Abstract
It was evaluated the inhibitory action of quercetin-3-O-β-D-glucuronopyranoside (QGC) on reflux esophagitis and gastritis in rats. QGC was isolated from the herba of Rumex Aquaticus. Reflux esophagitis or gastritis was induced surgically or by administering indomethacin, respectively. Oral QGC decreased ulcer index, injury area, gastric volume, and acid output and increased gastric pH as compared with quercetin. Furthermore, QGC significantly decreased gastric lesion sizes induced by exposing the gastric mucosa to indomethacin. Malondialdehyde levels were found to increase significantly after inducing reflux esophagitis, and were reduced by QGC, but not by quercetin or omeprazole. These results show that QGC can inhibit reflux esophagitis and gastritis in rats.
Keywords: Reflux esophagitis, Lipid peroxidation, Gastritis, Quercetin-3-O-β-D-glucuronopyranoside
INTRODUCTION
It is well known that flavonoids have anti-inflammatory, antioxidant, antiallergic, hepatoprotective, antithrombotic, antiviral, and anticarcinogenic activities, and it has been reported that the anti-inflammatory activities of flavonoids demonstrate their candidacy as therapeutic agents (Lewis, 1989). The flavonoids are typical phenolic compounds that act as potent metal chelators and free radical scavengers, and which modulate intracellular signaling caused by upstream binding partners, such as, regulatory kinases and receptors (Middleton et al., 2000; Williams et al., 2004).
Reflux esophagitis is a common disease entity whereby gastric juice gains access to the esophagus due to transient lower esophageal sphincter relaxation, a low esophageal clearance speed, a lack of mucosal resistance, and other factors, and is often associated with low LES pressure (Bell et al., 1992). If left untreated the erosion and ulceration of esophageal mucosa caused by gastric acid can induce several disease conditions, such as, chronic esophagitis, aspiration pneumonia, esophageal strictures, and Barrett's esophagus, the latter of which is a premalignant condition (Biancani et al., 1997). However, the severity of reflux esophagitis cannot be accurately predicted based on acid exposure, which suggests that other damaging factors or possibly impaired mucosal resistance are also involved.
Gastritis includes many disorders that induce inflammatory changes in the gastric mucosa, such as, erosive gastritis caused by noxious irritants, reflux gastritis due to exposure to bile and pancreatic fluids, hemorrhagic gastritis, infectious gastritis, and gastric mucosal atrophy. Stress, ethanol, bile, free radicals, oxidants, and non-steroidal anti-inflammatory drugs (NSAIDs) disrupt the gastric mucosal barrier and make it vulnerable to normal gastric secretions. Furthermore, Helicobacter pylori infection is the leading cause of peptic ulcer disease and is associated with virtually all ulcers not induced by NSAIDs.
Oxygen derived free radicals are known to play important roles in the pathogeneses of diseases that affect various tissues, including those of the digestive system (Naya et al., 1997), and may lead to acute gastric and esophageal mucosal injury as a result ischemia (Stein et al., 1990). The damage induced by ethanol is also induced in this manner (Pihan et al., 1987). Free radicals are also known to act as carcinogens because they cause DNA damage (Haegele et al., 1994). Furthermore, it has been demonstrated that free radical damage to the gastric or esophageal mucosa (Wetscher et al., 1995) can be prevented by the administration of free radical scavengers.
In this study, we evaluated the effects of quercetin-3-O-β-D-glucuronopyranoside (QGC), which was isolated from Rumex Aquaticus, on the development of reflux esophagitis induced surgically in rats, gastritis induced by indomethacin, on gastric secretion, and lipid peroxidation (a marker of oxidative stress).
METHODS
Animals
Male Sprague-Dawley rats of body weight of 200~250 g were used for the experiments. Animals were starved for 24 h before experiments, but had ad lib access to drinking water. Animals were cared for as recommended by the IACUC (Institutional Animal Care and Use Committee of Institute for Molecules-Based New Drug Development).
