Abstract
Teeth have been missing from birds (Aves) for at least 60 million years. However, in the chick oral cavity a rudiment forms that resembles the lamina stage of the mammalian molar tooth germ. We have addressed the molecular basis for this secondary loss of tooth formation in Aves by analyzing in chick embryos the status of molecular pathways known to regulate mouse tooth development. Similar to the mouse dental lamina, expression of Fgf8, Pitx2, Barx1, and Pax9 defines a potential chick odontogenic region. However, the expression of three molecules involved in tooth initiation, Bmp4, Msx1, and Msx2, are absent from the presumptive chick dental lamina. In chick mandibles, exogenous bone morphogenetic protein (BMP) induces Msx expression and together with fibroblast growth factor promotes the development of Sonic hedgehog expressing epithelial structures. Distinct epithelial appendages also were induced when chick mandibular epithelium was recombined with a tissue source of BMPs and fibroblast growth factors, chick skin mesenchyme. These results show that, although latent, the early signaling pathways involved in odontogenesis remain inducible in Aves and suggest that loss of odontogenic Bmp4 expression may be responsible for the early arrest of tooth development in living birds.
The developing murine molar tooth germ provides a powerful developmental system for identifying the genetic pathways involved in organogenesis (1, 2). Classical embryologic studies have shown that the developing tooth forms via a series of reciprocal inductive tissue interactions in which signals are exchanged between the dental epithelium and mesenchyme, resulting in a progressive specification of organ fate. In the developing molar dentition of the mouse, tooth inductive potential resides in the dental epithelium until embryonic day (E)12.5 (3). Thereafter, tooth inductive potential shifts to neural crest-derived dental mesenchyme, which acquires the ability to direct tooth formation in nonodontogenic tissues (3, 4).
These classical studies have been complemented by more recent experiments demonstrating that specific molecules function at particular steps in odontogenesis. Fibroblast growth factor (FGF)8 is expressed in the dental epithelium and has been proposed to act in conjunction with bone morphogenetic protein (BMP)4 antagonism to define the tooth-forming region and to act by inducing Pax9 expression, which in turn is required for tooth formation (5–7). In addition, BMP4 can substitute for other inductive functions of the dental epithelium, inducing morphologic changes in the dental mesenchyme and the expression of the homeobox genes Msx1 and Msx2 (8, 9), whereas inhibition of BMP4 signaling in the mandible produces alterations in spatial domains of gene expression and tooth fate (10). FGF8 and BMP4 each can differentially regulate the expression of Msx1 and Msx2 as well as that of the distal-less homeobox genes Dlx1 and Dlx2 in dental mesenchyme, and both Dlx and Msx genes function in dental mesenchymal induction (5, 11, 12).
Prior work has focused on the role of the homeobox genes Msx1 and Msx2 in tooth formation. Mutations in Msx1 are responsible for anodontia in both humans (13, 14) and mouse (15, 16). In mouse Msx1 mutants, tooth development arrests at the bud stage, when both tooth inductive potential and Bmp4 expression normally shift from dental epithelium to mesenchyme (8). In Msx1 mutants, dental epithelial Bmp4 expression is preserved but the subsequent mesenchymal phase of Bmp4 expression does not occur (9). This result suggests that Msx1 is required for the expression of inductive signaling molecules that then act back on the original inducing tissue to sustain the reciprocal inductive tissue interactions that characterize tooth morphogenesis. The validity of this model is supported by the finding that exogenous BMP4 partially rescues Msx1 mutant tooth development (ref. 9; M. Bei, K. Kratochwil, and R.M., unpublished work).
In mouse mutants genetically compounded for loss of function of both Msx1 and Msx2, tooth development can arrest at the dental lamina stage (11). Interestingly, the epithelial thickenings observed in Msx1-Msx2 double mutants morphologically resemble transient epithelial thickenings, which have been classically described in the chick oral cavity at day 5 in ovo and in the oral cavity of birds (17–19). Although modern birds and certain other lineages (e.g., turtles) lack dentition, all toothless vertebrates derived from ancestors that were once toothed (20). Ancient birds, such as the Jurassic bird Archaeopteryx and the late Cretaceous bird Hesperoronis, possessed teeth, and hence the phylogenetic derivation of modern birds indicates that the absence of dentition was a secondary event, occurring approximately 60 million or more years ago (20). Previous studies in which chick oral epithelium and mouse dental mesenchyme were recombined ostensibly resulted in enamelized teeth, suggesting that in chickens, some of the genes required for odontogenesis may have remained intact (21). However, the possibility of mouse dental epithelial contamination in these experiments makes this interpretation uncertain.
One hypothesis is that the loss of dentition in certain taxa reflects the evolutionary occurrence of mutations that inactivate the genetic pathways leading to tooth formation. The morphologic similarity between the arrested dental epithelium in Msx1-Msx2 double mutants and the rudimentary epithelial thickenings previously identified histologically in the oral cavity of birds prompted us to reconsider whether Aves might have retained some initial molecular steps in the odontogenic pathway, and whether the absence of dentition in modern birds might reflect a specific interruption of this pathway. In this report, we provide evidence consistent with this hypothesis.
Materials and Methods
Generation of Msx1-Msx2 Double Mutant Mice.
Msx1-Msx2 double mutant embryos were obtained from Msx1-Msx2 double heterozygous crosses (11). Genotyping was performed by PCR using genomic DNA from extra-embryonic membranes (9).
