Abstract
The dried roots of Danshen (Salvia miltiorrhiza) and Sanchi (Panax notoginseng) have been widely used in traditional Chinese medicine for promoting blood circulation as well as various other bodily functions. Here we investigated the effects of a mixture of aqueous extracts of Danshen and Sanchi, named PASEL, on blood pressure and vascular contractility in rats. Orally administered PASEL (62.5 mg/kg and 250 mg/kg, for 5 weeks) lowered the blood pressure of spontaneous hypertensive rats (SHR) but this was not observed in normal Wistar-Kyoto rats (WKR). We then investigated the effects of PASEL on the arterial contraction of the small branches of cerebral arteries (CAs) and large conduit femoral arteries (FAs) in rats. PASEL did not affect high-K (KCl 60 mM)- or phenyleprine (PhE)-induced contracture of FAs. The myogenic response, a reactive arterial constriction in response to increased luminal pressure, of small CA was dose-dependently suppressed by PASEL in SHR as well as control rats. Interestingly, the KCl-induced contraction of small CAs was slowly reversed by PASEL, and this effect was more prominent in SHR than control WKR. PASEL did not inhibit angiotensin-converting enzyme (ACE) activity. These results demonstrated that the antihypertensive effect of PASEL might be primarily mediated by altering the arterial MR, not by direct inhibition of L-type Ca2+ channels or by ACE inhibition.
Keywords: Myogenic response, Herbal extract, Blood pressure, Hypertension
INTRODUCTION
Danshen, the dried root of Salvia miltiorrhiza (Fam. Labiatae) has been widely reported to be useful in the treatment of cardiovascular diseases in China, Japan, and Korea. Its effects of "removing blood stasis" were documented in the ancient books of traditional Chinese medicine (TCM); furthermore, various scientific studies have shown that Danshen improved blood microcirculation, dilated the coronary arteries, and prevented myocardial ischemia (Zhou et al., 2005; Cheng, 2007). Apart from vasodilation, anti-hyperlipidemic and anti-homocysteinic actions have also been suggested as beneficial effects of Danshen (Cheng, 2007). Various mechanisms have been suggested for the vasodilatory activity of Danshen extracts; it has also been shown to be involved in the activation of K+ channels and inhibition of Ca2+ channels (Lam et al., 2006a; 2006b). However, such studies were mostly performed with large conduit arteries that might not directly reflect the in vivo effects on microcirculation or on blood pressure. While not as popular as Danshen, Sanchi (Panax notoginseng) has also been used in treating circulatory disorders in TCM (Sun et al., 2007; Chuang et al., 2008; Li et al., 2008).
Herbal drugs have been widely used in TCM and their beneficial effects have been identified by empirical usage. The combined extracts of multiple herbal materials are often used for the treatment of various diseases in TCM. Based on the background literature mentioned above, we tested the effects of a combination of aqueous extracts of Danshen and Sanchi. The extract was prepared by boiling the above-mentioned herbs in water and lyophilizing it; the resulting mixture was lyophilized and named PASEL (Korea Patent Nr. 0327894; USA Patent Nr. US6589572B2) here.
In the present study, we investigated the in vivo effects of PASEL on blood pressure and vascular contractility of cerebral and femoral arteries in rats. Inhibition of voltage-operated Ca2+ channels or receptor-operated Ca2+ channels have previously been suggested as possible mechanisms of vasodilatation induced by Danshen and Sanchi (Sun et al., 2007; Chuang et al., 2008; Li et al., 2008). In the present study, therefore, we examined the effects of PASEL on high K+-induced contraction and phenyleprine (PhE)-induced contraction of rat femoral arteries.