Evaluation of indomethacin-induced gastritis
Ulcers were induced by administering 50 mg/kg of indomethacin, suspended in sodium bicarbonate (NaHCO3) 3% per os (p.o.). The animals were sacrificed 5 hours after indomethacin administration and stomachs were excised, opened along the greater curvature, and spread on corkboard using pins. Total areas (mm2) of mucosal erosive lesions were determined using a dissecting microscope and a squared grid (×10; Olympus, Tokyo). QGC, or omeprazole was administered p.o. one hour before administering indomethacin. The volume of drug or vehicle administered was 2 ml/kg of body weight. The drugs were freshly prepared on each occasion.
Evaluation of reflux esophagitis
Under ether anesthesia, the abdomen was incised along the midline, and the pylorus and transitional region between the forestomach and corpus were simultaneously ligated (Nakamura et al., 1982). A longitudinal cardiomyotomy of length ~1 cm was performed across the gastroesophageal junction to enhance reflux. Six hours later, animals were sacrificed by cervical dislocation and esophagi were harvested. The total areas (mm2) of lesions that had developed in the esophagus were also determined under a dissecting microscope (×10), and graded as follows: 0, no visible lesions; 1, a few erosions; 2, total lesion area ≤30 mm2; 3, total lesion area ≥30 mm2; 4, perforation (Okabe et al., 1995). QGC dissolved in 0.01% dimethyl sulfoxide (DMSO) was administered immediately after ligation of the pylorus and limiting ridge. The volume of drug or vehicle administered was 1 ml/kg of body weight. Drugs were freshly prepared prior to administration.
Quantification of gastric acid secretion
Six hours after pylorus ligation, rats were sacrificed by cervical dislocation and the esophagi were clamped. Samples of gastric juice were collected in graduated conical centrifuge tubes and centrifuged at 3,000 g for 10 min at 4℃. After centrifugation, supernatant volumes (ml/rat), pH values (Toledo 320, Mettler, Swiss), and acidities (mEq/l) were measured. Total acidities were determined by automatic titration of gastric juice vs. 0.1 N NaOH to pH 7.0 (665 Dosimat, Metrohm, Swiss). Acid outputs are expressed as mEq/hr (Okabe et al., 1995).
TBARS assays
Lipid peroxidation was quantified by measuring the formation of thiobarbituric acid-reactive substances (TBARS) spectrophotometrically, as previously described (Buege and Aust, 1978). Esophageal mucosa was harvested, sonicated in 1 ml of Tris-HCl buffer (pH 7.0), and centrifuged at 600 g for 10 min at 4℃ (Micro17TR, Hanil, Korea). Trichloroacetic acid (TCA; 0.9 ml, 8%) was then added to 0.3 ml of supernatant, and after centrifugation at 10,000 g for 5 min at 4℃, 0.25 ml of TBA (1%) was added to 1 ml of this mixture and the resulting solution was heated at 100℃ for 20 min. The tubes were then cooled, 2 ml of n-butanol was added to each, and tubes were then vortexed for 90 sec, centrifuged at 3,000 g for 5 min at 4℃. 1 ml aliquots of the butanol phase were subjected to TBARS assays at 532 nm (UV-160A, Shimadzu, Japan) against a malonaldehyde bis (dimethyl acetal) standard. Results are expressed as ng/mg of protein. Protein assays were conducted using the Bradford method (Bradford, 1976) using bovine serum albumin as a standard.
Drugs
QGC was isolated and purified from Rumex Aquaticus. The purity of the isolated material was 96~97%. Quercetin, indomethacin, thiobarbituric acid, trichloroacetic acid, malonaldehyde bis (dimethyl acetal), bovine serum albumin, o-phthalaldehyde, diethylenetriaminepentaacetic acid, ascorbic acid, N-ethylmaleimide, and glutathione were purchased from Sigma (St. Louis, MO, USA). Potassium phosphate (dibasic) and potassium dihydrogenphosphate were purchased from Showa (Tokyo). Protein assay kits were purchased from BioRad (Richmond, CA).
Analysis of data
Values are expressed as means±SEM (standard error of means). The Student's t-test, ANOVA (analysis of variance), and Fischer's exact test were used to determine statistical significance. p values of <0.05 were considered significant.