In Situ Hybridization.
Whole-mount and tissue section in situ hybridization was carried out as described (9). Double labeling in situ hybridization was performed with digoxigenin and fluorescein-labeled probes, and expression was detected by using nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) and Fast Red substrates (Boehringer Mannheim). Staging of chick embryos was performed according to ref. 22.
Retroviral Infection.
Replication competent avian sarcoma (RCAS)-Bmp4 retrovirus was prepared as described (23). About 10 nl of RCAS-Bmp4 was injected into the right side of the mandible of stage 23 chick embryos, with the uninjected left side of the mandible used as control. Other RCAS viruses also were used as negative controls. Embryos were reincubated for 5 days before histological examination.
Tissue Recombination and Organ Culture.
Stage 27 chick mandibles and stage 34 chick back skin were dissected, and the epithelium and mesenchyme were separated by enzyme treatment as described (9). Epithelial-mesenchymal recombination was carried out on Nuclepore filters (0.4 μm pore size) in Trowell-type organ culture, and recombinants were cultured in DMEM with 10% FBS. Bead implantation experiments were performed as described (9). BMP4- or FGF-soaked beads (100 ng/ml) were placed on top of chick presumptive dental mesenchyme and cultured in Trowell-type organ culture in medium with 10% FBS for 24 h before fixation and whole-mount in situ hybridization. Chick mandibles also were cultured on Nuclepore filters in Trowell-type organ culture in medium (DMEM + 10% FBS) with or without addition of growth factors. Medium was changed every other day.
Histology and Histochemical Staining.
Standard histology procedures were performed. Tissues were fixed in 4% paraformaldehyde, embedded in wax at 60°C for several hours [sufficient to inactivate feather germ mesenchymal alkaline phosphatase (AP) activity], sectioned, and stained with hematoxylin and eosin. AP activity was detected by applying the AP substrate NBT/BCIP to tissue sections.
Results
Markers for Dental Lamina Formation Are Expressed in the Chick Mandible.
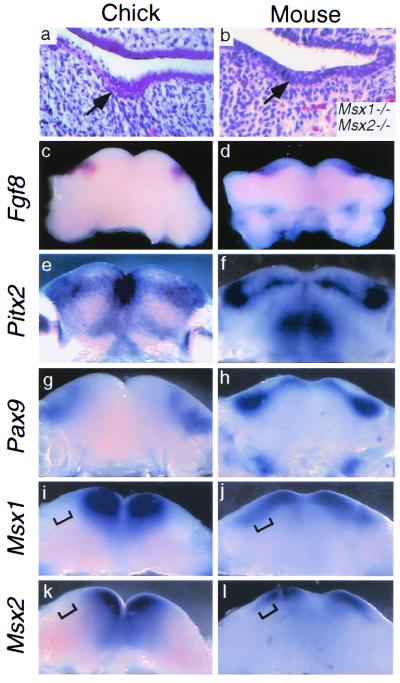
Rudimentary dental lamina-like epithelial structures are known to form transiently in the stage 27 chick jaw (17) and bear superficial resemblance to the early lamina stage of molar tooth development in mouse embryos (Fig. 1 a and b). This morphologic similarity suggests that the genetic program regulating tooth initiation is partly expressed in the avian embryonic jaw. To test this hypothesis, we tested for the presence of a potential chick dental lamina by examining the expression of several genes whose mammalian homologs either function in or are expressed during early tooth development. In mouse, Fgf8 and Pitx2 are early markers for dental epithelium, whereas Pax9, Barx1, Msx1, and Msx2 are early markers for dental mesenchyme (5, 6, 12, 24–27) (Fig. 1 c–l). Chick homologs of all six genes are expressed in the stage 27 chick mandible. Similar to mouse, transcripts of Fgf8 and Pitx2 localize to the region of the chick mandibular epithelium where lamina-like epithelial thickenings exist (Fig. 1 c and e), whereas Pax9 and Barx1 expression localizes to the underlying mesenchyme (Fig. 1g; and data not shown).
Figure 1.
Phenotypic and molecular comparison between putative chick dental lamina and mouse dental lamina. (a) Transverse section through stage 27 chick mandible showing a representative epithelial thickening (arrow), proposed to represent a vestigial dental lamina (16). Not all embryos examined exhibited such structures. (b) Transverse section of E14.5 Msx1-Msx2 double mutant molar tooth germ arrested at the lamina stage; the stage shown is developmentally equivalent to E11.5 in wild-type embryos. [Reproduced with permission from ref. 11. (Copyright 1998, Company of Biologists LTD).] (c–l) Molecular comparison between oral regions of stage 27 chick and E11.5 mouse mandibles. (c) Fgf8 expression in stage 27 chick mandibular epithelium. (d) Fgf8 expression in E11.5 mouse molar dental lamina. (e) Pitx2 expression in stage 27 chick mandibular epithelium. (f) Pitx2 expression in E11.5 mouse molar and incisor dental laminae. (g) Pax9 expression in stage 27 chick mandibular mesenchyme. (h) Pax9 expression in E11.5 mouse molar and incisor mesenchyme. (i–l) Differential Msx expression in chick and mouse mandibular mesenchyme. In chick, Msx1 (i) and Msx2 (k) expression is restricted to mesial mandibular mesenchyme and does not extend as far distally (bracketed). In mouse, Msx1 (j) and Msx2 (l) mesenchymal expression extends distally to the molar tooth-forming region (bracketed).
