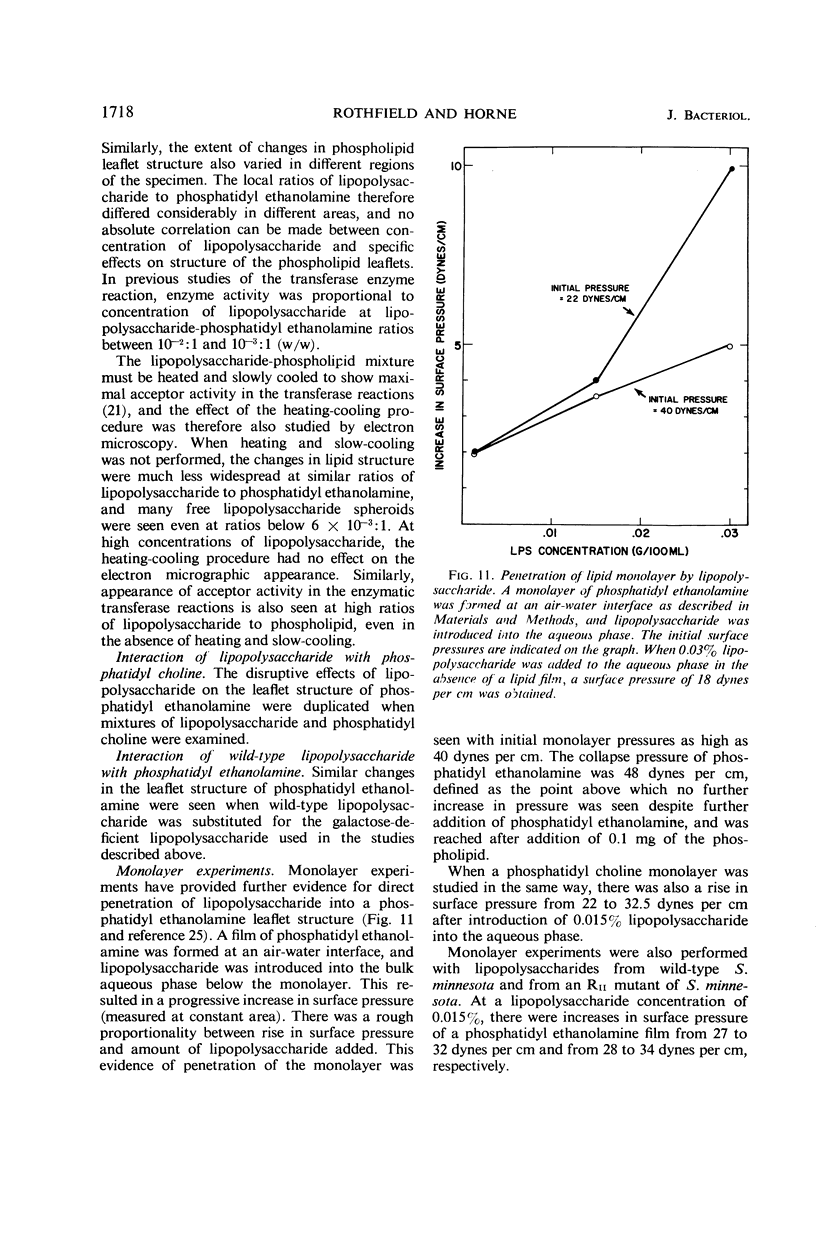
Abstract
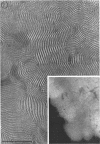
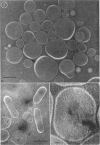
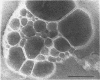
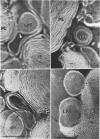
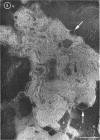
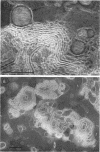
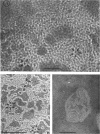
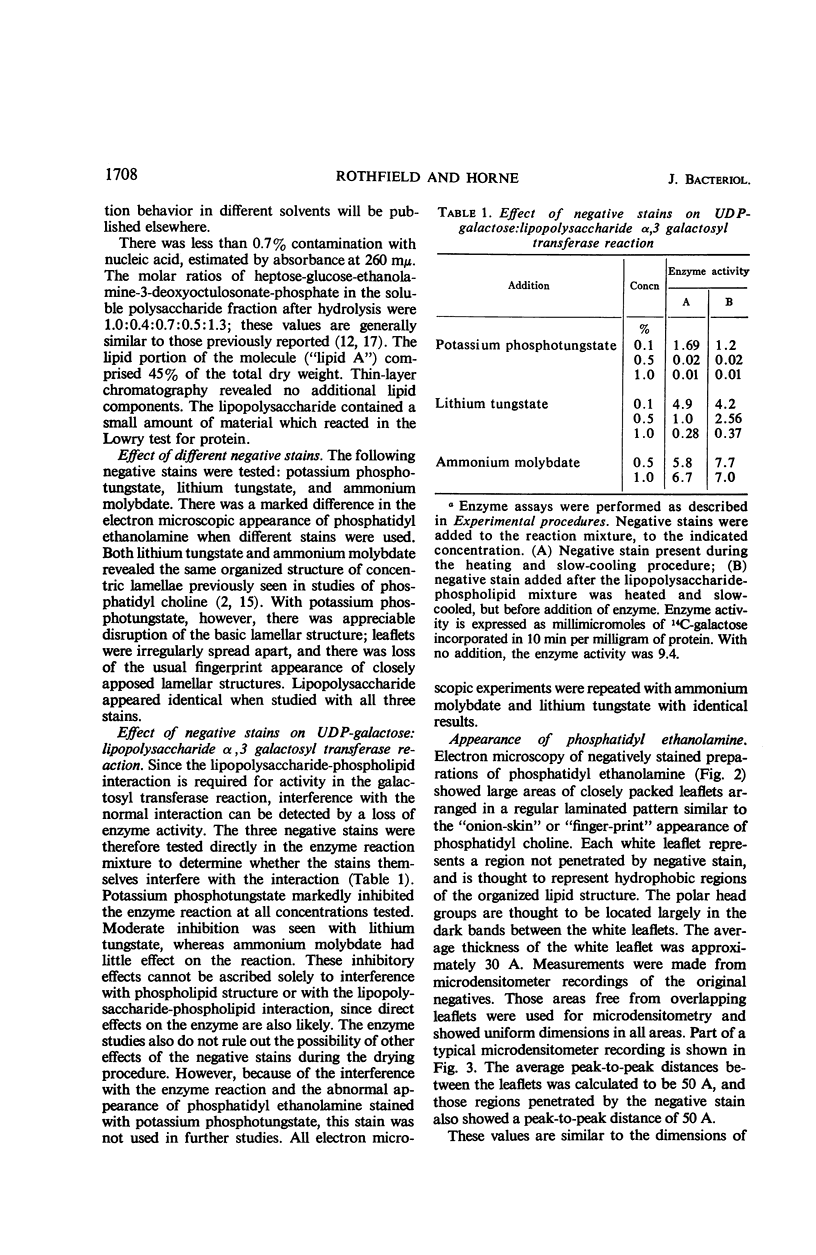
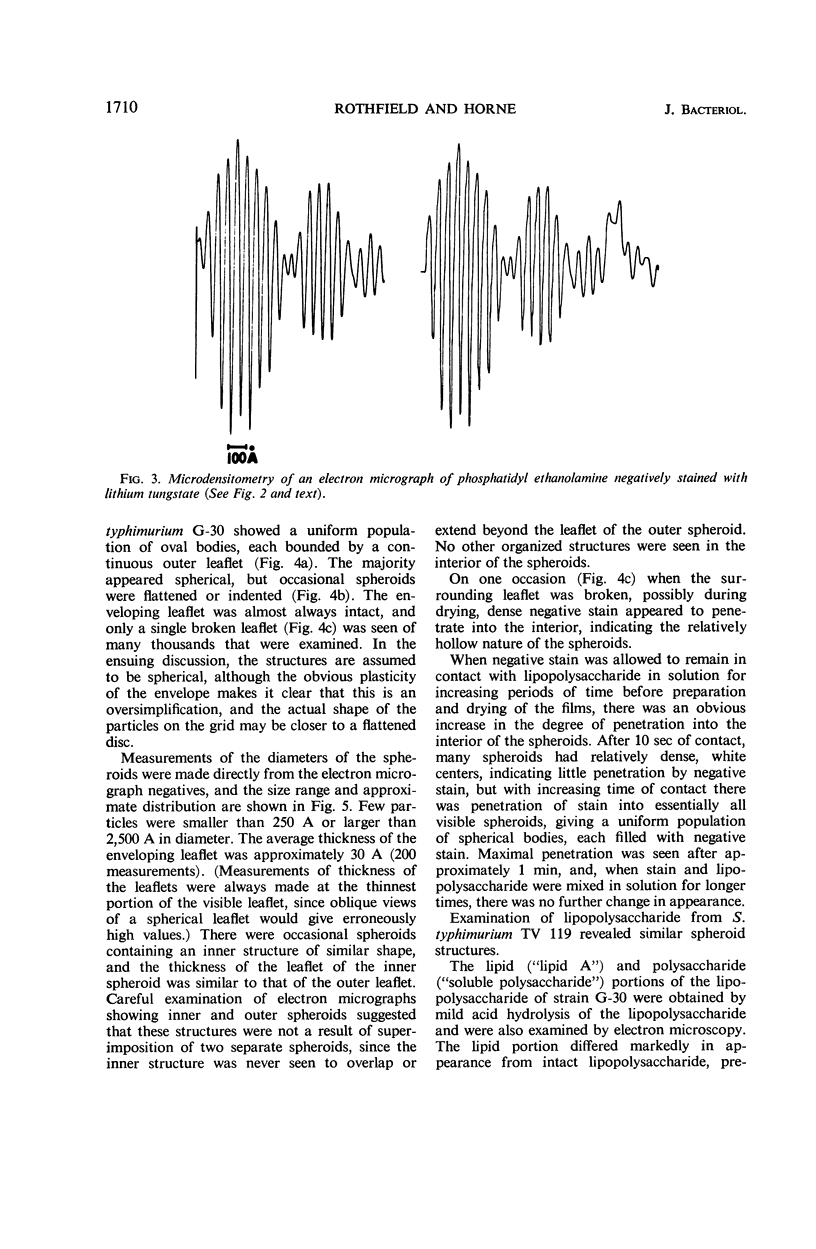
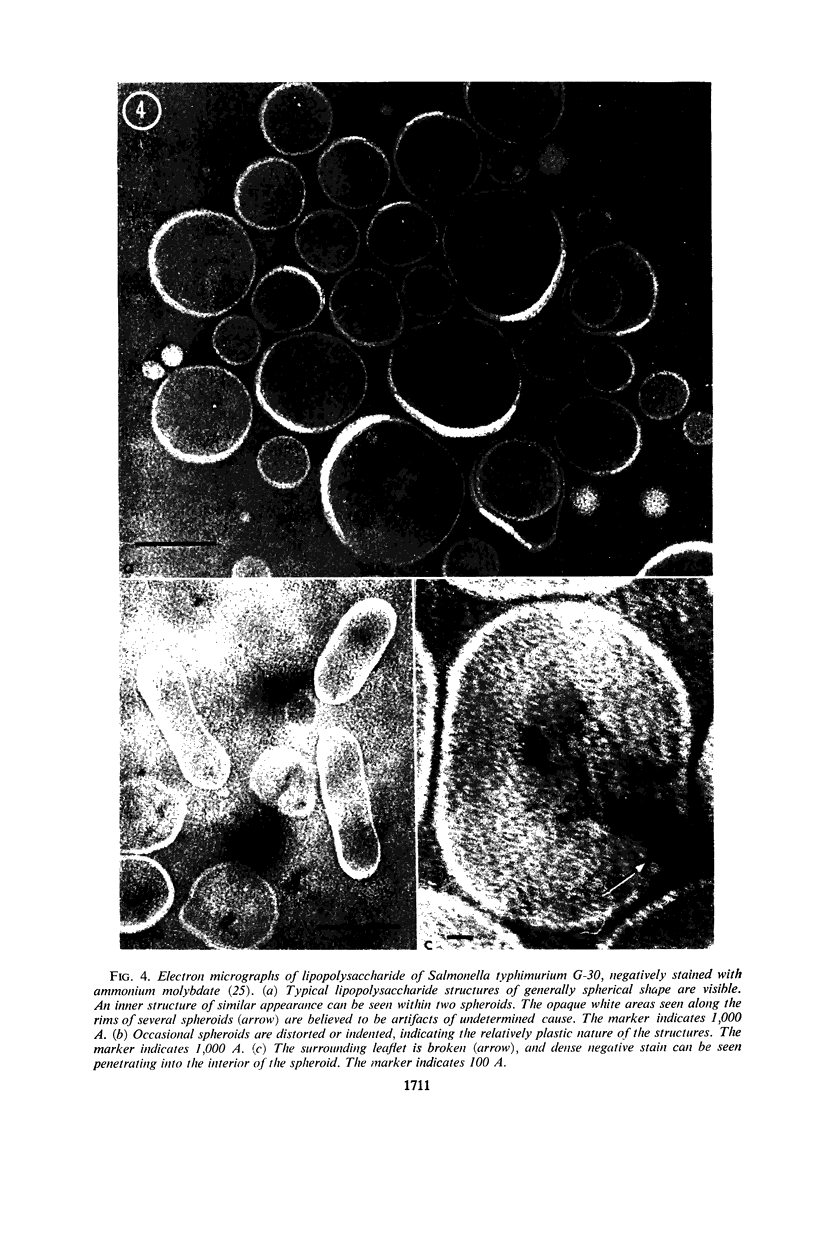
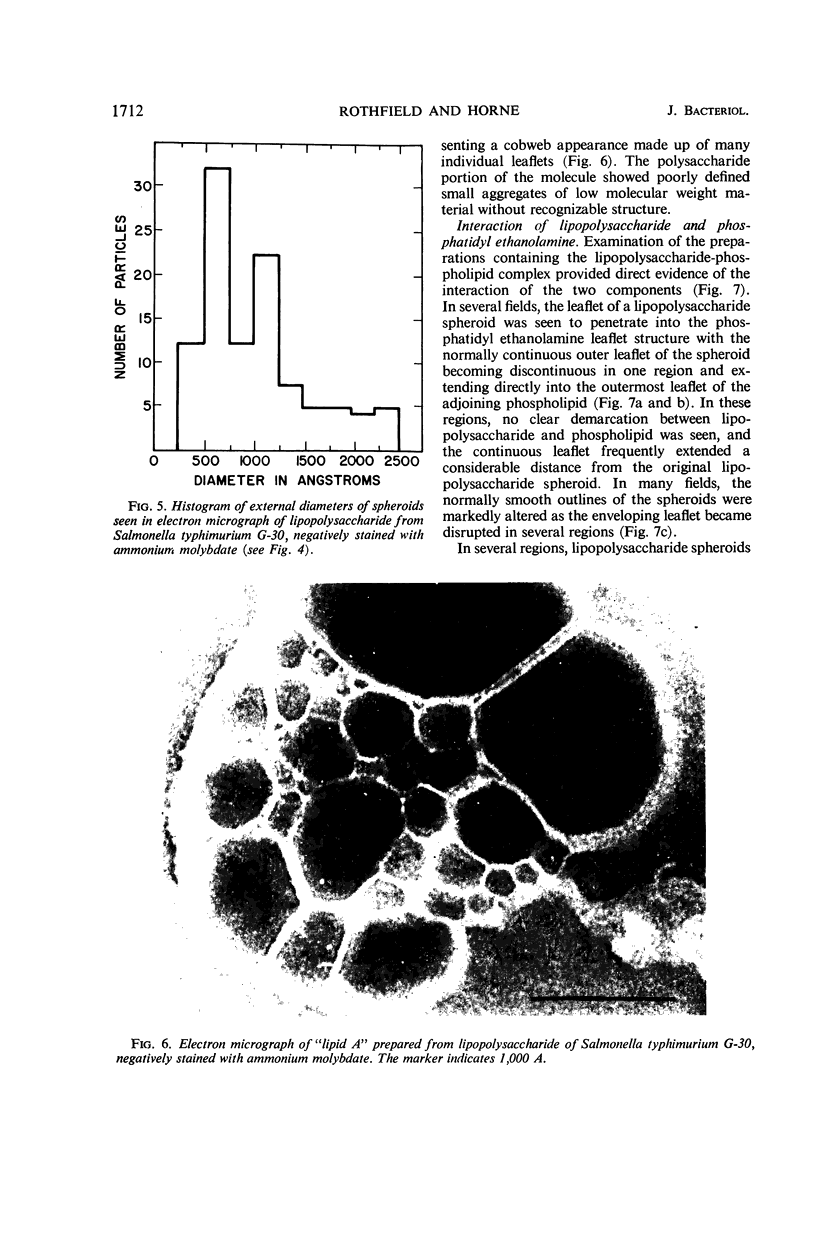
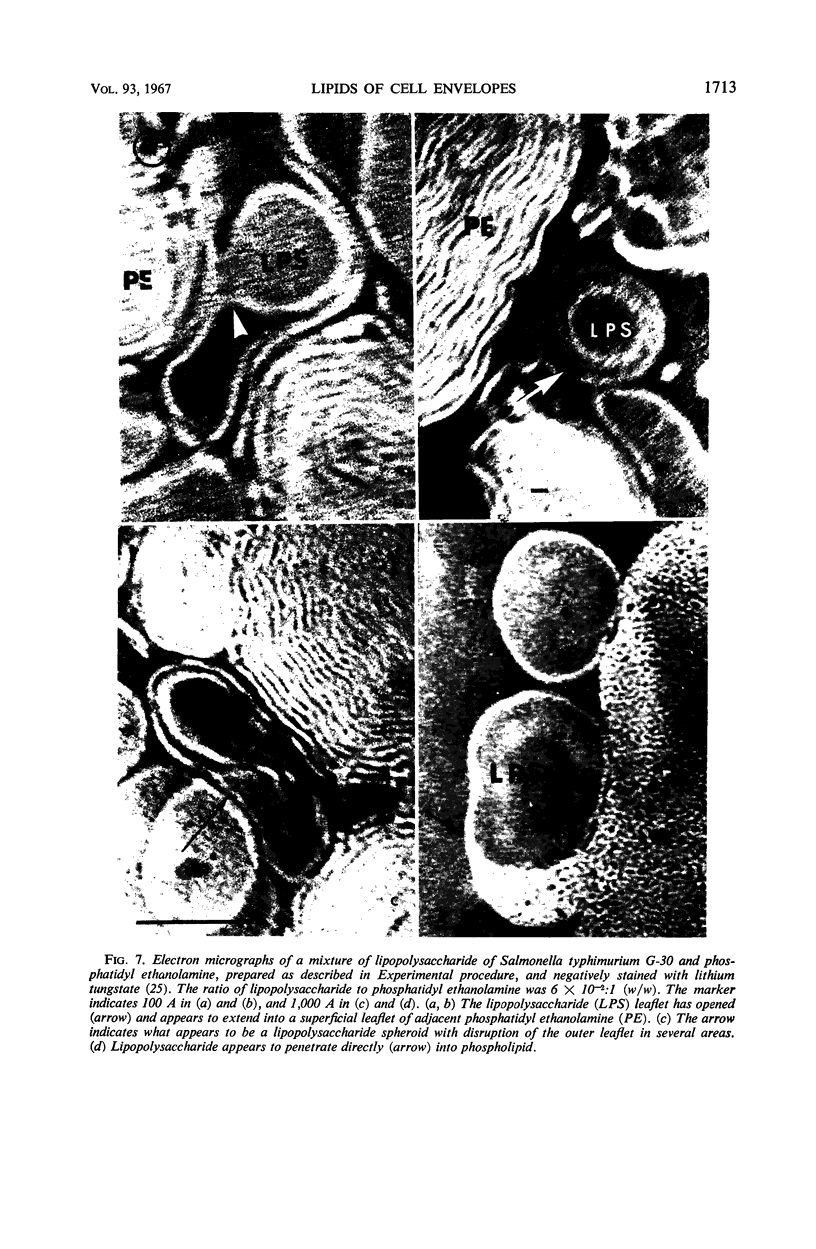
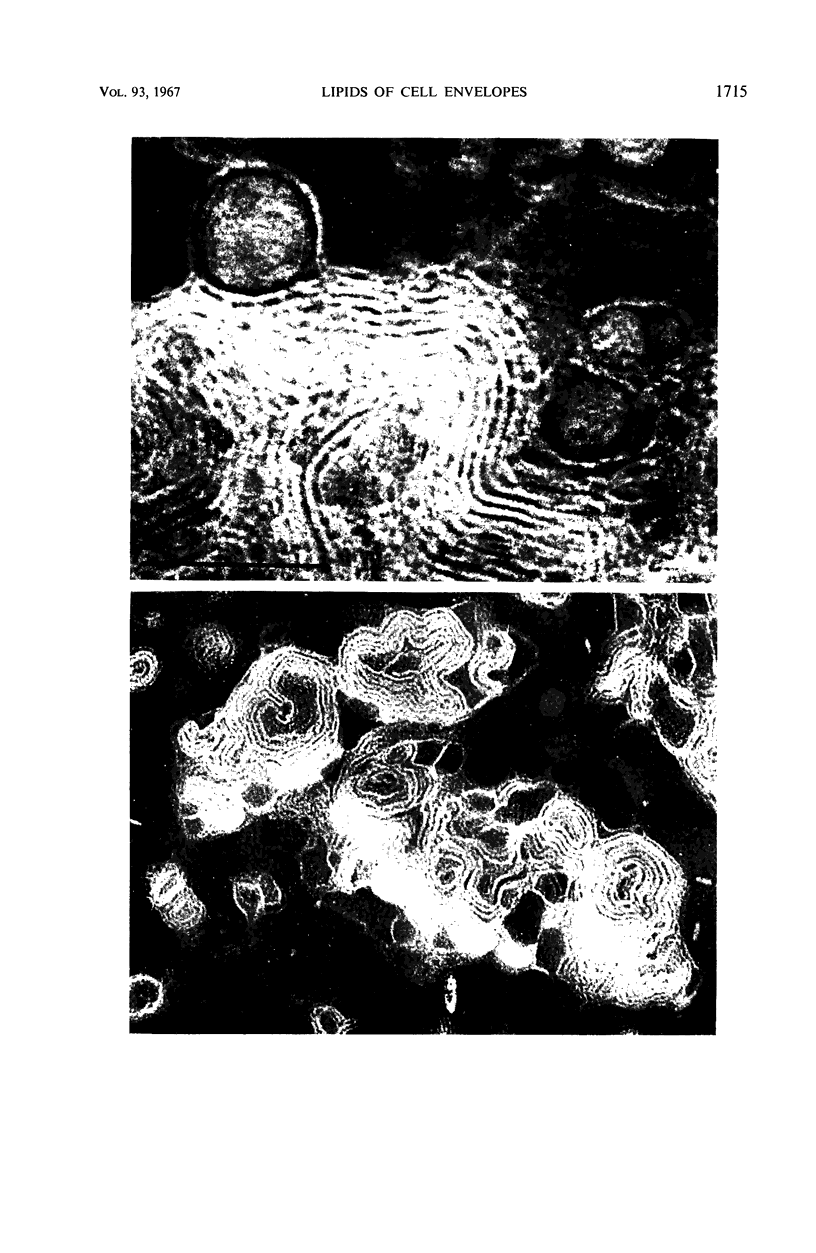
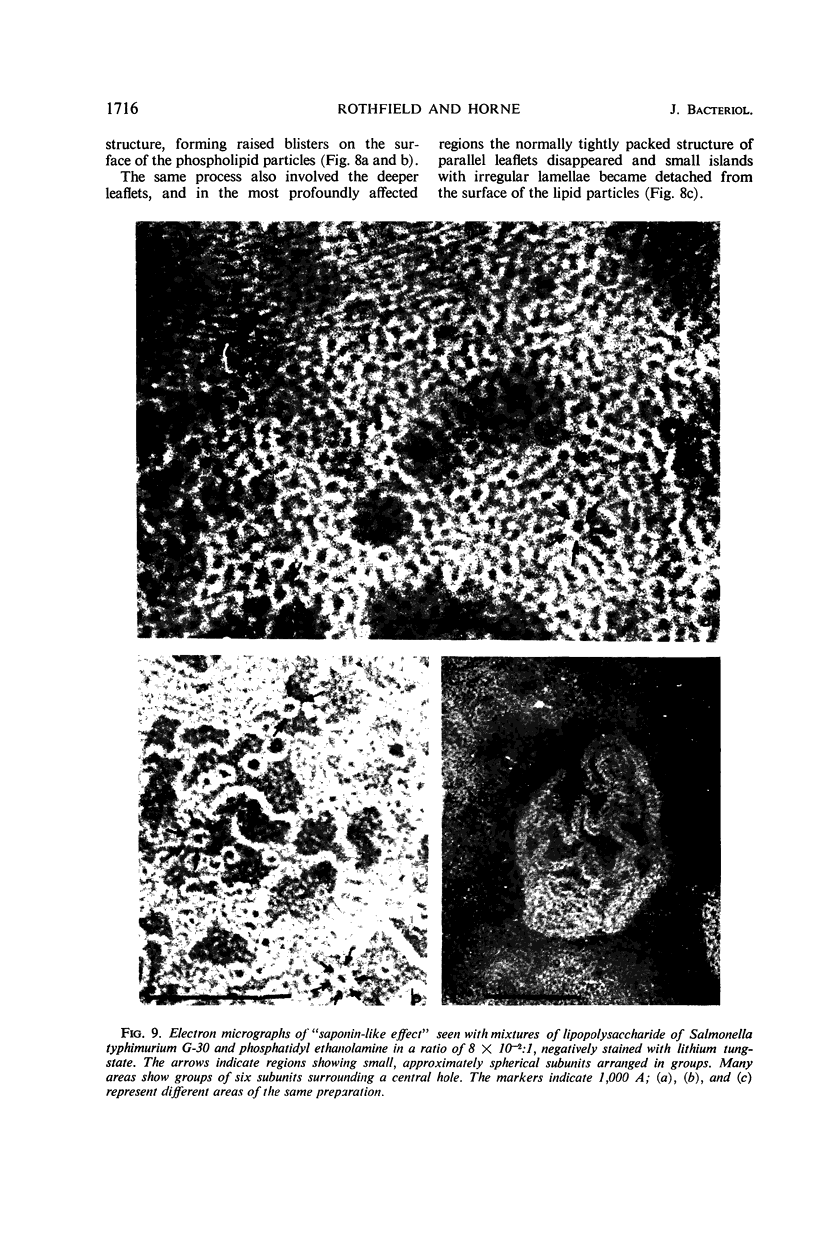
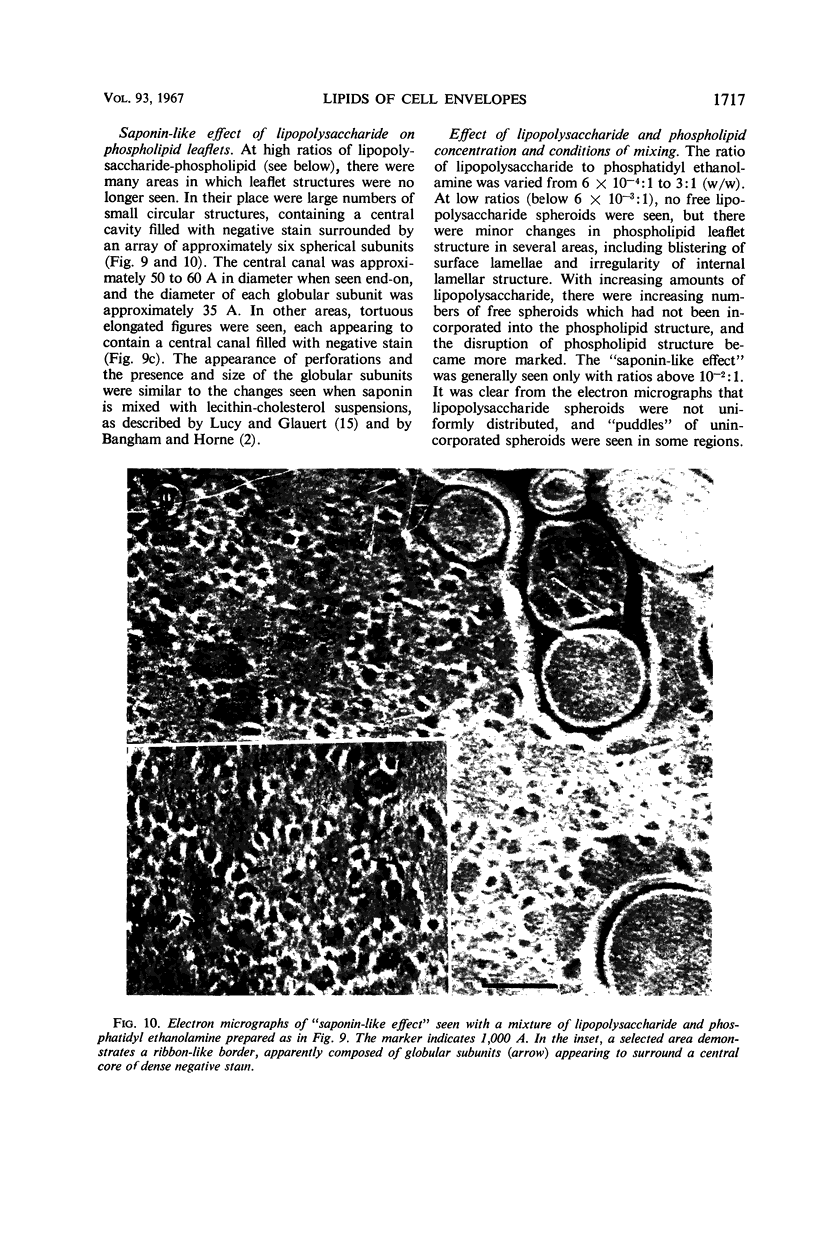
Phosphatidyl ethanolamine and lipopolysaccharide were extracted and purified from the cell envelope fractions of Escherichia coli and Salmonella typhimurium. The two components were studied