Abstract
Although anti-atherogenic effects of cilostazol have been suggested, its effects on the expression of SR in macrophages are unclear. This study investigated the role of cilostazol on CD36 expression of murine macrophages enhanced by HNE, a byproduct of lipid peroxidation. The stimulation of macrophages with HNE led to an increased expression of CD36, which was significantly attenuated by NAC, an antioxidant. Moreover, the increased production of ROS by HNE was completely abolished by NADPH oxidase inhibitors, DPI and apocynin, as well as by the 5-LO inhibitor, MK886, but not by inhibitors for other oxidases. This suggested that NADPH-oxidase and 5-LO were major sources of ROS induced by HNE. In addition, HNE-enhanced expression of CD36 was reduced by these inhibitors, which indicated a role for NADPH oxidase and 5-LO on CD36 expression. In our present study, cilostazol was a significant inhibitor of ROS production, as well as CD36 expression induced by HNE. An increase in NADPH oxidase activity by HNE was significantly attenuated by cilostazol, however cilostazol had no effect on HNE-enhanced 5-LO activity. Together, these results suggest that cilostazol attenuates HNE-enhanced CD36 expression on murine macrophages thorough inhibition of NADPH oxidase-derived ROS generation.
Keywords: Cilostazol, HNE, CD36, NADPH oxidase
INTRODUCTION
Oxidative stress is generally regarded as a key factor in atherogenesis, in that it is closely associated with inflammation and the formation of bioactive lipids (Berliner et al., 1995; Stocker and Keaney Jr, 2004; Leonarduzzi et al., 2005). Among the reactive aldehydes, 4-hydroxynonenal (HNE), a major end-product of lipid peroxidation (Esterbauer et al., 1991), is believed to promote the primary pathogenic effects of oxidative stress (Uchida, 2003; Lee et al., 2006; Sampey et al., 2007). In our previous study, elevated levels of HNE have been detected in atherosclerotic lesions (Yun et al., 2008), supporting an etiological role for HNE in the development of atherosclerosis.
One Of The Earliest Events In Atherogenesis Is The Accumulation Of Oxidized Low Density Lipoprotein (Oxldl) In The Intima Layer Of Blood Vessels And The Subsequent Uptake Of Modified Lipoprotein By Macrophages, Leading To Foam Cell Formation (Ross, 1999; Moore And Freeman, 2006). In Macrophages, Oxldl Is Ingested By Scavenger Receptors (Sr) Including Class A Sr (Sr-a) And Class B Sr (Cd36), Which Are Two Of The Most Important Sr For Foam Cell Formation (Greaves And Gordon, 2005). Among These Sr, The Macrophage Cd36 Receptor Was Shown To Be Involved In The Formation Of Foam Cells And Is Highly Expressed In Atherosclerotic Lesions (Nakata Et Al., 1999). Research Studies Indicate That The Expression Of Cd36 Is Stimulated By Phorbol Esters, Macrophage Colony Stimulating Factor (M-csf), And Possibly Ox-ldl (Yesner Et Al., 1996; Han Et Al., 1997; Yoshida Et Al., 1998). Moreover, Our Previous Study Demonstrated That Hne Directly Enhanced The Expression Of Cd36 On Murine Macrophages Via P38 Mapk-mediated Activation Of 5-lipoxygenase (5-lo), Which Leading To The Increased Uptake Of Oxldl Towards Foam Cell Formation (Yun Et Al., 2008; Yun Et Al., 2009).
Kimura et al. (1985) reported that cilostazol [6-[4-(1-cyclohexyl-1H-tetrazol-5-yl) butoxy]-3,4-dihydro-2(1H)-quinolinone] increased intracellular cAMP by blocking its hydrolysis by type III phosphodiesterase (PDE3) (Kimura et al., 1985). This resulted in reduced platelet aggregation and in peripheral vasodilation. In our previous study, cilostazol inhibited NAD(P)H oxidase-dependent superoxide formation and the release of cytokines concomitant with the suppression of atheromic plaque formation (Shin et al., 2004). In addition, cilostazol reduced atherosclerosis by inhibiting the formation of superoxide and TNF-α, and thereby reducing NF-κB activation/transcription of VCAM-1/MCP-1 expression, and monocyte recruitment of LDL receptor-null mice fed high cholesterol (Lee et al., 2005). Recently, Okutsu et al. (2009) reported that cilostazol inhibited the uptake of modified LDL and foam cell formation in mouse peritoneal macrophages. However, although anti-atherogenic effects of cilostazol have been suggested in the previous studies, the molecular mechanisms involved in macrophage foam cell formation have not been clarified.