Total blood pressure is determined from cardiac output and peripheral resistance of blood vessels. Small arteries and arterioles in vivo are partially contracted in response to luminal pressure. Such contraction is referred to as a myogenic response (MR) or myogenic tone because the vascular myocytes are believed to play roles as sensors as well as effectors. The physiological role of MR consists of regulating basal peripheral vascular resistance as well as local blood flow (Dora, 2005; Hill et al., 2006). A deranged MR has been suggested to be the result of pathophysiological changes in hypertension (Koller, 2002; Jarajapu and Knot, 2005, Ahn et al., 2007). Since MR is not observed in conduit arteries such as the femoral artery, we investigated the effects of PASEL on the MR of rat cerebral arteries.
METHODS
Preparation of PASEL
Mixed aqueous extracts of Danshen and Sanchi (1:1 by weight) were prepared by boiling the herbs under high pressure (121℃/1.5 kg/cm2, 1 hr). The aqueous fraction was separated by filtration using a 100-mesh housing filter, condensed to 2.5~3.0 brix by heating and thermo-lyophilization. The final product was named PASEL.
Experimental animals
The present investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and also conforms to Seoul National University College of Medicine guidelines for the care and use of animals. Male WKR or spontaneously hypertensive rats (200~300 g) were used in this study. The animals were anaesthetized with pentobarbital sodium (100 mg/kg) mixed with heparin (100 U/kg), and then sacrificed by heart puncture.
Blood pressure measurement
In order to estimate the effect of PASEL on blood pressure, fifteen spontaneously hypertensive rats (SHRs, 7~8 week old, male) were separated into three groups of five animals. Two groups of SHRs were orally administered with 62.5 mg/kg or 250 mg/kg of PASEL and one group of SHR and Wista-Kyoto rats (WKR, n=5, 7~8 week old, male) was used as the control and was administered with saline. Oral administration of the agents was performed everyday for 5 weeks and body weights were measured once a week. Blood pressures were measured once a week using the tail-cuff method (Coda 6 System, Kent Scientific, USA). The rats were placed in a warmed individual restrainer, and both an occlusion cuff and a volume pressure-recording cuff were placed close to the base of the tail. The pressure recording allowed a noninvasive measurement of systolic blood pressure and heart rate.
Video analysis of pressurized arteries
Small vessels (250~350 µm, i.d., passive) of rat middle cerebral arteries were dissected, and the arterial segments were placed in a glass-bottomed vessel chamber (Model CH/1/SH, Living System Instrument, Burlington, VT, USA). The chamber contained a HEPES-buffered physiological salt solution (pH 7.40±0.03; 140 mmol/l NaCl, 5.4 mmol/l KCl, 1.8 mmol/l CaCl2, 1 mmol/l MgCl2, 0.33 mmol/l NaH2PO4, 10 mmol/l glucose and 10 mmol/l HEPES), and its temperature was set at 37℃ using a temperature controller (Model TC-01, Living Systems Instrument, VT, USA). Two pipettes were utilized to cannulate the vessel that was secured with a 12-0 suture. To evaluate the pressure- induced myogenic response, the luminal flow was maintained at zero. After confirming vessel integrity, the intraluminal pressure (Plum) was set to 20 mmHg for at least an additional 60 min; a spontaneous tone was usually developed within this time frame. Vessel dimensions were measured using a video dimension analyzer (Model V94, Living Systems Instrument) and a data acquisition system (Digidata 1200 and Axoscope, Axon Instruments).
To assess myogenic behavior, internal diameters (Din) were measured during a step-like increase of Plum to 80 mmHg or 100 mmHg. On the step change in Plum, the arteriolar diameter was initially increased passively, and then constricted to attain a steady state within 2 to 3 min. After the step change, Plum was returned to 20 mmHg and the vessel concerned was allowed to re-equilibrate for a minimum of 5 min. At the end of each experiment, the maximum Din under the passive dilation was recorded at 80 mmHg and 100 mmHg in 0 Ca2+ solution containing 10 µM sodium nitroprusside. The MR in % scale was calculated using the following equation; MR (%)=100×(Din,0Ca-Din)/Din,0Ca.