RESULTS
The effects of QGC, quercetin, and omeprazole on reflux esophagitis
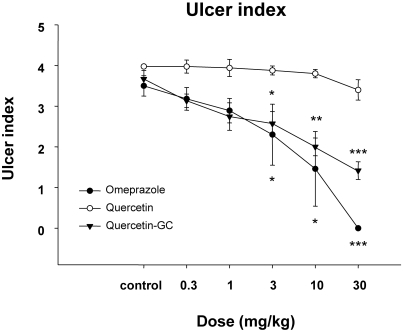
During the experiment of reflux esophagitis, six hours after pylorus ligation, severe ulcerations were observed in the esophagus of the control group. QGC and omeprazole, treated groups showed a significant and dose-dependent reduction in the development of reflux esophagitis (Fig. 1). This result showed that QGC and omepraolze inhibit reflux esophagitis in rats.
Fig. 1.
The effect of QGC on reflux esophagitis induced surgically in rats. Mean lesion area in the control group was 4 (0, no lesion; 1, ulcer area; 2, ulcer area <30 mm2; 3, ulcer area >30 mm2; 4, complete lesion (perforation of the esophageal mucosa). QGC treated animals showed significantly inhibition on reflux esophagitis. Data are means±SEMs of 6~8 animals. *Significantly different from the corresponding controls. *p<0.05, **p<0.01, and ***p<0.001 vs. the control.
The effects of QGC, quercetin, and omeprazol on gastric secretion during reflux esophagitis
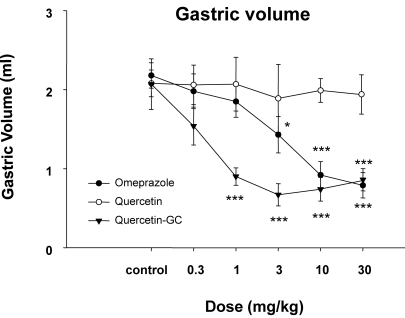
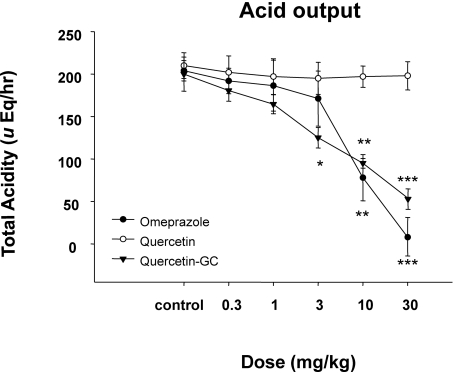
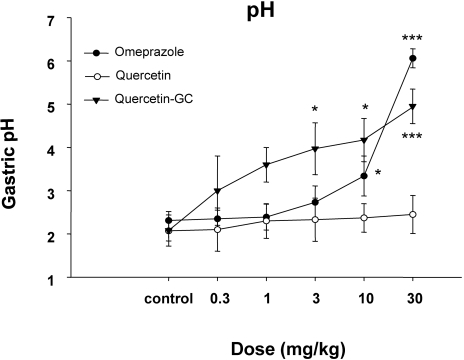
In experiments on gastric acid secretion, we measured gastric volumes, the pH values of gastric contents, and acid outputs (Fig. 2~4) after inducing reflex esophagitis. QGC significantly decreased gastric volume (Fig. 2), whereas omeprazole cause no observable change. The pH values of gastric contents increased dose dependently for all three drugs (Fig. 3). QGC and omeprazol decreased gastric volumes, and QGC dose-dependently inhibited gastric acid outputs (Fig. 4), which could decrease ulceration of gastric and esophageal mucosa.
Fig. 2.
The effect of QGC on gastric volume. QGC significantly and dose-dependently decreased gastric volume. Data are means±SEM. *p<0.05, **p<0.01, and ***p<0.001 vs. the control (n=6~8).
Fig. 4.
The inhibitory effect of QGC on gastric acid output. QGC significantly increased when compared to the acidity of quercetin. Data are means±SEM. *Significantly different from the corresponding control. *p<0.05, **p<0.01, and ***p<0.001 vs. the control (n=6~8).