In both stage 27 chick and E11.5 mouse embryos, Msx1 and Msx2 are expressed in mesial mandibular mesenchyme. However, in chick (in contrast to mouse) Msx1 and, to a lesser extent, Msx2 expression is absent from distal mandibular mesenchyme (Fig. 1 i–l, brackets). Significantly, some mouse embryos doubly homozygous for loss of function in the Msx1 and Msx2 homeobox genes exhibit an arrest of tooth development at the early lamina stage (11) that resembles the lamina-like epithelial structure present in the distal chick mandibular arch (Fig. 1 a and b). This phenotypic similarity suggests that tooth development in the distal mandible of birds could arrest at a lamina stage because of an evolutionarily acquired loss of odontogenic Msx expression.
BMP4 Can Induce Msx Expression in the Distal Chick Mandible.
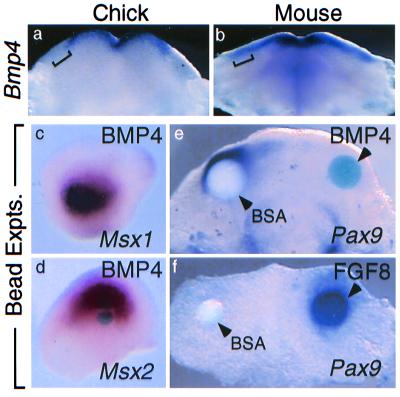
To define the factors responsible for the absence of Msx1 and Msx2 expression in chick distal mandibular mesenchyme, we examined chick mandibular epithelium for Bmp4 expression because BMP4 can induce Msx1 and Msx2 expression in mouse dental mesenchyme (8, 9). Bmp4 transcripts were detected in E11.5 mouse molar epithelium, but in the chick mandibular epithelium, as for Msx1 and Msx2, expression did not extend as far distally along the mesial-distal axis (Fig. 2 a and b, brackets). This observation was further confirmed by double-label, whole-mount in situ hybridization experiments that showed that Bmp4 expression did not extend distally to the Fgf8 expression domain (data not shown). To test whether the absence of Bmp4 expression from the chick oral epithelium in the putative odontogenic region could account for the absence of Msx1 and Msx2 expression in underlying mesenchyme, BMP4-soaked beads were tested for their ability to induce Msx expression in chick mandibular mesenchyme. BMP4-soaked beads strongly induced expression of Msx1 and Msx2 in chick mandibular mesenchyme (n = 12 each) (Fig. 2 c and d), supporting the idea that, although quiescent in chick, the odontogenic signaling pathway involving Bmp and Msx genes nonetheless is conserved between mouse and chick and capable of being activated.
Figure 2.
Expression of Bmp4 is absent from distal chick mandibular epithelium. (a) In the stage 27 chick mandible, Bmp4 is expressed in mesial oral epithelium and does not extend distally to the region of epithelial thickening (bracketed). (b) In contrast, in E11.5 mouse mandible, Bmp4 expression extends distally to the molar tooth-forming region (bracketed). (c and d) BMP4-soaked beads can induce Msx1 and Msx2 expression in stage 27 chick mandibular mesenchyme. BSA-soaked beads do not induce Msx1 or Msx2 expression (not shown). (e) BMP4 bead (Right) represses endogenous Pax9 expression in day 5 chick mandibular explants, whereas BSA bead (Left) does not. (f) FGF8 bead (Right) activates Pax9 expression in de-epithelialized day 4.5 chick mandibular explants, whereas BSA bead (Left) does not.
In addition, also similar to mouse (6), BMP4 beads repressed endogenous Pax9 expression in chick mandibular explants (n = 22/24), whereas FGF8 beads activated Pax9 expression in chick mandibular explants that were first de-epithelialized to eliminate endogenous Pax9 expression (n = 19/24) (Fig. 2 e and f). FGF8 also can activate Msx1 expression in mouse dental mesenchyme (5, 11); however, interruption of Bmp4 signaling is sufficient to produce a loss of Msx1 expression (10, 28). Thus, our results suggest that in chick a defect in Bmp4 signaling could explain the quiescence of the signaling pathways that control tooth initiation.
BMP and FGF Promote the Development of Sonic hedgehog (Shh)-Expressing Epithelial Structures in the Chick Mandible.
