the potency is similar to of olanzapine and other atypical antipsychotic drugs.
In the present study, all compounds tested including YKP1447 inhibited DOI-induced head-twitch responses in mice. The head-twitch response has been suggested to be mediated via the activation of central 5-HT2A receptors (Barnes and Sharp, 1999) and is blocked by mixed 5-HT2A/2C receptor antagonist such as ritanserin and mianserin, selective 5-HT2A antagonist ketanserin and MDL-100907 (Darmani et al., 1990; Schreiber et al., 1995; Bartoszyk et al., 2003; Egashira et al., 2004). YKP1447 and atypical antipsychotic drugs have high affinities for 5-HT2A and 2C receptors (Table 4), implicating 5-HT2A and/or 5-HT2C receptors in mediation of this response.
Table 4.
Ki values of YKP1447 and atypical antipsychotic drugs to 5-HT2A and 5-HT2C receptors
All Ki values from Kroeze et al., 2003 except YKP1447.
The behavioral studies using rats, antagonism of dopamine agonist-induced hyperlocomotion (Janssen et al., 1965; Ogren et al., 1984; Gustafsson and Christensson, 1990; Moore and Kenyon, 1994) and conditioned avoidance response (CAR) paradigm (Cook et al., 1955; Davidson and Weidley, 1976; Arnt, 1982; for review, see Wadenberg et al., 1999) have traditionally been used to predict the antipsychotic efficacy of novel agent. In the present study, YKP1447 dose-dependently inhibited d-amphetamine-induced hyperlocomotion activity in rats, indicating the antipsychotic action of YKP1447. The atypical antipsychotics, including olanzapine, risperidone, clozapine, quetiapine and aripiprazole, also inhibited hyperlocomotion induced by d-amphetamine. In the CAR, YKP1447 showed an excellent effect with lower ED50 value which is similar to that of risperidone. YKP1447, however, induced catalepsy (CAT) only at the high dose group (150 mg/kg). The relative ratio (e.g. Therapeutic index: TI) of the ED50 for CAT to the ED50 for a sensitive pharmacological screen for APs, such as conditioned avoidance responding (CAR) in rats, has been used to predict the relative ability of a compound to induce extra- pyramidal symptoms (Parkinson-like symptoms and tardive dyskinesia) in man; higher values suggest less likely induction of such symptoms following prolonged dosing (Wadenberg et al., 2000). The TI value (CAT/CAR) for YKP1447 was over 100, being much higher than 2 of olanzapine, 1.5 of risperidone, 3 of clozapine, 1 of quetiapine and 1.7 of arpiprazole (i.p.). It appears, therefore, that the potential of YKP1447 to induce motor symptoms in man may be very low.
In addition, it was reported that pretreatment of with high concentration of YKP1447 causes antipsychotic effect on apomorphine-induced impairment, suggesting that the compound could potentially be used to treat cognitive impairment due to increased dopaminergic receptor binding (Yoon et al., 2008).
In summary, YKP1447 shows general profiles of an atypical antipsychotic drug and has efficacies on negative and/or cognitive symptoms of schizophrenia patients. Furthermore, it has better safety than currently available antipsychotic drugs.
ACKNOWLEDGEMENTS
The research was supported by a grant from the Korea Health Industry Development Institute (KHIDI).
ABBREVIATIONS
- 5-HT
serotonin
- DOI
1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane
- CAR
conditioned avoidance response
- D-Amp
dextroamphetamine
- CAT
catalepsy
- TI
therapeutic index
References
- 1.Arnt J. Pharmacological specificity of conditioned avoidance response inhibition in rats. Inhibition by neuroleptics and correlation to dopamine receptor blockade. Acta Pharmacol Toxicol (Copenh) 1982;51:321–329. doi: 10.1111/j.1600-0773.1982.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 2.Bartoszyk GD, van Amsterdam C, Böttcher H, Seyfried CA. EMD 281014, a new selective serotonin 5-HT2A receptor antagonist. Eur J Pharmacol. 2003;473:229–230. doi: 10.1016/s0014-2999(03)01992-7. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on the formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 4.Cook L, Morris RW, Mattis PA. Neuropharmacological and behavioral effects of chlorpromazine (thorazine hydrochloride) J Pharmacol Exp Ther. 1955;113:11–12. [Google Scholar]
- 5.Costentin J, Protais P, Schwartz JC. Rapid and dissociated changes in sensitivities of different dopamine receptors in mouse brain. Nature. 1975;257:405–407. doi: 10.1038/257405a0. [DOI] [PubMed] [Google Scholar]
- 6.Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- 7.Davidson AB, Weidley E. Differential effects of neuroleptic and other psychotropic agents on acquisition of avoidance in rats. Life Sci. 1976;18:1279–1284. doi: 10.1016/0024-3205(76)90205-8. [DOI] [PubMed] [Google Scholar]