Given that HNE is a sensitive marker for oxidative stress and increased CD36 expression on macrophages, it is likely hypothesized that cilostazol may regulate CD36 expression induced by HNE. In order to verify the role of cilostazol on CD36 expression, we determined 1) the stimulatory effects of HNE on CD36 expression of the murine macrophage, 2) the underlying mechanism(s) involved in HNE-dependent regulation of CD36 expression, and 3) the inhibitory effect of cilostazol on the mechanism regulating CD36 expression.
METHODS
Chemicals and antibodies
HNE was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). 2',7'-Dichlorofluorescein diacetate (DCFH-DA), lucigenin, N-acetylcysteine (NAC), NADPH, diphenylamine iodonium (DPI), rotenone, stigmatellin, allopurinol, indomethacin, nordihydroguaiaretic acid (NDGA), and arachidonic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Apocynin was supplied by Calbiochem (La Jolla, CA, USA). MK886 and baicalein were obtained from Biomol (Plymouth Meeting, PA, USA).
Cell culture and isolation of mouse peritoneal macrophages
J774A.1 macrophages (a murine macrophage cell line; ATCC, Rockville, MD, USA) were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) and antibiotic-antimycotic at 37℃ in 5% CO2. After reaching confluence, the cells were detached from the surface of T75 culture flasks by gentle scraping. The detached cells were then washed and resuspended in complete medium. Cells between passages 2 and 5 were used for experiments.
Immunoblot analysis
To determine the expression of CD36 at the protein level, 30 µg of protein extracted from macrophages were separated using 10% SDS-PAGE, then transferred electrophoretically to a nitrocellulose membrane (Hybond; Amersham Biosciences, Piscataway, NJ, USA). The membranes were incubated with anti-CD36 antibody (Santa Cruz Biotechnology; Santa Cruz, CA, USA). Horseradish peroxidase (HRP)-conjugated IgG antibody (Santa Cruz Biotechnology) was used as the secondary antibody. The immunoblot was visualized using SuperSignal West Dura extended duration substrate kit (Pierce Chemical; Rockford, IL, USA). As an internal control, this membrane was reblotted with anti-β-actin antibody (MP Biomedicals; Aurora, Ohio, USA). The blots were scanned using ScanJet 4C (Hewlett-Packard; Palo Alto, CA, USA) and analyzed using UN-SCAN-IT GEL™ software (version 5.1, Silk Scientific Inc; Orem, Utah, USA).
Analysis of mRNA expression
The oligonucleotide primers used to perform RT-PCR were 5'-TCG-GAA-CTG-TGG-GCT-CAT-TG-3' and 5'-CCT-CGG-GGT-CCT-GAG-TTA-TAT-TTT-C-3' for CD36, and 5'-CTG-CCA-TTT-GCA-GTG-GCA-AAG-TGG-3' and 5'-TTG-TCA-TGG-ATG-ACC-TTG-GCC-AGG-3' for GAPDH. After macrophages were stimulated with HNE, total RNA from the cells was analyzed by RT-PCR using a Qiagen OneStep RT-PCR kit (Qiagen; Hilden, Germany).
Quantitating ROS generation
Changes in intracellular reactive oxygen species (ROS) levels were evaluated by measuring the oxidative conversion of DCFH-DA to fluorescent DCF as described previously (Amer et al., 2003). Cells grown in 12-well plates were loaded with 10 µM DCFH-DA for 45 min at 37℃, then incubated with HNE under the indicated conditions. The cells were washed with PBS and harvested by gentle scraping. DCF fluorescence of 10,000 cells was detected using FACScaliber flow cytometer (Becton Dickinson; San Jose, CA, USA) and analyzed using the CellQuest Software (version 3.3, Becton Dickinson).
Quantitating NADPH oxidase activity
NADPH-oxidase activity was measured using a lucigenin-enhanced chemiluminescence assay (Manea et al., 2007). Cells were washed briefly with PBS, and then harvested. After low spin centrifugation, the pellet was lysed in phosphate buffer (20 mM monobasic potassium phosphate [pH 7.0], 1 mM EGTA, 10 µM aprotinin, 0.5 µg/ml leupeptin, 0.7 µg/ml pepstatin, and 0.5 mM phenylmethlysulfonyl fluoride [PMSF]). The cellular lysates were centrifuged for 10 min at 13,000 rpm and the supernatant was used for the assay. The total protein concentration was determined using a bicinchoninic acid protein assay kit (Sigma Chemical Co.). The reaction mixture comprised a Krebs/HEPES buffer, pH 7.0, lucigenin (5 µM) as the electron acceptor, and NADPH (100 µM) as the substrate. The reaction was initiated by the addition of 25 µg protein, and photon emission was measured every second for 10 min time period using a microtiterplate luminometer (Microlumat LB96P; EG and G Berthold, Germany). The activity was expressed as relative light units (RLU) per second per milligram of total protein (RLU/sec/mg protein).