separately and after recombination, by use of electron microscopy and monolayer techniques, and by measuring their ability to participate in the enzyme-catalyzed uridine diphosphate-galactose:lipopolysaccharide α, 3 galactosyl transferase reaction, which requires a lipopolysaccharide-phospholipid complex as substrate. Electron microscopy of purified lipopolysaccharide showed a uniform population of hollow spheres, with each sphere bounded by a continuous leaflet. The diameter of the spheres was approximately 500 to 1,000 A, and the thickness of the enveloping leaflet was approximately 30 A. Phosphatidyl ethanolamine showed a regular lamellar structure. When lipopolysaccharide and phosphatidyl ethanolamine were mixed under conditions of heating and slow-cooling, the leaflet of the lipopolysaccharide spheroids appeared to extend directly into the phosphatidyl ethanolamine structure, with continuity between the two leaflets. Various stages of penetration were seen. At high concentrations of lipopolysaccharide, there were disruptive changes in phosphatidyl ethanolamine leaflets similar to those seen when saponin acts on cholesterol-lecithin leaflets. Monolayer experiments indicated that lipopolysaccharide penetrated a monomolecular film of phosphatidyl ethanolamine at an air-water interface, as revealed by an increase in surface pressure. The results indicate that a common leaflet structure containing lipopolysaccharide and phosphatidyl ethanolamine may be formed in vitro, and suggest that a similar leaflet may exist in the intact bacterial cell envelope.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., HORNE R. W. NEGATIVE STAINING OF PHOSPHOLIPIDS AND THEIR STRUCTURAL MODIFICATION