Fig. 3.
The effects of QGC on gastric pH. QGC administration changed the pH of reflux esophagitis in rats. QGC significantly increased when compared with the pH of quercetin. Data are means±SEM. *p<0.05, ***p<0.001 vs. the control (n=6~8).
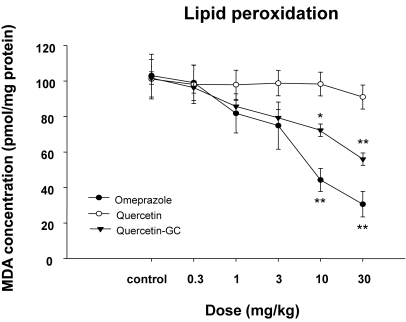
The inhibitory effect of QGC on lipid peroxidation
In the control group (Compound dose 0=sham operated rats), amounts of malondialdehyde (MDA, the end product of lipid peroxidation) were significantly higher than in normal controls (Fig. 5). QGC significantly reduced MDA formation in a dose dependent manner.
Fig. 5.
The inhibitory effects of QGC on lipid peroxidation. In the gastritis control group levels of lipid peroxidation were elevated as compared with the normal group. QGC administration significantly decreased the amount of MDA formation, suggesting that it inhibited gastritis induced lipid peroxidation. Column represent means±SEMs of 6~8 animals. *p<0.05 and **p<0.01 vs. control.
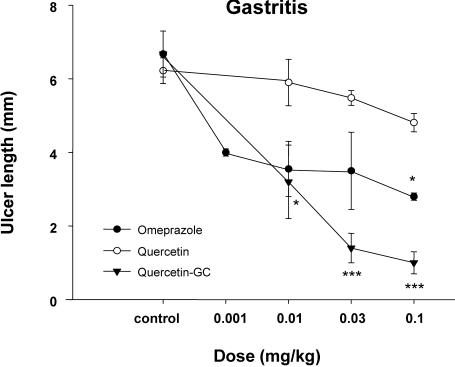
The preventive effects of QGC on indomethacin-induced gastritis
The administration of indomethacin (50 mg/kg) to rats induced gastric mucosal ulceration. Ulcer total areas significantly reduced after QGC administration, whereas omeprazole had no effect (Fig. 6). Furthermore, this inhibitory effect of QGC was dose-dependent in the range 0.001~0.1 mg/kg p.o. These findings suggest that QGC can inhibit the formation of indomethacin-induced gatric lesions.
Fig. 6.
The inhibitory effect of QGC on indomethacin-induced gastritis. Mean lesion area in the control group was 4.0±0.7 cm2. QGC (0.001~0.01 mg/kg, p.o.) dose-dependently inhibited the formation of indomethacin-induced lesions. Data are means±SEM of 6~8 animals. *Significantly different from the corresponding control. *p<0.05 and ***p<0.001 vs control.
DISCUSSION
Gastro-esophageal reflux disease is commonly encountered, and it is well known that gastric acid plays a key causative role in mucosal damage. In fact, mainstay of medical treatment is the suppression of gastric acid secretion (Bell et al., 1992; Wolfe et al., 2000). In a previous study, we showed that apigenin- and luteolin-contained flavonoids have a beneficial effect on esophagitis and gastritis in rats (Min et al., 2005), and the findings of the present study suggest that QGC has an inhibitory effect on reflux esophagitis and indomethacin-induced gastritis, and that these inhibitions are caused by reductions in gastric secretions and oxidative stress. In addition, our findings support the antiulcer, gastroprotective, and gastric antisecretory effects of QGC and quercetin.