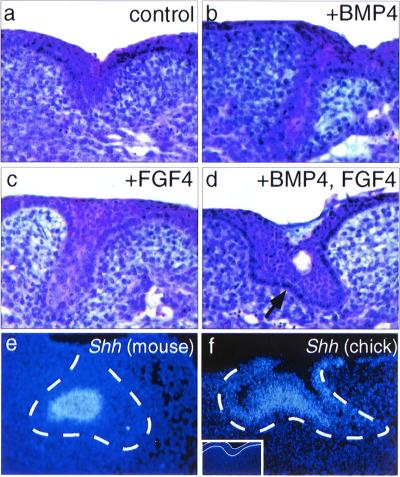
Experiments in mouse have shown that Msx1 is required for the shift of Bmp4 expression from dental epithelium to mesenchyme and that exogenous BMP4, mimicking the function of mesenchymal BMP4, can bypass the requirement for Msx1 function and act back on the dental epithelium to permit developmental progression of the tooth germ (ref. 9; M. Bei, K. Kratochwil, and R.M., unpublished work). We therefore asked whether, in analogous fashion, exogenous BMP4 also could act upon chick oral epithelium to promote the development of epithelial appendage structures in the chick oral cavity that resembled tooth germs. When stage 27 chick mandibles were cultured for 6 days in vitro, the chick oral epithelium either failed to invaginate or invaginated only slightly into the underlying mesenchyme (Fig. 3a, Table 1). In contrast, when chick mandibles were cultured in the presence of BMP4, the oral epithelium invaginated into the underlying mesenchyme to generate epithelial bud structures (Fig. 3b, Table 1). The ability of BMP4 to induce mandibular epithelial invaginations also was reproduced in 2 of 10 embryos by Bmp4 overexpression in chick mandibular mesenchyme by using a RCAS-Bmp4 retroviral vector; no invaginations were observed on the uninfected sides of the mandible or in control RCAS vector infections (Table 1).
Figure 3.
Induction of chick oral epithelial appendages by BMP and/or FGF. (a) Section through a control, untreated chick mandible after 6 days of culture showing region of thickened epithelium. (b and c) Bud-like structures induced in chick mandibles after 6 days of culture with 100 ng/ml of exogenous BMP4 (b) or FGF4 (c). (d) More advanced epithelial structure induced to form in chick mandibles after 6 days of culture with BMP4 and FGF4 (100 ng/ml each). Note convoluted epithelium (arrow). The clear space is a cyst. (e) Localization of Shh transcripts in the enamel knot of an E14.5 mouse molar tooth germ. (f) Shh expression induced in the central portion of the epithelial structure by addition of BMP4 and FGF4 to chick mandibles in explant culture. The dotted line in e and f indicates the location of the basal lamina separating epithelium and mesenchyme. (Inset) Shh is not expressed in control explants; the epithelium resides between the white lines.
Table 1.
Induction of oral epithelial structures in chick mandibles
| Bud stage | Advanced stage | Shh positive | AP positive | |
|---|---|---|---|---|
| Induction by soluble factors in organ culture | ||||
| Control | 0/14 | 0/14 | 0/12 | nd |
| BMP4 (100 ng/ml) | 8/18 | 0/18 | nd | nd |
| RCAS-Bmp4 (in ovo) | 2/10* | 0/10 | nd | nd |
| FGF4 (100 ng/ml) | 2/12 | 0/12 | nd | nd |
| BMP4 + FGF4 (100 ng/ml each) | 13/20 | 5/20 | 3/5† | nd |
| Induction by skin mesenchyme in organ culture | ||||
| Mandibular epithelium (distal, oral) + skin mesenchyme | 3/9 | 6/9 | 15/18† | nd |
| Mandibular epithelium (oral non-Fgf8 exp.) + skin mesenchyme | 10/14 | 0/14 | 1/6 (weak) | nd |
| Skin epithelium + mandibular mesenchyme | 0/8 | 0/8 | nd | nd |
| Induction by skin mesenchyme in organ culture + CAM | ||||
| Mandibular epithelium (distal, oral) + skin mesenchyme (exp. I) | 23/42 | 12/42 | nd | nd |
| Mandibular epithelium (distal, oral) + skin mesenchyme (exp. II) | 4/11 | 3/11 | nd | 6/7 |
| Skin epithelium + skin mesenchyme | 0/24‡ | 0/24‡ | nd | 5/20 (weak) |
Bud stage is defined by invagination of the oral epithelium only (e.g. Fig. 3b). Advanced stage is defined by the presence of epithelial invagination and convolution (e.g. Figs. 3d and 4c and e). nd, not determined. See Materials and Methods for details.
RCAS-Bmp4: retroviral virus expressing BMP4; these experiments were performed in vivo. Buds were observed only on the infected side.
Independent experiments from those in which morphology was scored.
In many of these experiments feather germs formed; these were not assigned a developmental stage.
Several FGFs also are expressed in the mouse tooth germ and are implicated as signaling molecules regulating tooth initiation and morphogenesis (5, 6, 11, 29). To test whether FGFs acting together with BMPs could promote further epithelial development, stage 27 chick mandibles were cultured in media supplemented with FGF4 (as a surrogate for mesenchymal FGFs) or with both BMP4 and FGF4. Although less effective than BMP4, FGF4 also stimulated invagination of the chick oral epithelium (Fig. 3c, Table 1). However, when added together, FGF4 and BMP4 acted synergistically to enhance the development of chick mandibular epithelium beyond the bud stage to form structures in which the epithelium was convoluted, thus resembling the cap stage of mouse tooth development (Fig. 3d, Table 1). Moreover, expression of Shh, a molecular marker for the dental epithelial enamel knot at the cap stage of mouse tooth germ development (30, 31), also is activated in these explants, predominantly in the central part of the epithelium (Fig. 3 e and f, Table 1). Shh transcripts were not detected in the epithelium of chick mandibles cultured without BMP4 and FGF4 (Fig. 3f, Inset).
Induction of Distinct Epithelial Appendages in Heterotypic, Heterochronic Recombinations Between Chick Oral Epithelium and Skin Mesenchyme.