- 8.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 9.Egashira N, Mishima K, Uchida T, Hasebe N, Nagai H, Mizuki A, Iwasaki K, Ishii H, Nishimura RJ, Shoyama Y, Fujiwara M. Anandamide inhibits the DOI-induced head-twitch response in mice. Psychopharmacology. 2004;171:382–389. doi: 10.1007/s00213-003-1611-y. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson B, Christensson E. Amperozide, a new putative antipsychotic drug with a limbic mode of action on dopamine mediated behavior. Pharmacol Toxicol. 1990;66(Suppl 1):12–17. doi: 10.1111/j.1600-0773.1990.tb01600.x. [DOI] [PubMed] [Google Scholar]
- 11.Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 12.Janssen PAJ, Niemegeers CJE, Smellekens KHL. Is it possible to predict the clinical effects of neuroleptic drugs (major tranquilizers) from animal data? Drug Res. 1965;15:56–69. [PubMed] [Google Scholar]
- 13.Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav. 2002;71:555–568. doi: 10.1016/s0091-3057(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 14.Matthysse S. Antipsychotic drug actions: a clue to the pathology of schizophrenia? Fed Proc. 1973;32:200–205. [PubMed] [Google Scholar]
- 15.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–1172. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Moore NA, Tye NC, Axton MS, Risius FC. The behavioral pharmacology of olanzapine, a novel "atypical" antipsychotic agent. J Pharmacol Exp Ther. 1992;262:545–551. [PubMed] [Google Scholar]
- 17.Moore S, Kenyon P. Atypical antipsychotics, clozapine and sulpiride do not antagonize amphetamine-induced stereotyped locomotion. Psychopharmacology. 1994;114:123–130. doi: 10.1007/BF02245453. [DOI] [PubMed] [Google Scholar]
- 18.Ogren SO, Hall H, Kohler C, Magnusson O, Lindbom LO, Angeby K, Florvall L. Remoxipride, a new potential antipsychotic compound with selective antidopaminergic actions in the rat brain. Eur J Pharmacol. 1984;102:459–474. doi: 10.1016/0014-2999(84)90567-3. [DOI] [PubMed] [Google Scholar]
- 19.Park WK, Jeong DY, Yun CW, Lee SH, Cho HY, Kim GD, Koh HY, Pae AN, Cho YS, Choi KI, Jung JY, Jung SH, Kong JY. Pharmacological actions of a novel and selective dopamine D3 receptor antagonist, KCH-1110. Pharmacological Research. 2003;48:615–622. doi: 10.1016/s1043-6618(03)00242-1. [DOI] [PubMed] [Google Scholar]
- 20.Peroutka SJ, Snyder SH. Relationship of neuroleptic drug effects at brain dopamine, serotonin, a-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry. 1980;137:1518–1522. doi: 10.1176/ajp.137.12.1518. [DOI] [PubMed] [Google Scholar]
- 21.Protais P, Constentin J, Schwartz JC. Climbing behavior induced by apomorphine in mice: a simple test for the study of dopamine receptors in striatum. Psychopharmacology. 1976;50:1–6. doi: 10.1007/BF00634146. [DOI] [PubMed] [Google Scholar]
- 22.Protais P, Costentin J, Schwartz JC. Climbing behavior induced by apomorphine in mice: a simple test for the study of dopamine receptors in striatum. Psychopharmacology (Berlin) 1976;50:1–6. doi: 10.1007/BF00634146. [DOI] [PubMed] [Google Scholar]
- 23.Reavill C, Kettle A, Holland V, Riley G, Blackburn TP. Attenuation of haloperidol-induced catalepsy by a 5-HT2C receptor antagonist. Br J Pharmacol. 1999;126:572–574. doi: 10.1038/sj.bjp.0702350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane)-induced headtwitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- 25.Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 26.Sibley DR, Monsma FJ, Jr, Shen Y. Molecular neurobiology of dopaminergic receptors. Int Rev Neurobiol. 1993;35:391–415. doi: 10.1016/s0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- 27.Snyder SH, Banergee SP, Yamamura HI, Greenberg D. Drugs, neurotransmitters and schizophrenia. Science. 1974;184:1243–1245. doi: 10.1126/science.184.4143.1243. [DOI] [PubMed] [Google Scholar]
- 28.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor D3 as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 29.Sokoloff P, Schwartz JC. Novel dopamine receptors half a decade later. Trends Pharmacol Sci. 1995;16:270–275. doi: 10.1016/s0165-6147(00)89044-6. [DOI] [PubMed] [Google Scholar]
- 30.Stahl SM. Psychopharmacology of antidepressants. 1st ed. London: Martin Dunitz Ltd; 1997. pp. 31–38. [Google Scholar]
- 31.Van Rossum JM. The significance of dopamine-receptor blockade for the action of neuroleptic drugs. Arch Int Pharmacodyn Ther. 1966;160:492–494. [PubMed] [Google Scholar]
- 32.Wadenberg ML, Hicks PB. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev. 1999;23:851–862. doi: 10.1016/s0149-7634(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 33.Wadenberg ML, Kapur S, Soliman A, Jones C. Dopamine D2 receptor occupancy predicts catalepsy and the suppression of conditioned avoidance response behavior in rats. Psychopharmacology. 2000;150:422–429. doi: 10.1007/s002130000466. [DOI] [PubMed] [Google Scholar]
- 34.Willins DL, Meltzer HY. Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther. 1997;282:699–706. [PubMed] [Google Scholar]
- 35.Yoon BS, Park EH, Park EH, Kwon JT, Hong SB, Dong SM, Kim YH, Heo J, Ji MK, Kim YG, Kwak BS, Choi JS, Kim HT. Effects of a new antipsychotic drug (YKP1447) on impaired prepulse inhibition induced by apomorphine and phencyclidine in rats. Korean J Psychopharmacol. 2008;19:38–45. [Google Scholar]