Quantitation of LTB4 production
Leukotriene B4 (LTB4) production was measured in cell-free supernatants using a commercially available LTB4 assay kit (R&D Systems; Minneapolis, MN, USA). Briefly, after macrophages were stimulated with HNE (10 µM) in the presence of exogenous arachidonic acid (40 µM), the conditioned media was harvested and then concentrated using Vivaspin 2 (Sartorius Biolab; Goettingen, Germany). The amount of LTB4 in the concentrated media was measured using ELISA (Bio-Tek Instrument Inc; Winooski, VT, USA) following the manufacturer's instructions.
Statistical analysis
The results were expressed as means±SE. Statistical significance was estimated by Student's t-test for unpaired observations between two groups or by ANOVA with Bonferroni correction for comparisons of multiple groups. p<0.05 was regarded as significant.
RESULTS
HNE enhanced CD36 expression in J774A.1 macrophages
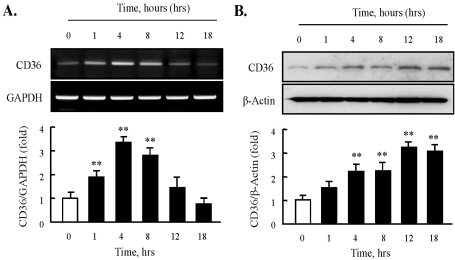
SR family proteins play an important role in cellular lipid accumulation and in macrophage foam cell formation. Therefore, we first investigated whether HNE could modulate the expression of CD36, a major SR, in J774A.1 macrophages. The effect of HNE on CD36 mRNA expression was examined by semi-quantitative RT-PCR analysis. As shown in Fig. 1A, HNE (10 µM) significantly increased CD36 mRNA in a time-dependent manner for up to 8 hrs. Moreover, immunoblot analysis showed that CD36 protein levels were enhanced significantly in murine macrophages upon exposure to HNE for the indicated time (Fig. 1B).
Fig. 1.
Effect of HNE on the expression of CD36 in J774A.1 macrophages. (A) Total RNA was extracted from macrophages treated with 10 µM HNE for the indicated time (0~18 hrs). 2 µg RNA was subjected to RT-PCR. Band intensity for CD36 mRNA was quantified by densitometric scanning and normalized to that of GAPDH. (B) Macrophages were incubated with 10 µM HNE, and cellular lysates were analyzed by Western blotting using anti-CD36 antibody. β-actin as used to normalize density. Each photograph is the representative of 5 independent experiments. Data is presented as mean±SE from 5 independent experiments. **p<0.01 vs. value at time 0.
Role of ROS on HNE-induced CD36 expression
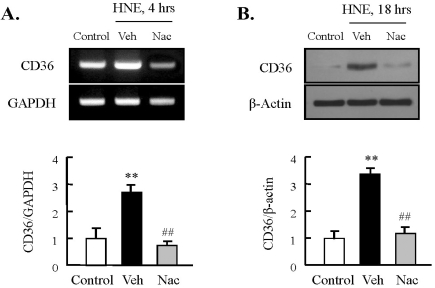
To determine the involvement of ROS in HNE-induced expression of CD36, we pretreated macrophages with NAC, an antioxidant, followed by stimulation with HNE (10 µM) for the indicated time. As shown in Fig. 2, the increase in HNE-induced CD36 expression at the mRNA and protein levels was attenuated significantly by treatment with NAC, indicating participation of ROS in HNE-enhanced CD36 expression.
Fig. 2.
Role of ROS on HNE-induced CD36 expression in J774A.1 macrophages. Macrophages were treated with 10 µM HNE for the indicated time in the absence or presence of N-acetylcysteine (Nac, 5 mM). The levels of CD36 mRNA and protein were determined by RT-PCR (A) and Western blot analysis (B), respectively. Each fig is the representative of 4 independent experiments. Data was presented as mean±SE from 5 independent experiments. **p<0.01 vs. control; ##p<0.01 vs. veh (vehicle).