Angiotensin converting enzyme (ACE)
ACE activity was measured using following protocol. Briefly, different concentrations of 10 µl PASEL (1, 10, and 100 mg/ml) were added to a mixed solution (490 µl) composed of 0.04 M sodium borate buffer (pH 8.3), 0.9 M NaCl, and 5 mM Hip-His-Leu (Hippuryl-Histidyl-Leucine), and incubated for five minutes at 37℃. Then angiotensin-converting enzyme (Sigma, USA) was added and allowed to react for 15 min at 37℃). Finally, the reaction was stopped by applying 1.2 ml of 0.34 N NaOH. The product, His-Leu, was measured fluorimetrically at 365 nm excitation and 495 nm emission with a fluorescence spectrophotometer (Hitachi, model F-2000, Tokyo, Japan) as follows. After 100 µl of O-phthaldehyde (20 mg/ml) in methanol was added to the reaction solution for 10 min, the solution was acidified with 200 µl of 3 N HCl and centrifuged at 3,000 rpm (Model Union 32 R Plus, Hanil, Seoul, Korea) for 10 min at room temperature. To correct the intrinsic fluorescence of plasma, a time zero blank was prepared by adding plasma after NaOH treatment. Captopril was used as a positive control.
Drugs and chemicals
All drugs and chemicals were purchased from Sigma Chemical (St. Louis, MO). PASEL was dissolved in DMSO (dimethyl sulfoxide) to prepare stock solutions. The final amount of DMSO in the bath solution was less than 0.1%.
Statistical analysis
Results are expressed as mean±S.E.M. Statistical significance was evaluated using the paired or unpaired Student's t-test (*p value<0.05). N numbers in the Results section and in the figure legends indicate numbers of vessels tested.
RESULTS
Effects of orally applied PASEL on the blood pressure of rats
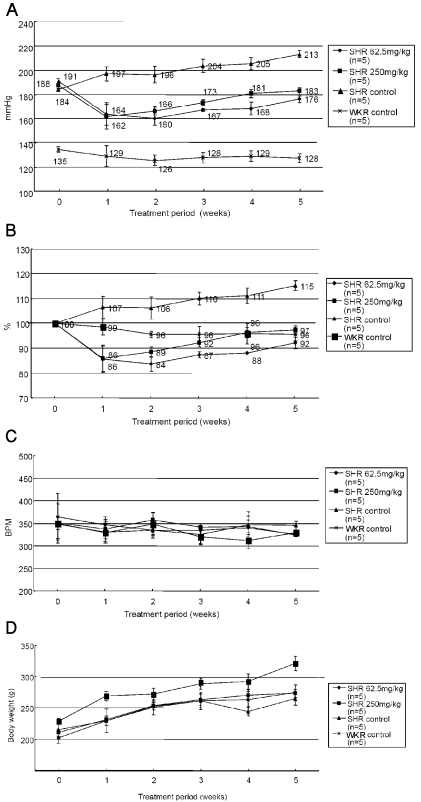
Blood pressure (BP) and heart rate (beat per minute, BPM) were measured in control Wistar-Kyoto Rats (WKR) and spontaneous hypertensive rats (SHR) every week. Two levels of PASEL (62.5 mg/kg and 250 mg/kg, per oral) were daily applied using a sonde for five weeks. The BP of SHR was decreased from the first week of PASEL application, and this effect was maintained during the tested period of application (Fig. 1A). However, the BP of SHR was not completely reversed to the normal level of control WKR. Normalized percentage change in BP was analyzed (Fig. 1B). The BP was normalized (%) to the initial level in each rat, and the analyzed result demonstrated the BP lowering effect of PASEL. The BP in untreated SHR was continuously increased during the tested period. Interestingly, the BP of WKR was not altered after PASEL treatment. Neither the heart rate (BPM) nor the body weight was affected by PASEL application (Fig. 1C, D).
Fig. 1.
Effects of PASEL treatment on blood pressure (BP) of SHR and control rats. (A) BP measurements were conducted using tail-cuff methods in WKY control and SHR (n=5, respectively) for five weeks. PASEL was applied daily (62.5 and 250 mg/kg, p.o.) for five weeks. The BP of SHR was lowered from the first week of PASEL application, while not completely normalized to the control level. The mean BP values are indicated in Fig. (B) The BP of each animal was normalized (100%) to the initial level and the mean values are summarized. (C, D) Summary of the heart rates (beat per minute, BPM) and body weights (g).