Omeprazole, contains a sulfinyl group in a bridge between its substituted benzimidazole and pyridine ring and is an irreversible inhibitor of H+, K+ - ATPase. Flavonoids exhibit antiulcer, gastroprotective, and gastric antisecretory activities (Alvarez et al., 1999), They are naturally occurring plant polyphenols and are found in abundance in diets rich in fruit and vegetables, and in plant-derived beverages like tea (Lewis, 1989). The effect of antisecretory therapy on reflux esophagitis can be predicted by determining the duration of intragastric acidity suppression above a pH of 4.0 (Bell et al., 1992). This effect can be caused by different mechanisms. For example, Parmar and Hennnings (1984) showed that gastric antisecretory activity is as effective at reducing gastric acid secretion as cimetidine (Parmar and Hennings, 1984), and it has been reported that acid output inhibition by greater than 40% is sufficient to prevent the development of esophagitis (Okabe et al., 1995). Furthermore, it has been proposed that gastric acid is essential for esophageal mucosal damage (Bell et al., 1992). It appeared to have anti-ulcerogenic properties in rats and guinea pigs, and such properties are of interest with respect to the adverse effect of gastric ulceration, which commonly develops in subjects taking anti-inflammatory drugs (Gambhir et al., 1987).
Flavonoids are potent bioactive molecules and have been proposed to have antioxidative and modulatory effects in cells and anti-inflammatory and anticancer properties. Many of the biological actions of flavonoids have been attributed to their antioxidant properties either to their reducing capacities or to their possible influences on redox status (Williams et al., 2004). It should also be emphasized that the reducing properties of flavonoids might contribute to redox regulation in cells, and, flavonoids might protect against cell aging, for example, by working in concert with the intracellular network (Rice-Evans, 2001).
Quercetin is a natural flavone with various bioactivities. Quercetin was found to be highly efficient at scavenging free radicals in cell-free systems (Rice-Evans et al., 1996), and to be more active in this respect than the traditional antioxidants vitamins C and E (Gordon and Roedig-Penman, 1998; Ishige et al., 2001; Geetha et al., 2005). Gerritsen et al. (1995) found that flavonoids, especially an apigenin, blocked the cytokine-induced expressions of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin on human endothelial cells (Gerritsen et al., 1995). Quercetin was also found to be an active anti-inflammatory agent in a rat paw carrageenan model and to reduce contact sensitivity in mice. In a study on gastric secretion, the oral administration of QGC reduced gastric content significantly and dose-dependently, and when QGC inhibited gastric acid output, the flavonoids extensively prevented the development of reflux esophagitis. These results suggest that QGC has inhibitory effects on reflux esophagitis and gastritis in rats, and our findings support the antiulcer, gastroprotective, and gastric anti-secretory activities of QGC. Furthermore, in feline esophageal epithelial cells, QGC was found to have a protective effect on ethanol induced cell damage by inhibiting ROS generation, activation downstream of ERK (Sohn et al., 2009), and downstream signal transduction induced by interleukin-1 beta (Lee et al., 2009).
Our results show that QGC significantly decreased lipid peroxide levels in experimental esophagitis models. Some authors have demonstrated that oxygen free radicals are directly implicated in the pathogenesis of indomethacin-induced mucosal damage (Kvietys et al., 1990). Our results show that QGC has both an antioxidative effect and free radical scavenging activity, for example, MDA contents were higher in esophageal mucosa after the induction of reflux esophagitis. Furthermore, it has been shown that reflux esophagitis in rats is mediated by oxygen-derived free radicals or superoxide anions (Wetscher et al., 1995). Superoxide anions produced by inflammatory cells (neutrophils, macrophages, and monocytes) play an important part in the pathogenesis of acid and pepsin induced esophagitis in rabbits (Naya et al., 1997), and in a recent study, the production of free radicals and lipid peroxidation were found to be positively associated with degree of esophagitis, and these were found to be highest in patients with Barrett's esophagus, a premalignant condition (Wetscher et al., 1995). Recently omeprazole was shown to prevent by acting as an anti-inflammatory agent and by preventing neutrophil infiltration, and mucosal damage (Kobayashi et al., 2002). Moreover, it has also been found that omeprazole acts not only as proton pump inhibitor but also as an antioxidant and hydroxyl radical scavenger (Bandyopadhyay et al., 2002; Biswas et al., 2003). However, it has not been established whether QGC and omeprazole act via an antioxidant mechanism.