HNE enhanced ROS production in J774A.1 macrophages
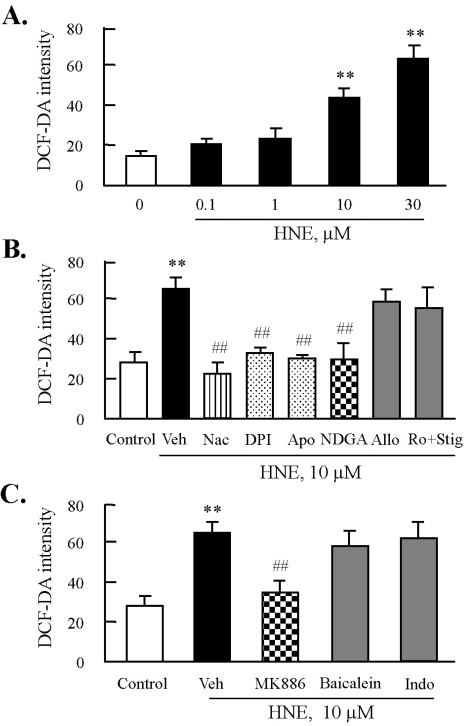
To examine the level of intracellular ROS by treatment with HNE in J774A.1 macrophages, cells were labeled with DCFH-DA, and then incubated with HNE for 45 min. This was followed by flow cytometric analysis. As shown in Fig. 3A, after stimulation with increasing concentrations of HNE ranging from 0.1 to 30 µM, macrophages showed a concentration-dependent increase in the level of intracellular ROS. To further elucidate enzymatic sources of HNE-induced ROS generation, macrophages were stimulated with 10 µM HNE for 45 min in the presence of various inhibitors for ROS-generating enzymes. As shown in Fig. 3B, HNE-induced ROS generation was almost completely inhibited by DPI and apocynin, specific inhibitors for NADPH-oxidase, as well as NDGA, a LO inhibitor, but not by inhibitors of other enzyme source including xanthine oxidase and mitochondrial oxidases (Fig. 3B). Furthermore, since NDGA was not selective to individual LO because it inhibited most of eicosanoids including 5-LO, 12-LO, and cyclooxygenase (COX), we examined the individual role of eicosanoid pathways in HNE-induced ROS production. As shown in Fig. 3C, HNE-induced ROS generation was significantly attenuated by MK886, a 5-LO inhibitor, but not by baicalein, a 12-LO inhibitor, and indomethacin, a COX inhibitor.
Fig. 3.
Effects of inhibitors of NADPH oxidase or lipoxygenase on HNE-induced ROS production in J774A.1. macrophages. (A) Cells were treated with different concentrations of HNE for 45 min, and then ROS generation was measured by flow cytometry (FACS) using DCF fluorescence. Fluorescence intensity was analyzed by CellQuest Software. Data was presented as mean±SE from 5 independent experiments. **p<0.01 vs. value at concentration 0. (B) Cells were incubated with 10 µM HNE for 45 min after pre-treatment with various inhibitors for ROS including N-acetylcysteine (Nac, 5 mM), diphenylamine iodonium (DPI, 10 µM), apocynine (Apo, 300 µM), nordihydroguaiaretic acid (NDGA, 10 µM), allopurinol (Allo, 100 µM), or rotenone (Ro, 1 µM) plus stigmatellin (Stig, 1 µM) for 30 min. (C) Cells were pre-treated with eicosanoid inhibitors including MK886 (10 µM), baicalein (10 µM), or indomethacin (Indo, 100 µM) for 30 min, and then stimulated with 10 µM HNE for 45 min. ROS generation was analyzed by FACS. Data was presented as mean±SE from 5 independent experiments. **p<0.01 vs. control; ##p<0.01 vs. veh (vehicle).
Role of NADPH oxidase and 5-LO on HNE-induced CD36 expression
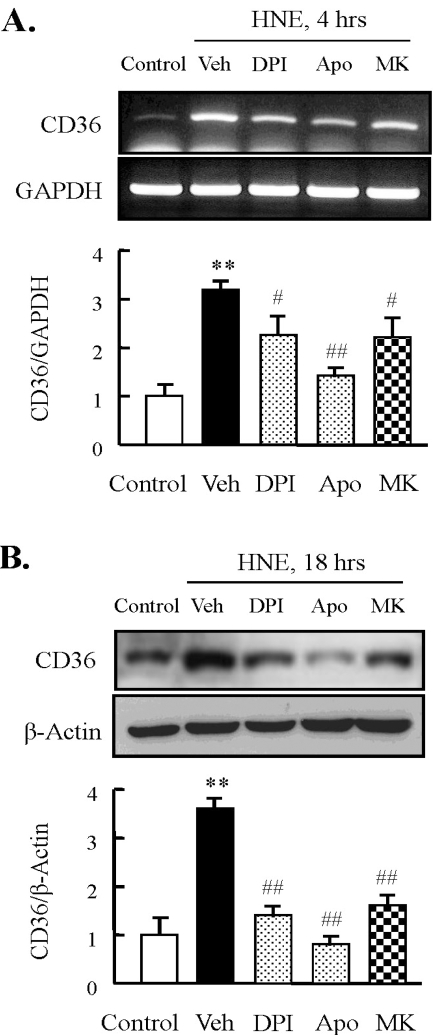
Next, we investigated whether enzymes involved in HNE-induced ROS generation play a role in regulation of HNE-enhanced CD36 expression. Similar to the effects of HNE-induced ROS generation, inhibitors for NADPH oxidase and 5-LO attenuated the increase in HNE-induced CD36 expression (Fig. 4). These results suggested that both NADPH oxidase and 5-LO were essential for HNE-induced expression of CD36 in murine macrophages.