In vitro effects of PASEL on arterial contraction
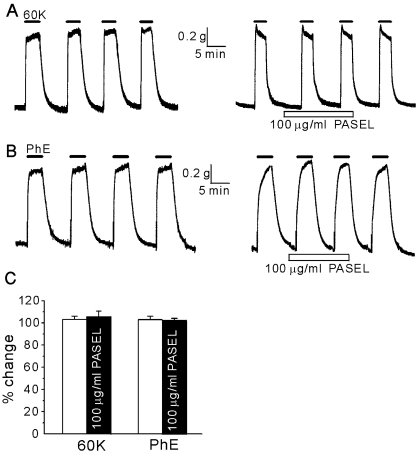
Initially, we studied the effects of PASEL on the contraction of large conduit arteries. The isometric contractile force (g) of femoral arteries from SHR was measured using a modified myograph system. An increase in extracellular [K+] (60 mM) levels induced a sustained contraction that was repeatedly induced in a reversible manner. The application of PASEL up to 100 µg/ml had no effect on the high K+-induced contraction (Fig. 2A, C). We also tested whether PASEL inhibited agonist-induced contraction of arteries. Phenyleprine (5 µM) was applied to stimulate alpha-adrenergic receptors, inducing the contraction of the femoral artery. PASEL (100 µg/ml) application had no effect on the phenyleprine-induced contraction (Fig. 2B, C).
Fig. 2.
No significant effect of PASEL on high K+- and phenyleprine-induced contraction of large femoral artery. (A, B) Representative traces of isometric contraction of femoral arteries induced by 60 mM KCl (60 K) or 5 µM of phenyleprine (PhE). After confirming the consistent contractile responses to repetitive application of KCl or PhE (left panels), PASEL was applied (right panels). Neither 60 K- nor PhE-induced contraction was affected by pretreatment of the animals with 100 µg/ml PASEL. (C) Summary of the contractile responses in the absence (open bars) or the presence of PASEL (100 µg/ml, closed bars). Contractile responses were normalized (%) to the initial control amplitude. The number of tested arteries is indicated above each bar.
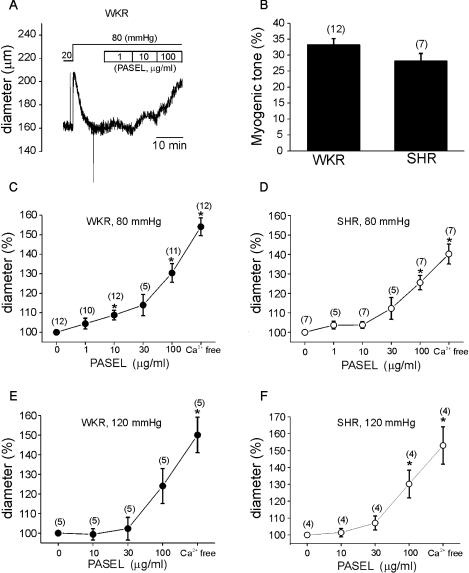
The lack of effect of PASEL on KCl-induced contractions indicated that its anti-hypertensive effect might not have been due to a direct inhibition of the voltage-operated Ca2+ channels. We thus postulated that the MR of small resistance arteries might be affected by PASEL. The middle cerebral arteries of WKR and SHR were cannulated and a constant pressure was applied (see Methods). To evoke MR, the Plum was increased from 20 mmHg to 80 mmHg. The Din was initially increased by the step increase of Plum and then slowly recovered to the previous Din or below (Fig. 3A). Such a contraction in response to increased Plum was regarded as MR. At the end of each experiment, the maximum passive Din was confirmed under Ca2+-free conditions. The amplitudes of MR were slightly smaller in SHR but not statistically different from those recorded in WKR (Fig. 3B). After confirming the MR at 80 mmHg, PASEL was applied to the bath solution. The effects of PASEL were evaluated by normalizing the Din at each concentration of PASEL to the control Din. The Din was increased by PASEL in a dose-dependent manner in both normal control (WKR, n=12) and SHR (Fig. 3C, D, n=7).