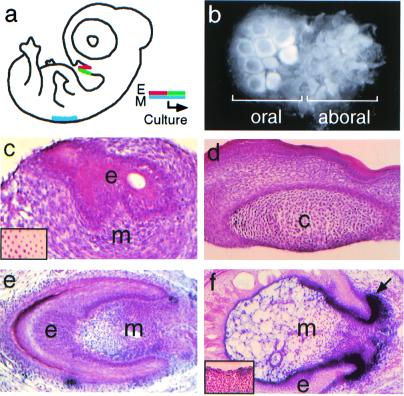
Because exogenous growth factors may not fully mimic the effects of endogenous growth factors, we next tested whether an embryonic mesenchymal tissue expressing BMP4 and FGF could more effectively induce the formation of chick oral epithelial structures. Previously, we found that experimental recombinations between stage 34 chick dorsal skin mesenchyme, an abundant source of BMP4 and FGF4, and stage 34 chick dorsal skin epithelium generated feather buds in explant culture (32). Therefore, to test the odontogenic potential of chick oral epithelium, we isolated individual pieces of stage 27 chick mandibular epithelium that included in each piece both the oral surface of the chick mandible and the aboral surface that normally forms feathered skin, and these were recombined with stage 34 chick dorsal skin mesenchyme (Fig. 4 a and b). As predicted, after in vitro culture for 5 days, the part of the mandibular epithelium isolated from the aboral surface formed typical feather buds. However, the portion of the mandibular epithelium isolated from the oral surface formed plaque-like epithelial appendages distinct from feather buds (Fig. 4 a and b). These plaque-like epithelial appendages then were analyzed further by histology.
Figure 4.
Chick oral epithelial appendage structures induced in vitro by heterotypic, heterochronic recombination. (a) Scheme for recombinations in which a single piece of stage 27 chick mandibular epithelium, including both oral (red) and aboral (green) surfaces, was recombined with stage 34 chick dorsal skin mesenchyme (blue) and cultured for 5 days. (b) Whole mount showing that different epithelial appendages form from oral and aboral chick mandibular epithelia. Feather germs form from aboral epithelium (Right), whereas novel epithelial appendages form from oral epithelium (Left). (c) Histology of oral epithelial structures in the preceding experiment. A small cyst is present. (Inset) Whole-mount in situ demonstrating Bmp4 expression in stage 34 chick dorsal feather follicle mesenchyme. (d) Control recombination between stage 34 chick skin epithelium and stage 27 mandibular mensenchyme, which does not produce epithelial structures and in some cases (shown) results in cartilage formation (indicated by c). (e) Epithelial appendage structure formed after recombination of stage 27 chick mandibular epithelium with stage 35 chick skin mesenchyme after 2 days in organ culture and 6 days on CAM. Note condensation of mesenchymal cells within the epithelium. (f) AP activity detected (black, arrow) in condensed mesenchyme of a CAM-cultured chick mandibular epithelium-chick skin mesenchyme recombinant. (Inset) Absence of AP activity in naïve chick mandibular tissue. e, epithelium; m, mesenchyme.
In 3 of 9 recombinants, the oral epithelium invaginated into the underlying skin mesenchyme to form bud structures, whereas in the other six recombinants both invagination and convolution of the epithelium occurred, resulting in a more developmentally advanced structure resembling the cap stage of odontogenesis (Fig. 4c, Table 1). In a separate set of experiments, these epithelial structures were again found to be strongly Shh-positive; in contrast, control reciprocal recombinations between mandibular mesenchyme and skin epithelium yielded no epithelial in-growth, and in some cases cartilage formed (Fig. 4d). Additional control recombinations between non-Fgf8-expressing regions of oral mandibular epithelium and skin mesenchyme showed minor degrees of epithelial in-growth, but Shh expression was detected in only 1 of 6 cases (Table 1).
To further test the developmental potential of these chick mandibular epithelial appendages, recombinants were cultured for 2 days in organ culture, then transferred to chick chorio-allantoic membrane (CAM) cultures and cultured for 6 additional days. Although epithelial bud structures were again observed in about half the cases (23 of 42), morphologically advanced structures were observed in 12 of 42 cases, and in many the mesenchyme was condensed within the surrounding epithelium (Fig. 4e, Table 1). These epithelial appendage structures were not typical tooth or feather germs, but exhibited some morphologic features consistent with both fates.
Because specific markers for chick tooth development do not exist, these structures were analyzed histochemically for AP activity, a marker in mouse tooth germs for dental papillary mesenchyme and odontoblasts (33). In control combinations of chick skin epithelium and skin mesenchyme, mesenchymal AP activity was detected weakly in only 1 case of 20 in the feather germs that formed (Table 1), probably because feather germ AP activity is heat labile and therefore inactivated during embedding (32, 34). Weak epithelial AP staining also was observed in five cases, including that in which the weak mesenchymal activity was observed (Table 1). In contrast, in a set of 11 CAM-cultured recombinants of chick oral mandibular epithelium and chick skin mesenchyme, epithelial structures developed in seven and AP activity was strongly induced in the condensed mesenchyme surrounding the epithelium in six of these (Fig. 4e, Table 1), consistent with the conclusion that these epithelial appendage structures exhibited some characteristics of early stages of tooth morphogenesis.