Fig. 4.
Effect of inhibitors for NADPH oxidase or 5-lipoxygenase on HNE-induced CD36 expression in J774A.1. macrophages. Macrophages were treated with diphenylamine iodonium (DPI, 10 µM), apocynin (Apo, 300 µM), or MK886 (10 µM) for 30 min prior to HNE application. Cells were stimulated with 10 µM HNE for the indicated time. The levels of CD36 mRNA and protein were determined by RT-PCR (A) and Western blot analysis (B), respectively. Each fig is the representative of 5 independent experiments. Data was presented as mean±SE from 5 independent experiments. **p<0.01 vs. control; #p<0.05; ##p<0.01 vs. veh (vehicle).
Effects of cilostazol on HNE-enhanced CD36 expression and ROS production
To evaluate the effect of cilostazol on HNE-induced CD36 expression, we stimulated macrophages with 10 µM HNE for the indicated time in the absence or presence of cilostazol. HNE-enhanced CD36 expression at the mRNA and protein levels was suppressed by cilostazol (50 and 100 µM) in a concentration-dependent manner (Fig. 5). Likewise, HNE-induced ROS production was also markedly reduced in the presence of cilostazol (Fig. 6), suggesting that intracellular ROS signaling pathways are involved in the inhibitory effect of cilostazol on HNE-enhanced CD36 expression.
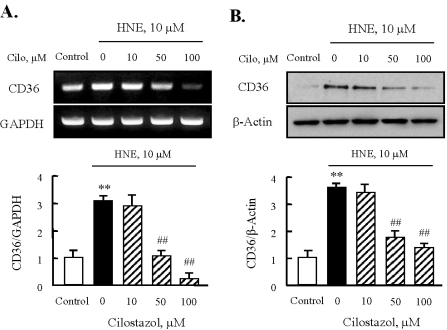
Fig. 5.
Effects of cilostazol on the expression of CD36 in J774A.1. macrophages. Effects of cilostazol on the expression of CD36 mRNA (A) and protein (B) were analyzed using RT-PCR and Western blot, respectively. Each fig is the representative of 4 independent experiments. Data was presented as mean±SE from 4 independent experiments. **p<0.01 vs. control; ##p<0.01 vs. HNE alone.
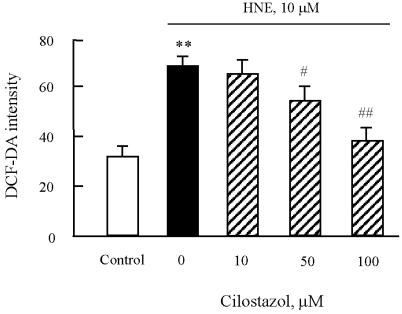
Fig. 6.
Effects of cilostazol on the generation of ROS in J774A.1. macrophages. After pre-treatment of various concentrations of cilostazol, macrophages were stimulated with 10 µM HNE for 45 min. ROS generation was measured by flow cytometry (FACS) using DCF fluorescence. Data was presented as mean±SE from six independent experiments. **p<0.01 vs control; #p<0.05; ##p<0.01 vs. HNE alone.
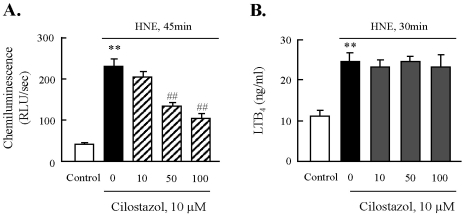
Cilostazol inhibited NADPH oxidase activity, not 5-LO activity
Then, we evaluated the effect of cilostazol on the activities of NADPH-oxidase and 5-LO enhanced by HNE. After J774A.1 macrophages were exposed to HNE (10 µM) for 45 min, NADPH oxidase activity was measured by lucigenin-enhanced chemiluminescence assay. Our data showed that the activity of NADPH-oxidase was increased significantly in response to HNE, which was concentration dependently suppressed by cilostazol (Fig. 7A). Furthermore, to determine the activity of 5-LO, we measured a 5-LO metabolite, LTB4, as a marker for 5-LO activity. As shown in Fig. 7B, the incubation of macrophages with HNE (10 µM) for 30 min in presence of 40 µM arachidonic acid as exogenous substrate (to circumvent the need for phosphatase activity) significantly elevated 5-LO activity. However, cilostazol had no effect on HNE-induced 5-LO activation. These results suggested that the inhibitory effect of cilostazol on HNE-mediated expression of CD36 was mediated by inhibition of NADPH-oxidase, but not by 5-LO.