Fig. 3.
Effects of PASEL on MR of rat cerebral arteries. (A) A representative trace of diameter (Din) measured during application of PASEL (1~100 µg/ml) at 80 mmHg in the cerebral artery of WKR. (B) Summary of MR in % scale (see Methods) between WKR and SHR. There was no statistical significance between both groups. (C, D) Summary of the effects of 1~100µg/ml PASEL on MR of WKR and SHR at 80 mmHg, respectively. Normalized diameters (% values of control Din at 80 mmHg) were averaged. The numbers in parenthesis above each point indicate numbers of tested arteries. (E, F) Summary of the effects of 10~100 µg/ml PASEL on MR of WKR and SHR at 120 mmHg, respectively (*p value<0.05).
The relaxing effects of PASEL on MR were also observed at higher Plum (120 mmHg) but this effect appeared less potent than that observed at 80 mmHg (Fig. 3E).
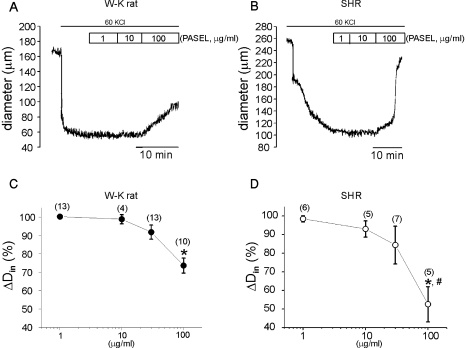
Different from the effects on the high K+-contraction of the large conduit artery, PASEL treatment suppressed the high K+-induced constriction of pressurized small CAs (Fig. 4). This inhibitory effect was notably slow, and dependent on the concentration of PASEL. Interestingly, the inhibition of high K+-induced constriction of CAs by 100 µg/ml of PASEL was more prominent in SHR than control animals (Fig. 4E).
Fig. 4.
Effects of PASEL on high K+-induced constriction of rat cerebral arteries. (A, B) Representative traces of inner diameter (Din) showing the high K+-constriction and the inhibition by PASEL (1~100 µg/ml) treatment in WKR and SHR. (C, D) Summary of the concentration-dependent effects of PASEL in WKR and SHR, respectively. Changes in Din (ΔDin%) are expressed in percent values versus the extent of high-K+ constriction ΔDin at 40 mmHg as 100%. The extent of dilation by 100 µg/ml PASEL was more prominent in SHR than WKR (p<0.05, #in D).
No effect of PASEL on angiotensin converting enzymes (ACE)
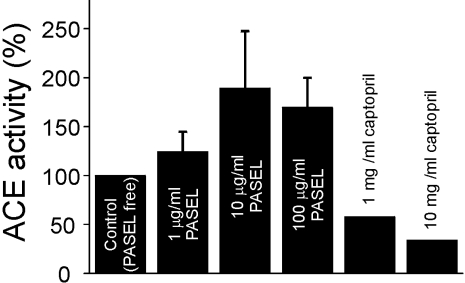
Some studies have suggested that the antihypertensive effect of Danshen might be due to the inhibition of ACEs (Kang et al., 2002; 2003). However, in our study, the effect of PASEL on ACE activity was inconsistent, showing an apparently positive effect that was statistically insignificant (p>0.05). In contrast, captopril, a positive control, showed the expected inhibitory effects (Fig. 5).
Fig. 5.
Effect of PASEL on angiotensin converting enzyme activity. PASEL did not show a significant effect on ACE activity in contrast to the inhibitory effect of the positive control, captopril. The effects of captopril were confirmed twice and the effects of PASEL were tested four times (n=4).