Our results confirm that QGC at a dose of 0.03 mg/kg protects against indomethacin-induced gastritis. Furthermore, QGC significantly reduced the gastric mucosal damage produced by oral indomethacin administration at all doses tested.
QGC might be promising drug for the treatment of reflux esophagitis and gastritis. There are two reasons why QGC showed efficacy against ulcers. The bioavailabilities and health effects of dietary flavonoids were reviewed by Hollman and Katan (Hollman and Katan, 1998). They found that quercetin glycosides from onions were more readily absorbed than the pure aglycone. Furthermore, absorbed quercetin was found to be eliminated slowly from the blood, which suggests that the enterohepatic circulation may be operative (Hollman et al., 1997). The suggestion was also made that glycosides may be absorbed via the intestinal sugar uptake route (Hollman et al., 1997; Jung et al., 2001), and it was suggested that the effect of glucuronic acid in exercised rats involves reduce tissue peroxidation, which acts as an antioxidative defense mechanism and promotes recovery of muscle fatigue. For these two reasons, the administration of QGC reduced ulcer indices, amounts of malondialdehyde, gastric volume, and gastric acid output. This may provide a useful model of reflux esophagitis and gastritis, when the former is induced by surgery and the latter by a chemical agent.
We conclude that QGC can inhibit the reflux esophagitis and gastritis of rats.
ACKNOWLEDGEMENTS
This study was supported by Post-Doctoral Grants from Chung-Ang University in 2006~2007.
ABBREVIATIONS
- LES
lower esophageal sphincter
- NSAIDs
non-steroidal anti-inflammatory drugs
- QGC
quercetin-3-O-β-D-glucuronopyranoside
- TBARS
thiobarbituric acid-reactive substances
- TBA
thiobarbituric acid
- MDA
malondialdehyde
- TCA
trichloroacetic acid
References
- 1.Alvarez A, Pomar F, Sevilla, Montero MJ. Gastric antisecretory and antiulcer activities of an ethanolic extract of Bidens pilosa L. var. radiata Schult. Bip. J Ethnopharmacol. 1999;67:333–340. doi: 10.1016/s0378-8741(99)00092-6. [DOI] [PubMed] [Google Scholar]
- 2.Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt H. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59–67. doi: 10.1159/000200917. [DOI] [PubMed] [Google Scholar]
- 3.Bell NJ, Hunt RH. Role of gastric acid suppression in the treatment of gastro-oesophageal reflux disease. Gut. 1992;33:118–124. doi: 10.1136/gut.33.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biancani P, Sohn UD, Rich HG, Harnett KM, Behar J. Signal transduction pathways in esophageal and lower esophageal sphincter circular muscle. Am J Med. 1997;103:23S–28S. doi: 10.1016/s0002-9343(97)00316-1. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid, sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 7.Gambhir SS, Goel RK, Das Gupta G. Anti-inflammatory & anti-ulcerogenic activity of amentoflavone. Indian J Med Res. 1987;85:689–693. [PubMed] [Google Scholar]
- 8.Geetha T, Malhotra V, Chopra K, Kaur IP. Antimutagenic and antioxidant/prooxidant activity of quercetin. Indian J Exp Biol. 2005;43:61–67. [PubMed] [Google Scholar]
- 9.Gerritsen ME, Carley WW, Ranges GE, Shen CP, Phan SA, Ligon GF, Perry CA. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 1995;147:278–292. [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon MH, Roedig-Penman A. Antioxidant activity of quercetin and myricetin in liposomes. Chem Phys Lipids. 1998;97:79–85. doi: 10.1016/s0009-3084(98)00098-x. [DOI] [PubMed] [Google Scholar]