Fig. 7.
Effect of cilostazol on the activities of NADPH oxidase and 5-lipoxygenase. After pre-treatment with various concentrations of cilostazol, macrophages were stimulated with 10 µM HNE for the indicated time. The activities of NADPH oxidase (A) and 5-LO (B) were quantified by chemiluminescence assay and ELISA, respectively. Data was presented as mean±SE from 5 independent experiments. **p<0.01 vs. control; ##p<0.01 vs. HNE alone.
DISCUSSION
The present study demonstrated that HNE-enhanced ROS produces a subsequent increase in CD36 expression in murine macrophages. This response was blunted by NAC, an antioxidant, as well as by inhibitors of NADPH-oxidase and 5-LO. Linked to these results, cilostazol significantly inhibited ROS production, as well as CD36 expression in murine macrophages stimulated with HNE. An increase in NADPH-oxidase activity by HNE was attenuated significantly by cilostazol, however, cilostazol had no effect on HNE-enhanced 5-LO activity. Thus, it is suggested that cilostazol attenuates HNE-enhanced CD36 expression in murine macrophages through inhibition of NADPH-oxidase-derived ROS generation.
An increasing number of studies have suggested the potential pathophysiological role of HNE, a major electrophilic end-product of lipid peroxidation in atherosclerotic lesion formation (Leonarduzzi et al., 2005). In support of these hypotheses, an immunohistochemical analysis of atherosclerotic lesions demonstrated that intense HNE immunoreactivity in atherosclerotic lesions was associated with cells, primarily macrophages (Kumagai et al., 2004; Yun et al., 2008). In our previous study, consistent with the report in which HNE enhanced CD36 expression in murine macrophages (Ishii et al., 2004). In addition, HNE caused an increased expression of CD36 in murine macrophages with an increased foam cell formation (Yun et al., 2008). This was mediated via p38-MAPK pathway activated by 5-LO metabolites in macrophages (Yun et al., 2009). Although HNE was suggested as an inducer of macrophage foam cell formation, however, the precise mechanism involving the expression of scavenger receptors on macrophages remains unclear.
Consistent with other reports showing that lipid peroxidation products, including HNE and other reactive aldehydes, stimulated ROS formation in various types of cells (Knobel et al., 2006; Lee et al., 2006; Raza and John, 2006; Forman et al., 2008), a significant increase in ROS generation by HNE was observed in J774A.1 macrophages. Although ROS has been reported to be generated by a variety of enzymatic sources (Thannickal et al., 2000), our present study demonstrates that ROS production in NHE-stimulated macrophages was attenuated by inhibition of either NADPH-oxidase or 5-LO, but not by inhibitors of other pro-oxidant enzymes, including xanthine oxidase and mitochondrial oxidases. Thus, it was suggested that HNE-induced ROS generation in macrophages occurred exclusively through activation of both NADPH oxidase and 5-LO. Similar to the effects of HNE-induced ROS generation, inhibitors for NADPH oxidase and 5-lipoxygenase attenuated the increase in HNE-induced CD36 expression. These results suggest that both NADPH oxidase and 5-LO were essential for HNE-induced expression of CD36 in murine macrophages. Considering the facts that both these enzyme inhibitors had similar magnitudes of inhibition during ROS generation and CD36 expression, it was also suggested that there was an interaction between these 2 enzymes in ROS generation in macrophages.
Arachidonic acid is converted to LT by 5-LO, and the major products formed in the neutrophils are 5-HETE, LTA4 (the precursor of LTB4) and cysteinyl LT (CysLT), such as LTC4, LTD4 and LTE4 (Shimizu et al., 1984; Samuelsson et al., 1987; Funk, 2001; Peters-Golden and Brock, 2003). In contrast to prostaglandin F2a (PGF2a), a COX metabolite, which stimulates NADPH-oxidase through transcriptional upregulation of NOX-1, a subunit of NADPH oxidase (Katsuyama, 2007; Cevik, 2008), LTB4 activated NADPH oxidase through phosphorylation and translocation of p47phox to the membrane, a process that was dependent on PKC activity (Woo et al., 2002; Serezani et al., 2005). These observations suggest both a short-term and a delayed activation of the NADPH-oxidase system by eicosanoids. The short-term activation may involve membrane translocation of cytosolic subunits by 5-LO-derived LTB4 formation, while the delayed long-term activation may involve a transcriptional up-regulation of the NADPH oxidase subunits by PGF2a. In this regard, our present study is of particular interest, as it shows the HNE-enhanced synthesis and release of LTB4 in murine macrophages. However, since indomethacin, an unspecific COX inhibitor, had no influence on ROS formation by HNE, it was suggested that PGF2a formation might not be involved in the early phase of NADPH-oxidase activation.