DISCUSSION
Here, we showed that a mixture of aqueous extracts of Salvia miltiorrhiza and Panax notoginseng lowered the blood pressure of SHR and also inhibited the arteriolar myogenic tone in a concentration-dependent manner. Because neither the high-K+ contraction nor ACE activity was affected by PASEL, it was suggested that the antihypertensive effects of PASEL might be due to its inhibitory effects on the myogenic tone, a unique property of resistance arteries.
In industrialized countries, the risk of becoming hypertensive (blood pressure >140/90 mmHg) during a lifetime exceeds 90%. Essential hypertension can be defined as a rise in blood pressure of unknown cause that increases the risk for cerebral, cardiac, and renal events (Messerli et al., 2007). All antihypertensive drugs are expected to lower blood pressure and this decline is the best determinant of cardiovascular risk reductions. However, differences between drugs exist with respect to reduction of target-organ disease and prevention of major cardiovascular events. Most hypertensive patients need two or more drugs for blood-pressure control and a concomitant statin treatment for risk factor reduction. Patients are exposed to antihypertensive treatment for decades; yet, the long-term safety of these drugs is not well-reported.
As mentioned in Introduction, Danshen and Sanchi are popular herbal drugs in TCM for improving various aspects of cardiovascular diseases. Linked to the vasodilatory effects of Danshen and Sanchi, previous studies on their biological action mechanisms have revealed interesting cellular effects: 1) inhibition of receptor-operated Ca2+ entry by a ginsenoside from Sanchi (Guan et al., 2006); 2) inhibition of leukocyte adhesion and ROS (reactive oxygen species) production by saponins from Sanchi (Sun et al., 2007); 3) activation of BKCa type K+ channels by salvianolic acid B from Danshen (Lam et al., 2006b); 4) inhibition of Ca2+ channels by Danshen extracts (Lam et al., 2006a); 5) ACE inhibition by lithospermic acid B from Danshen (Kang et al., 2003); 6) antioxidant and antithrombotic effects of trilinolein from Sanchi (Chan et al., 2002). Although the exact molecular target is unidentified, the inhibition of myogenic responses in the resistance of the small artery (Fig. 3) might provide a novel mechanism for the beneficial effects of PASEL on cardiovascular functions.
In the present study, PASEL had no significant effect on the high-K+ contraction of femoral arteries. These results are inconsistent with a report by Lam et al. (2006a) showing that the extracts of Danshen concentration-dependently inhibited the high-K+ contraction of rat femoral arteries. In the study by Lam et al. (2006a), however, the concentration of Danshen extract used was much higher than that used in the present study. We kept the concentration of PASEL lower than 100 µg/ml because the color of the solution darkened above this concentration, and such a high concentration might not be a true reflection of the in vivo effects. Moreover, the study by Lam et al. (2006a) demonstrated that the inhibition of high-K+ contraction was more potently inhibited by a lipid-soluble extract rather than an aqueous extract of Danshen. Since our compounds, PASEL, are aqueous extracts of both Danshen and Sanchi, it was likely that the effects of the same amount of PASEL on voltage-operated Ca2+ channels would be relatively weak.
The contractility of small arteries and arterioles are affected by luminal pressure (Plum), and the resistance arteries show partial contraction in vivo. The Plum-dependent contraction is referred to as the myogenic response (MR) because the sensing mechanisms are intrinsic to the arterial smooth muscle cells. MR is critical for setting the peripheral vascular resistance and autoregulation of blood flow (Dora, 2005; Hill et al., 2006), derangement of which could promote hypertension (Koller, 2002; Jarajapu and Knot, 2005, Ahn et al., 2007). A number of signaling pathways have been suggested such as mechanosensitive nonselective cation channels (NSCms), Rho kinase (ROCK), and protein kinase C (PKC) (Hill et al., 2006; Shubert et al., 2008). More proximal to the above mechanisms, altered synthesis of 20-hydroxyeicosatetraenoic acid (20-HETE, Gebremedhin et al., 2000), sphingosine-1 phosphate (Keller et al., 2006), and mechanosensitive G-protein coupled receptors (Mederos y Schnitzler et al., 2008) have been proposed to be involved. At this point, we are unable to suggest a specific target mechanism or signaling pathway that drives the inhibition of MR by PASEL. We do suggest, however, the involvement of multiple pathways as PASEL is composed of a large number of chemical agents.