Discussion
It is well known that, unlike their toothed ancestors of the Jurassic and Cretaceous periods, modern birds lack dentition. Gardiner (17) was the first to call attention to the residual tooth germ in the embryonic chick oral cavity, a finding subsequently confirmed in other Aves (18, 19). Here we present evidence consistent with the existence of an avian tooth primordia. Our results suggest that chick tooth initiation in the distal mandible is arrested at a lamina-like stage because of a defect in epithelial-mesenchymal interactions (35), and that this defect could be caused by the absence of Bmp4 expression and hence that of Msx1 and Msx2 in the distal mandible. This idea is supported by the findings that: (i) BMP4-soaked beads induce Msx1 and Msx2 expression in chick mandibular mesenchyme, and (ii) exogenous BMP4 induces an epithelial bud that invaginates into the underlying mesenchyme. Although other odontogenic factors are also likely to be missing in Aves, the fact that epithelial appendages can be induced in chick oral epithelium is consistent with the idea that the chick oral epithelium retains odontogenic potential.
In addition to the phylogenetic absence of dentition in certain groups such as Aves and Testudines (turtles), developmental regressions of tooth primordia occur in certain species that also could be potentially explained by an evolutionarily acquired inactivation of the genetic pathways controlling tooth formation (20). For example, the diastema region of the mouse oral cavity exhibits transient epithelial thickenings that resemble dental laminae but subsequently undergo apoptosis (36, 37). These structures, which reside at locations considered homologous to tooth-forming regions in other eutherians, have been suggested to represent abortive attempts at tooth initiation (38). However, in contrast to the thickened epithelium in the chick mandible, these structures express Bmp4 and Msx1 (39). Thus, the genetic mechanisms for the absence of dentition in birds and the restricted loss in the diastema region in muroid rodents are presumably different.
Previously, it was shown that combinations of chick mandibular epithelium with mouse tooth mesenchyme formed dental structures containing enamel-secreting ameloblasts (21). Similar results appear to have been obtained by some (40–43) but not other investigators (44–47), and the positive results can be challenged in all cases by the possibility of tissue contamination (43, 47). In this study, only chicken-derived tissues were used, excluding this particular possibility. The expression of Shh in growth factor-induced chick oral epithelial buds and of AP activity in the interacting mesenchyme suggests that signaling pathways have been retained in Aves at a level sufficient to recapitulate some of the same early molecular steps that also are observed in formation of the mammalian tooth germ. We conclude that while latent, early odontogenic signaling pathways have been retained by Aves, and that an evolutionary loss of Bmp4 expression in avian dental epithelium could account for the resemblance to the arrest in tooth development observed in mouse Msx1-Msx2 double mutants.
The present work suggests that in Aves early steps in the odontogenic pathway potentially exist in latent form. It does not, however, demonstrate the formation in Aves of structures that can be called teeth. In addition, although the competence of avian tissues to support the terminal differentiation of dental tissues is unknown, it seems likely that unless expressed in other contexts, many of the genes that characterize the hard mineralized dentition of toothed vertebrates would have sustained inactivating mutations and therefore represent pseudogenes in birds. For example, amelogenin-like sequences have not been identified in the chicken genome by degenerate PCR, despite the fact that such sequences can be detected in actinopterygian (ray-finned) fishes (48) and amphibia (49). Interestingly, a dentin matrix protein 1 gene, DMP1, has been identified in chicken, but its conservation is limited to three short segments, the rest being highly diverged (50). Thus, even if present in the chicken genome, amelogenin or DMP1 sequences may be unable to participate in enamel or dentin formation.
Whereas previous results imply that only neural crest-derived mesenchyme retains the capacity to participate in tooth formation (4), the data presented here would suggest that skin mesenchyme also might possess this ability. However, it should be noted that unlike the experiments involving recombinations of mouse neural crest and mouse mandibular epithelia (6), we did not detect mineralized tooth formation and, for the reasons mentioned above, it seems unlikely that this could occur in chick. It is possible that skin mesenchyme retains only a rudimentary capacity to induce early steps in odontogenesis and hence, the two sets of results are not necessarily inconsistent. Nonetheless, it has been shown that chick and mouse mandibular mesenchymes exhibit similar molecular responses when recombined with mouse odontogenic epithelium (51). In our oral epithelium-skin mesenchyme recombinants, despite the induction of mesenchymal heat-stable AP expression, the fate of the skin mesenchyme is unclear compared with that of the oral epithelium, in which Shh expression is clearly induced. Additional studies will be required to accurately categorize the epithelial appendage structures that form in the skin mesenchyme-oral epithelium tissue recombinations.
Epithelial appendages form in successive stages, which can been categorized as: (i) induction, when the decision to form an appendage is made, (ii) morphogenesis, when the different epithelial organ phenotypes are established, and (iii) differentiation, when organ-specific gene products are expressed (52). During morphogenesis, a number of signaling molecules and components of the induction cascade, including BMP, FGF, and Msx, are expressed in common in different developing appendages, and the fates of nascent epithelial appendages are capable of interconversion (53). In this work we show that, given an appropriate stimulus, avian oral epithelium can reactivate a latent molecular pathway to form morphologically distinct epithelial appendages that share some features in common with mammalian tooth germs.