The previous report noted that cilostazol strongly inhibited the uptake of modified LDL by decreasing the protein expression of SR-A, but not CD36 (Okutsu et al., 2009). However, in the present study using J774A.1 macrophages stimulated with HNE, cilostazol significantly suppressed HNE-induced CD36 expression. Moreover, cilostazol attenuated HNE-induced activation of NADPH oxidase in murine macrophages, as well as an increased generation of ROS. This is supported by previous reports in which cilostazol reduced atherosclerosis by inhibition of NAD(P)H oxidase-dependent superoxide production, thereby reducing NF-κB activation/transcription of VCAM-1/MCP-1 expressions, and monocyte recruitments in LDL receptor-null mice fed a high cholesterol diet. (Shin et al., 2004; Lee et al., 2005).
In conclusion, the present study demonstrates that cilostazol suppresses HNE-stimulated CD36 expression in a significant manner, as well as ROS generation in murine macrophages through inhibition of NADPH-oxidase. Moreover, the identification of a role for cilostazol in the activation of NADPH-oxidase by HNE suggests that cilostazol has an anti-atherogenic action in which ROS might be responsible for vascular pathophysiology.
ACKNOWLEDGEMENTS
This work was supported for two years by a Pusan National University Research Grant.
ABBREVIATIONS
- HNE
4-Hydroxynonenal
- ROS
reactive oxygen species
- Ox-LDL
oxidized low density lipoprotein
- SR
scavenger receptor
- NAC
N-acetylcysteine
- 5-LO
5-Lipoxygenase
- DPI
diphenylamine iodonium
- NDGA
nordihydroguaiaretic acid
- COX
cyclooxygenase
- LT
leukotrienes
References
- 1.Amer J, Goldfarb A, Fibach E. Flowcytometric measurement of reactive oxygen species production by normal and thalassaemic red blood cells. Eur J Haematol. 2003;70:84–90. doi: 10.1034/j.1600-0609.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 2.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 3.Cevik MO, Katsuyama M, Kanda S, Kaneko T, Iwata K, Ibi M, Matsuno K, Kakehi T, Cui W, Sasaki M, Yabe-Nishimura C. The AP-1 site is essential for the promoter activity of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme: Possible involvement of the ERK1/2-JunB pathway. Biochem Biophys Res Commun. 2008;379:351–355. doi: 10.1016/j.bbrc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 5.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 7.Greaves DR, Gordon S. Thematic review series: the immune system and atherogenesis. Recent insights into the biology of macrophage scavenger receptors. J Lipid Res. 2005;46:11–20. doi: 10.1194/jlr.R400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Han J, Hajjar DP, Febbraio M, Nicholson AC. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor. J Biol Chem. 1997;272:21654–21659. doi: 10.1074/jbc.272.34.21654. [DOI] [PubMed] [Google Scholar]
- 9.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 10.Katsuyama M, Ozgur CM, Arakawa N, Kakehi T, Nishinaka T, Iwata K, Ibi M, Matsuno K, Yabe-Nishimura C. Myocyte enhancer factor 2B is involved in the inducible expression of NOX1/NADPH oxidase, a vascular superoxide-producing enzyme. FEBS J. 2007;274:5128–5136. doi: 10.1111/j.1742-4658.2007.06034.x. [DOI] [PubMed] [Google Scholar]
- 11.Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985;35:1144–1149. [PubMed] [Google Scholar]
- 12.Knöbel Y, Glei M, Osswald K, Pool-Zobel BL. Ferric iron increases ROS formation, modulates cell growth and enhances genotoxic damage by 4-hydroxynonenal in human colon tumor cells. Toxicol In Vitro. 2006;20:793–800. doi: 10.1016/j.tiv.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Oh GT, Park SY, Choi JH, Park JG, Kim CD, Lee WS, Rhim BY, Shin YW, Hong KW. Cilostazol reduces atherosclerosis by inhibition of superoxide and tumor necrosis factor-formation in low-density lipoprotein receptor-null mice fed high cholesterol. J Pharmacol Exp Ther. 2005;313:502–509. doi: 10.1124/jpet.104.079780. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Jung GY, Heo HJ, Yun MR, Park JY, Bae SS, Hong KW, Lee WS, Kim CD. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol Lett. 2006;166:212–221. doi: 10.1016/j.toxlet.2006.07.305. [DOI] [PubMed] [Google Scholar]