Danshen has been used primarily in traditional Chinese medicine for the treatment of coronary heart diseases, particularly angina pectoris and myocardial infarction (Wang et al., 2007). It is generally accepted that MR is better developed in the small arteries supplying vital organs such as heart, brain and kidney. In this respect, the relatively potent inhibition of MR by PASEL might partly explain the beneficial effects of Danshen on coronary blood flow.
Although the high K+-contraction of FAs was unaffected, the high-K+ induced constriction of pressurized CAs was reversed by PASEL (Fig. 4). Interestingly, this effect was more potent in SHR than control rats (Fig. 4E). Considering the lack of effect of PASEL on the high-K+ contraction in femoral arteries, the small CA-specific inhibition implied that PASEL might have a direct effect on the contractile mechanisms distal to the L-type Ca2+ channels, such as rho-kinase. A recent study in SHR demonstrated an enhanced MR that was mediated by the specific activation of rhoA/rho-kinase signaling pathway (Ahn et al., 2007). However, we cannot exclude a possibility that the diverse effects of PASEL on the high-K+ constriction might be due to different types of experimental conditions; isometric tone measurement (FAs) vs. pressurized artery diameter measurement (CAs). More direct investigation is definitely necessary to elucidate the precise mechanism of SHR-specific vasodilation by PASEL.
In summary, a mixed aqueous extract of Danshen and Sanchi showed anti-hypertensive effects in SHR. We report, for the first time, that PASEL inhibited the MR of resistance arteries, and suggest that this might contribute toward a novel mechanism of anti-hypertensive and anti-anginal effects of the medicinal herbs. Further investigation with specific bioactive constituents in terms of their precise mechanisms is required in order to broaden the clinical use of these plants.
ACKNOWLEDGEMENTS
This work was partly supported by the IT R&D program of MKE/IITA [2008-F-029-01, Development of e-Organ system based on Cyber Computing], and by the Joint Technology Development Supporting Business among Industry, Academy and Research Institute (2007).
ABBREVIATIONS
- MR
myogenic response
- CA
cerebral artery
- FA
femoral artery
- PhE
phenyleprine
- SHR
spontaneously hypertensive rat
- WRK
Wista-Kyoto rat
- BP
blood pressure
- BPM
beat per minute
- Din
inner diamter
- Plum
luminal pressure
References
- 1.Ahn DS, Choi SK, Kim YH, Cho YE, Shin HM, Morgan KG, Lee YH. Enhanced stretch-induced myogenic tone in the basilarartery of spontaneously hypertensive rats. J Vasc Res. 2007;44:182–191. doi: 10.1159/000100374. [DOI] [PubMed] [Google Scholar]
- 2.Chan P, Thomas GN, Tomlinson B. Protective effects of trilinolein extracted from Panax notoginseng against cardiovascular disease. Acta Pharmacol Sin. 2002;23:1157–1162. [PubMed] [Google Scholar]
- 3.Chuang CM, Hsieh CL, Lin HY, Lin JG. Panax Notoginseng Burk attenuates impairment of learning and memory functions and increases ED1, BDNF and beta-secretase immunoreactive cells in chronic stage ischemia-reperfusion injured rats. Am J Chin Med. 2008;36:685–693. doi: 10.1142/S0192415X08006156. [DOI] [PubMed] [Google Scholar]
- 4.Dora KA. Does arterial myogenic tone determine blood flow distribution in vivo? Am J Physiol Heart Circ Physiol. 2005;289:H1323–H1325. doi: 10.1152/ajpheart.00513.2005. [DOI] [PubMed] [Google Scholar]