What distinguishes these structures from the early stages of epithelial appendage formation that are common to the early development of many organs that form via epithelial-mesenchymal interactions? The molecular markers we have used, Shh and AP, although consistent with a tooth fate, both are expressed in other developmental contexts, including developing feather germs (32, 34). Although the structures that formed in the heterotypic recombinants do not resemble feather germs nor do they express the same heat-stable AP isoform, these markers are general to other epithelial-mesenchymal interactions. Perhaps the best evidence that these structures are compatible with an odontogenic fate comes from their ability to form from the oral surface of the chick mandible. Thus, in the chick mandible, initial stages of epithelial appendage formation can be activated that are consistent with an odontogenic fate, and interruption of this pathway provides a plausible basis for the absence of dentition in birds.
Acknowledgments
We thank members of the Maas laboratory and the reviewers for helpful comments and Dr. H. Peters for translating refs. 18 and 19. This work was supported by National Institutes of Health grants to R.M. (RO1DE11697) and C.-M.C., a Medical Research Council grant to P.F.-W., and National Institutes of Health (R01 DE12329) and National Science Foundation (IBN-979632) grants to Y.P.C.
Abbreviations
- BMP
bone morphogenetic protein
- FGF
fibroblast growth factor
- E(n)
embryonic day
- AP
alkaline phosphatase
- CAM
chorio-allantoic membrane
- Shh
Sonic hedgehog
- RCAS
replication competent avian sarcoma
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160245097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160245097
References
- 1.Maas R, Bei M. Crit Rev Oral Biol Med. 1997;8:4–39. doi: 10.1177/10454411970080010101. [DOI] [PubMed] [Google Scholar]
- 2.Thesleff I, Sharpe P. Mech Dev. 1997;67:11–23. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 3.Mina M, Kollar E J. Arch Oral Biol. 1987;32:123–127. doi: 10.1016/0003-9969(87)90055-0. [DOI] [PubMed] [Google Scholar]
- 4.Lumsden A G S. Development (Cambridge, UK) 1988;103,Suppl.:155–169. doi: 10.1242/dev.103.Supplement.155. [DOI] [PubMed] [Google Scholar]
- 5.Kettunen P, Thesleff I. Dev Dyn. 1998;211:256–268. doi: 10.1002/(SICI)1097-0177(199803)211:3<256::AID-AJA7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 6.Neubüser A, Peters H, Balling R, Martin G R. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- 7.Peters H, Neubüser A, Kratochwil K, Balling R. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vainio S, Karavanova I, Jowett A, Thesleff I. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- 9.Chen Y P, Bei M, Woo I, Satokata I, Maas R. Development (Cambridge, UK) 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- 10.Tucker A S, Matthews K L, Sharpe P T. Science. 1998;282:1136–1138. doi: 10.1126/science.282.5391.1136. [DOI] [PubMed] [Google Scholar]
- 11.Bei M, Maas R L. Development (Cambridge, UK) 1998;125:4325–4333. doi: 10.1242/dev.125.21.4325. [DOI] [PubMed] [Google Scholar]
- 12.Thomas B L, Tucker A S, Qiu M, Ferguson C A, Hardcastle Z, Rubenstein J L, Sharpe P. Development (Cambridge, UK) 1997;124:4811–4818. doi: 10.1242/dev.124.23.4811. [DOI] [PubMed] [Google Scholar]
- 13.Vastardis H, Karimbux N, Guthua S W, Seidman J G, Seidman C E. Nat Genet. 1996;13:417–421. doi: 10.1038/ng0896-417. [DOI] [PubMed] [Google Scholar]
- 14.van den Boogard M-J H, Dorland M, Beemer F A, van Amstel H K P. Nat Genet. 2000;24:342–343. doi: 10.1038/74155. [DOI] [PubMed] [Google Scholar]
- 15.Satokata I, Maas R. Nat Genet. 1994;6:348–356. doi: 10.1038/ng0494-348. [DOI] [PubMed] [Google Scholar]
- 16.Houzelstein D, Cohen A, Buckingham M E, Robert B. Mech Dev. 1997;65:123–133. doi: 10.1016/s0925-4773(97)00065-8. [DOI] [PubMed] [Google Scholar]
- 17.Gardiner E G. Arch J Mikr Anat. 1884;24:289–338. [Google Scholar]
- 18.Röse C. Anat Anz. 1892;7:748–758. [Google Scholar]
- 19.Carlsson A. Anat Anz. 1896;12:72–75. [Google Scholar]
- 20.Huysseune A, Sire J-Y. Eur J Oral Sci. 1998;106, Suppl. 1:437–481. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- 21.Kollar E J, Fisher C. Science. 1980;207:993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- 22.Hamburger V, Hamilton H L. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 23.Duprez D, Bell E J, Richardson M K, Archer C W, Wolpert L, Brickell P M, Francis-West P H. Mech Dev. 1996;57:145–157. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- 24.Mucchielli M-L, Mitsiadis T A, Raffo S, Brunet J F, Proust J, Goridis C. Dev Biol. 1997;189:275–284. doi: 10.1006/dbio.1997.8672. [DOI] [PubMed] [Google Scholar]