- 11.Haegele AD, Briggs SP, Thompson HJ. Antioxidant status and dietary lipid unsaturation modulate oxidative DNA damage. Free Radic Biol Med. 1994;16:111–115. doi: 10.1016/0891-5849(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 12.Hollman PC, Katan MB. Bioavailability and health effects of dietary flavonols in man. Arch Toxicol Suppl. 1998;20:237–248. doi: 10.1007/978-3-642-46856-8_21. [DOI] [PubMed] [Google Scholar]
- 13.Hollman PC, van Trijp JM, Buysman MN, van der Gaag MS, Mengelers MJ, de Vries JH, Katan MB. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- 14.Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–446. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 15.Jung SY, Choi S, Ko YS, Park CS, Oh S, Koh SR, Oh U, Oh JW, Rhee MH, Nah SY. Effects of ginsenosides on vanilloid receptor (VR1) channels expressed in Xenopus oocytes. Mol Cells. 2001;12:342–346. [PubMed] [Google Scholar]
- 16.Kvietys PR, Twohig B, Danzell J, Specian RD. Ethanol-induced injury to the rat gastric mucosa. Role of neutrophils andxanthine oxidase-derived radicals. Gastroenterology. 1990;98:909–920. doi: 10.1016/0016-5085(90)90015-s. [DOI] [PubMed] [Google Scholar]
- 17.Lee SE, Jang HS, Song HJ, Hwang WK, Sohn UD. M1904 Downstream Signal Transduction Induced By Interleukin-1 Beta-Stimulated ROS Generation and Anti-Oxidative Effects of Quercetin-3-O-[beta]-D-Glucuronopyranoside (QGC) in Feline Esophageal Epithelial Cells. Gastroenterology. 2009;136:A442–A443. [Google Scholar]
- 18.Lewis DA. Anti-inflammatory drugs from plant, marine sources. Agents Actions Suppl. 1989;27:3–373. [PubMed] [Google Scholar]
- 19.Min YS, Yim SH, Bai KL, Choi HJ, Jeong JH, Song HJ, Park SY, Ham I, Whang WK, Sohn UD. The effects of apigenin-7-O-beta-D-glucuronopyranoside on reflux oesophagitis and gastritis in rats. Auton Autacoid Pharmacol. 2005;25:85–91. doi: 10.1111/j.1474-8673.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K, Ozawa Y, Furuta Y, Miyazaki H. Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol. 1982;32:445–456. doi: 10.1254/jjp.32.445. [DOI] [PubMed] [Google Scholar]
- 21.Naya MJ, Pereboom D, Ortego J, Alda JO, Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut. 1997;40:175–181. doi: 10.1136/gut.40.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okabe S, Takinami Y, Iwata K, Yanagawa T. Mucosal protective effect of leminoprazole on reflux esophagitis induced in rats. Jpn J Pharmacol. 1995;69:317–323. doi: 10.1254/jjp.69.317. [DOI] [PubMed] [Google Scholar]
- 23.Parmar NS, Hennings G. The gastric antisecretory activity of 3-methoxy-5,7,3'4'-tetrahydroxyflavan (ME)--a specific histidine decarboxylase inhibitor in rats. Agents Actions. 1984;15:143–145. doi: 10.1007/BF01972340. [DOI] [PubMed] [Google Scholar]
- 24.Pihan G, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987;32:1395–1401. doi: 10.1007/BF01296666. [DOI] [PubMed] [Google Scholar]
- 25.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 26.Sohn UD, Cho JH, Song HJ, Sun YH, Hwang WK. M1903 Protective Effects of Quercetin-3-O-[beta]-D-Glucuronopyranoside (QGC) On Ethanol-Induced Cell Damage Involve Inhibitions of ROS Generation and Downstream Activation of the ERK in Feline Esophageal Epithelial Cells. Gastroenterology. 2009;136:A442. [Google Scholar]
- 27.Stein HJ, Hinder RA, Oosthuizen MM. Gastric mucosal injury caused by hemorrhagic shock and reperfusion: protective role of the antioxidant glutathione. Surgery. 1990;108:467–473. [PubMed] [Google Scholar]
- 28.Wetscher GJ, Perdikis G, Kretchmar DH, Stinson RG, Bagchi D, Redmond EJ, Adrian TE, Hinder RA. Esophagitis in Sprague-Dawley rats is mediated by free radicals. Dig Dis Sci. 1995;40:1297–1305. doi: 10.1007/BF02065542. [DOI] [PubMed] [Google Scholar]
- 29.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]