- 5.Feihl F, Liaudet L, Levy BI, Waeber B. Hypertension and microvascular remodelling. Cardiovasc Res. 2008;78:274–285. doi: 10.1093/cvr/cvn022. [DOI] [PubMed] [Google Scholar]
- 6.Gebremedhin D, Lange AR, Lowry TF, Reza Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 7.Guan YY, Zhou JG, Zhang Z, Wang GL, Cai BX, Hong L, Qiu QY, He H. Ginsenoside-Rd from panax notoginseng blocks Ca2+ influx through receptor- and store-operated Ca2+ channels in vascular smooth muscle cells. Eur J Pharmacol. 2006;548:129–136. doi: 10.1016/j.ejphar.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc. 2006;34:67–79. [PubMed] [Google Scholar]
- 9.Jarajapu YP, Knot HJ. Relative contribution of Rho kinase and protein kinase C to myogenic tone in rat cerebral arteries in hypertension. Am J Physiol Heart Circ Physiol. 2005;289:H1917–H1922. doi: 10.1152/ajpheart.01012.2004. [DOI] [PubMed] [Google Scholar]
- 10.Kang DG, Oh H, Chung HT, Lee HS. Inhibition of angiotensin converting enzyme by lithospermic acid B isolated from radix Salviae miltiorrhiza Bunge. Phytother Res. 2003;17:917–920. doi: 10.1002/ptr.1250. [DOI] [PubMed] [Google Scholar]
- 11.Kang DG, Yun YG, Ryoo JH, Lee HS. Anti-hypertensive effect of water extract of danshen on renovascular hypertension through inhibition of the renin angiotensin system. Am J Chin Med. 2002;30:87–93. doi: 10.1142/S0192415X02000107. [DOI] [PubMed] [Google Scholar]
- 12.Keller M, Lidington D, Vogel L, Peter BF, Sohn HY, Pagano PJ, Pitson S, Spiegel S, Pohl U, Bolz SS. Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. FASEB J. 2006;20:702–704. doi: 10.1096/fj.05-4075fje. [DOI] [PubMed] [Google Scholar]
- 13.Koller A. Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation. 2002;9:277–294. doi: 10.1038/sj.mn.7800142. [DOI] [PubMed] [Google Scholar]
- 14.Lam FF, Yeung JH, Cheung JHY, Or PM. Pharmacological evidence for calcium channel inhibition by Danshen (Salvia miltiorrhiza)on rat isolated femoral artery. J Cardiovasc Pharmacol. 2006a;47:139–145. doi: 10.1097/01.fjc.0000197540.12685.ce. [DOI] [PubMed] [Google Scholar]
- 15.Lam FFY, Seto SW, Kwan YW, Yeung JH, Chan P. Activation of the iberiotoxin-sensitive BKCa channels by salvianolic acid B of the porcine coronary artery smooth muscle cells. Eur J Pharmacol. 2006b;546:28–35. doi: 10.1016/j.ejphar.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Deng CQ, Chen BY, Zhang SP, Liang Y, Luo XG. Total saponins of Panax Notoginseng modulate the expression of caspases and attenuate apoptosis in rats following focal cerebral ischemia-reperfusion. J Ethnopharmacol. 2009;121:412–418. doi: 10.1016/j.jep.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Mederos Y, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. G(q)-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370:591–603. doi: 10.1016/S0140-6736(07)61299-9. [DOI] [PubMed] [Google Scholar]
- 19.Schubert R, Lidington D, Bolz SS. The emerging role of Ca2+ sensitivity regulation in promoting myogenic vasoconstriction. Cardiovasc Res. 2008;77:8–18. doi: 10.1016/j.cardiores.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Sun K, Wang CS, Guo J, Horie Y, Fang SP, Wang F, Liu YY, Liu LY, Yang JY, Fan JY, Han JY. Protective effects of ginsenosideRb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 2007;81:509–518. doi: 10.1016/j.lfs.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of tanshen. Med Res Rev. 2007;27:133–148. doi: 10.1002/med.20077. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Zuo Z, Chow MS. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]