BY SURFACE-ACTIVE AGENTS AS OBSERVED IN THE ELECTRON MICROSCOPE. J Mol Biol. 1964 May;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D. PHYSICAL STRUCTURE AND BEHAVIOR OF LIPIDS AND LIPID ENZYMES. Adv Lipid Res. 1963;1:65–104. doi: 10.1016/b978-1-4831-9937-5.50008-9. [DOI] [PubMed] [Google Scholar]
- BLADEN H. A., MERGENHAGEN S. E. ULTRASTRUCTURE OF VEILLONELLA AND MORPHOLOGICAL CORRELATION OF AN OUTER MEMBRANE WITH PARTICLES ASSOCIATED WITH ENDOTOXIC ACTIVITY. J Bacteriol. 1964 Nov;88:1482–1492. doi: 10.1128/jb.88.5.1482-1492.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON A. J., CARTER H. E. PURIFICATION AND CHARACTERIZATION OF THE LIPID A COMPONENT OF THE LIPOPOLYSACCHARIDES FROM ESCHERICHIA COLI. Biochemistry. 1964 Mar;3:411–418. doi: 10.1021/bi00891a018. [DOI] [PubMed] [Google Scholar]
- ELBERS P. F., PIETERS J. ACCURATE DETERMINATION OF MAGNIFICATION IN THE ELECTRON MICROSCOPE. J Ultrastruct Res. 1964 Aug;11:25–32. doi: 10.1016/s0022-5320(64)80090-3. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ-MORAN H. Cell-membrane ultrastructure. Low-temperature electron microsopy and x-ray diffraction studies of lipoprotein components in lamellar systems. Circulation. 1962 Nov;26:1039–1065. doi: 10.1161/01.cir.26.5.1039. [DOI] [PubMed] [Google Scholar]
- FINEAN J. B. X-ray diffraction studies on the polymorphism of phospholipids. Biochim Biophys Acta. 1953 Mar;10(3):371–384. doi: 10.1016/0006-3002(53)90268-6. [DOI] [PubMed] [Google Scholar]
- GROLLMAN A. P., OSBORN M. J. O-PHOSPHORYLETHANOLAMINE: A COMPONENT OF LIPOPOLYSACCHARIDE IN CERTAIN GRAM-NEGATIVE BACTERIA. Biochemistry. 1964 Oct;3:1571–1574. doi: 10.1021/bi00898a031. [DOI] [PubMed] [Google Scholar]