- 16.Leonarduzzi G, Chiarpotto E, Biasi F, Poli G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–1049. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- 17.Manea A, Manea SA, Gafencu AV, Raicu M. Regulation of NADPH oxidase subunit p22 (phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- 18.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 19.Nakata A, Nakagawa Y, Nishida M, Nozaki S, Miyagawa J, Nakagawa T, Tamura R, Matsumoto K, Kameda-Takemura K, Yamashita S, Matsuzawa Y. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 1999;19:1333–1339. doi: 10.1161/01.atv.19.5.1333. [DOI] [PubMed] [Google Scholar]
- 20.Okutsu R, Yoshikawa T, Nagasawa M, Hirose Y, Takase H, Mitani K, Okada K, Miyakoda G, Yabuuchi Y. Cilostazol inhibits modified low-density lipoprotein uptake and foam cell formation in mouse peritoneal macrophages. Atherosclerosis. 2008 Nov 17; doi: 10.1016/j.atherosclerosis.2008.10.042. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Peters-Golden M, Brock TG. 5-lipoxygenase and FLAP. Prostaglandins Leukot Essent Fatty Acids. 2003;69:99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 22.Raza H, John A. 4-hydroxynonenal induces mitochondrial oxidative stress, apoptosis and expression of glutathione S-transferase A4-4 and cytochrome P450 2E1 in PC12 cells. Toxicol Appl Pharmacol. 2006;216:309–318. doi: 10.1016/j.taap.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 24.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 25.Sampey BP, Carbone DL, Doorn JA, Petersen DR. 4-Hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (Erk) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol Pharmacol. 2007;71:871–883. doi: 10.1124/mol.106.029686. [DOI] [PubMed] [Google Scholar]
- 26.Serezani CH, Aronoff DM, Jancar S, Peters-Golden M. Leukotriene B4 mediates p47phox phosphorylation and membrane translocation in polyunsaturated fatty acid-stimulated neutrophils. J Leukoc Biol. 2005;78:976–984. doi: 10.1189/jlb.1004587. [DOI] [PubMed] [Google Scholar]
- 27.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimizu T, Radmark O, Samuelsson B. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc Natl Acad Sci USA. 1984;81:689–693. doi: 10.1073/pnas.81.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin HK, Kim YK, Kim KY, Lee JH, Hong KW. Remnant lipoprotein particles induce apoptosis in endothelial cells by NAD(P)H oxidase-mediated production of superoxide and cytokines via lectin-like oxidized low-density lipoprotein receptor-1 activation: Prevention by cilostazol. Circulation. 2004;109:1022–1028. doi: 10.1161/01.CIR.0000117403.64398.53. [DOI] [PubMed] [Google Scholar]
- 30.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 31.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 32.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 33.Woo CH, You HJ, Cho SH. Leukotriene B(4) stimulates Rac-ERK cascade to generate reactive oxygen species that mediates chemotaxis. J Biol Chem. 2002;277:8572–8578. doi: 10.1074/jbc.M104766200. [DOI] [PubMed] [Google Scholar]
- 34.Yesner LM, Huh HY, Pearce SF, Siverstein RL. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol. 1996;16:1019–1025. doi: 10.1161/01.atv.16.8.1019. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, Quehenberger O, Kondratenko N, Green S, Steinberg D. Minimally oxidized low-density lipoproteinincreases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler Thromb Vasc Biol. 1998;18:794–802. doi: 10.1161/01.atv.18.5.794. [DOI] [PubMed] [Google Scholar]
- 36.Yun MR, Im DS, Lee SJ, Woo JW, Hong KW, Bae SS, Kim CD. 4-hydroxynonenal contributes to macrophage foam cell formation through increased expression of class a scavenger receptor at the level of translation. Free Radic Biol Med. 2008;45:177–183. doi: 10.1016/j.freeradbiomed.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Yun MR, Im DS, Lee SJ, Park HM, Bae SS, Lee WS, Kim CD. 4-Hydroxynonenal enhances CD36 expression on murine macrophages via p38 MAPK-mediated activation of 5-lipoxygenase. Free Radic Biol Med. 2009;46:692–698. doi: 10.1016/j.freeradbiomed.2008.12.013. [DOI] [PubMed] [Google Scholar]