- 25.Tissier-Seta J-P, Mucchielli M-L, Mark M, Mattei M-G, Goridis C, Brunet J-F. Mech Dev. 1995;52:3–15. doi: 10.1016/0925-4773(94)00343-l. [DOI] [PubMed] [Google Scholar]
- 26.MacKenzie A, Leeming G L, Jowett A K, Ferguson M W J, Sharpe P T. Development (Cambridge, UK) 1991;111:269–285. doi: 10.1242/dev.111.2.269. [DOI] [PubMed] [Google Scholar]
- 27.MacKenzie A, Ferguson M W J, Sharpe P T. Development (Cambridge, UK) 1992;115:403–420. doi: 10.1242/dev.115.2.403. [DOI] [PubMed] [Google Scholar]
- 28.Tucker A S, Khamis A A, Sharpe P T. Dev Dyn. 1998;212:533–539. doi: 10.1002/(SICI)1097-0177(199808)212:4<533::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Thesleff I, Sahlberg C. Semin Cell Dev Biol. 1996;7:185–193. [Google Scholar]
- 30.Koyama E, Yamaai T, Iseki S, Ohuchi H, Nohno T, Yoshioka H, Hayashi Y, Leatherman J L, Golden E B, Noji S, Pacifici M. Dev Dyn. 1996;206:59–72. doi: 10.1002/(SICI)1097-0177(199605)206:1<59::AID-AJA6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Vaahtokari A, Åberg T, Jernvall J, Keranen S, Thesleff I. Mech Dev. 1996;54:39–43. doi: 10.1016/0925-4773(95)00459-9. [DOI] [PubMed] [Google Scholar]
- 32.Chuong C-M, Widelitz R B, Ting-Bereth S, Jiang T-X. J Invest Dermatol. 1996;107:639–646. doi: 10.1111/1523-1747.ep12584254. [DOI] [PubMed] [Google Scholar]
- 33.Miyake T, Cameron A M, Hall B K. J Anat. 1997;190:239–260. doi: 10.1046/j.1469-7580.1997.19020239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crawford K, Weissig H, Bionette F, Millan J L, Goetinck P F. Dev Dyn. 1995;204:48–56. doi: 10.1002/aja.1002040107. [DOI] [PubMed] [Google Scholar]
- 35.Butler P M. Int J Dev Biol. 1995;39:25–34. [PubMed] [Google Scholar]
- 36.Peterkova R. J Craniofac Genet Dev Biol. 1983;3:133–142. [PubMed] [Google Scholar]
- 37.Tureckova J, Lesot H, Vonesch J L, Pertka M, Peterkova R, Ruch J V. Int J Dev Biol. 1996;40:483–489. [PubMed] [Google Scholar]
- 38.Ruch J V, Lesot H, Peterkova R, Peterka M. BioEssays. 1997;19:1041. doi: 10.1002/bies.950191115. [DOI] [PubMed] [Google Scholar]
- 39.Tureckova J, Sahlberg C, Aberg T, Ruch J V, Thesleff I, Peterkova R. Int J Dev Biol. 1995;39:459–468. [PubMed] [Google Scholar]
- 40.Lemus D, Fuenzalida M, Illanes J, Paz de la Vega Y. J Morphol. 1983;176:341–350. doi: 10.1002/jmor.1051760307. [DOI] [PubMed] [Google Scholar]
- 41.Lemus D, Coloma L, Fuenzalida M, Illanes J, Paz de la Vega Y, Ondarza A, Blanquez M J. J Morphol. 1986;176:341–350. [Google Scholar]
- 42.Fuenzalida M, Lemus R, Romero S, Fernandez-Valencia R, Lemus D. J Exp Zool. 1990;256:264–272. doi: 10.1002/jez.1402560305. [DOI] [PubMed] [Google Scholar]
- 43.Lemus D. Int J Dev Biol. 1995;39:291–297. [PubMed] [Google Scholar]
- 44.Cummings E G, Bringas P, Jr, Grodin M S, Slavkin H C. Differentiation. 1981;20:1–9. doi: 10.1111/j.1432-0436.1981.tb01149.x. [DOI] [PubMed] [Google Scholar]
- 45.Karcher-Djuricic V, Ruch J V. C R Soc Biol. 1973;167:915–918. [PubMed] [Google Scholar]
- 46.Ruch J V, Karcher-Djuricic V, Thiebold J. In: Extracellular Matrix Influences on Gene Expression. Slavkin H C, Greulich R C, editors. New York: Academic; 1975. p. 549. [Google Scholar]
- 47.Arechaga J, Karcher-Djuricic V, Ruch J V. Differentiation. 1983;25:142–147. doi: 10.1111/j.1432-0436.1984.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 48.Girondet M, Sire J-Y. Eur J Oral Sci. 1998;106, Suppl. 1:501–508. doi: 10.1111/j.1600-0722.1998.tb02213.x. [DOI] [PubMed] [Google Scholar]
- 49.Toyosawa S, O'hUigin C, Figueroa F, Tichy H, Klein J. Proc Natl Acad Sci USA. 1998;95:13056–13061. doi: 10.1073/pnas.95.22.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toyosawa S, Sato A, O'hUigin C, Tichy H, Klein J. J Mol Evol. 2000;50:31–38. doi: 10.1007/s002399910004. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y H, Upholt W B, Sharpe P T, Kollar E J, Mina M. Dev Dyn. 1998;213:386–397. doi: 10.1002/(SICI)1097-0177(199812)213:4<386::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Chuong C-M, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Austin, TX: Landes; 1998. pp. 3–14. [Google Scholar]
- 53.Zhou P, Byrne C, Jacobs J, Fuchs E. Genes Dev. 1995;9:700–713. doi: 10.1101/gad.9.6.700. [DOI] [PubMed] [Google Scholar]