- LUCY J. A., GLAUERT A. M. STRUCTURE AND ASSEMBLY OF MACROMOLECULAR LIPID COMPLEXES COMPOSED OF GLOBULAR MICELLES. J Mol Biol. 1964 May;8:727–748. doi: 10.1016/s0022-2836(64)80121-2. [DOI] [PubMed] [Google Scholar]
- LUCY J. A. GLOBULAR LIPID MICELLES AND CELL MEMBRANES. J Theor Biol. 1964 Sep;7:360–373. doi: 10.1016/0022-5193(64)90080-3. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., ZELEZNICK L. D., HORECKER B. L. LIPOPOLYSACCHARIDE OF THE GRAM-NEGATIVE CELL WALL. Science. 1964 Aug 21;145(3634):783–789. doi: 10.1126/science.145.3634.783. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. The molecular structure and contact relationships of cell membranes. Prog Biophys Mol Biol. 1960;10:343–418. [PubMed] [Google Scholar]
- ROTHFIELD L., HORECKER B. L. THE ROLE OF CELL-WALL LIPID IN THE BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. Proc Natl Acad Sci U S A. 1964 Oct;52:939–946. doi: 10.1073/pnas.52.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHFIELD L., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. II. INCORPORATION OF GLUCOSE AND GALACTOSE CATALYZED BY PARTICULATE AND SOLUBLE ENZYMES IN SALMONELLA. J Biol Chem. 1964 Sep;239:2788–2795. [PubMed] [Google Scholar]
- Rothfield L., Pearlman M. The role of cell envelope phospholipid in the enzymatic synthesis of bacterial lipopolysaccharide. Structural requirements of the phospholipid molecule. J Biol Chem. 1966 Mar 25;241(6):1386–1392. [PubMed] [Google Scholar]
- Rothfield L., Takeshita M., Pearlman M., Horne R. W. Role of phospholipids in the enzymatic synthesis of the bacterial cell envelope. Fed Proc. 1966 Sep-Oct;25(5):1495–1502. [PubMed] [Google Scholar]
- Rothfield L., Takeshita M. The role of cell envelope phospholipid in the enzymatic synthesis of bacterial lipopolysaccharide: binding of transferase enzymes to a lipopolysaccharide-lipid complex. Biochem Biophys Res Commun. 1965 Aug 16;20(4):521–527. doi: 10.1016/0006-291x(65)90611-x. [DOI] [PubMed] [Google Scholar]
- STOECKENIUS W. Some electron microscopical observations on liquid-crystalline phases in lipid-water systems. J Cell Biol. 1962 Feb;12:221–229. doi: 10.1083/jcb.12.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTPHAL O. [Recent research on the chemistry and biology of the endotoxins of gram-negative bacteria]. Ann Inst Pasteur (Paris) 1960 Jun;98:789–813. [PubMed] [Google Scholar]
- Work E., Knox K. W., Vesk M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Ann N Y Acad Sci. 1966 Jun 30;133(2):438–449. doi: 10.1111/j.1749-6632.1966.tb52382.x. [DOI] [PubMed] [Google Scholar]