Abstract
Spontaneous hypertensive rats (SHR) are an established model of genetic hypertension. Vascular smooth muscle cells (VSMC) from SHR proliferate faster than those of control rats (Wistar-Kyoto rats; WKY). We tested the hypothesis that induction of heme oxygenase (HO)-1 induced by aprotinin inhibits VSMC proliferation through cell cycle arrest in hypertensive rats. Aprotinin treatment inhibited VSMC proliferation in SHR more than in normotensive rats. These inhibitory effects were associated with cell cycle arrest in the G1 phase. Tin protoporphyrin IX (SnPPIX) reversed the anti-proliferative effect of aprotinin in VSMC from SHR. The level of cyclin D was higher in VSMC of SHR than those of WKY. Aprotinin treatment downregulated the cell cycle regulator, cyclin D, but upregulated the cyclin-dependent kinase inhibitor, p21, in VSMC of SHR. Aprotinin induced HO-1 in VSMC of SHR, but not in those of control rats. Furthermore, aprotinin-induced HO-1 inhibited VSMC proliferation of SHR. Consistently, VSMC proliferation in SHR was significantly inhibited by transfection with the HO-1 gene. These results indicate that induction of HO-1 by aprotinin inhibits VSMC proliferation through cell cycle arrest in hypertensive rats.
Keywords: Aprotinin, Hypertension, Proliferation, Heme oxygenase-1, Cell cycle arrest
INTRODUCTION
Vascular smooth muscle cell (VSMC) proliferation contributes to the arterial remodeling that occurs in hypertension (Intengan et al., 2001). Indeed, VSMC from hypertensive rats (SHR) proliferate faster than normotensive rats (Wistar-Kyoto rats; WKY) (Resink, 1987). This phenomenon has been attributed to differences in cell cycle regulation in SHR and WKY (Tanner et al., 2003). Mammalian cell proliferation is governed by the cell cycle (Sherr, 1996). Cell cycle progression is a tightly controlled event regulated positively by cyclin-dependent kinases (CDK) and their cyclin-regulatory subunits (Sherr CJ, 1993), and negatively by CDK inhibitors and tumor suppressor genes (Hunter, 1993).
Aprotinin is a broad-spectrum serine protease inhibitor with a molecular weight of 6512 d. It is widely used in congenital cardiac surgery for its hemostatic and anti-inflammatory benefits (Westaby, 1993; Levy, 2001), although its safety when used in adults undergoing coronary artery surgery has been questioned (Mangano et al., 2006). We previously found that aprotinin inhibited VSMC inflammation and proliferation via induction of heme oxygenase (HO)-1 (Lee et al., 2009). Expression of HO-1 in blood vessels by hemin treatment in vivo reduces vasoconstriction and normalizes blood pressure in SHR (Ndisang et al., 2002; Wang et al., 2006). Similarly, HO inhibitors increase blood pressure and peripheral resistance, suggesting a critical vasoregulatory role for HO (Ndisang et al., 2003). Interestingly, induction of HO-1 by chronic hemin treatment of SHR inhibits proliferation of VSMC (Chang et al., 2008). We previously observed that induction of HO-1 by hemin inhibited proliferation of VSMC through the regulation of cell cycle arrest in SHR (Jeon et al., 2009). Thus genetic approaches targeting HO-1 or pharmacological interventions using HO-1 inducers may offer a promising therapeutic modality in the treatment of hypertension.
Based on these ideas, we investigated the effects of aprotinin on VSMC proliferation. Here, we have shown that aprotinin inhibits VSMC proliferation of SHR and induces G1/G0 cell arrest through regulation of cyclin D and p21 via the induction of HO-1.
METHODS
Reagents
Cell culture reagents were purchased from Hyclone. Acrylamide and Western blot reagents were purchased from Bio-Rad. Cell cycle regulator antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA), and Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The antibody against HO-1 was purchased from Stressgen Bioreagents (Victoria, BC, Canada). Tin protoporphyrine IX (SnPPIX) was purchased from Calbiochem (La Jolla, CA, USA). Lipofectamine 2000 was purchased from R&D Systems (Minneapolis, MN, USA). PRO-PREP protein extract solution was purchased from iNtRON Biotechnology (Sungnam, Korea). All other chemicals, including aprotinin, 3-(4,5 dimethylthiazole 2yl)-2,5-diphenyl tetrazolium bromide thiazoyl blue (MTT), propidium iodide (PI) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Rats
Specific pathogen-free male inbred WKY and SHR, 12 to 16 weeks of age, were purchased from Japan SLC Inc. (Shizuoka, Japan). All experimental animals received autoclaved food and bedding to minimize exposure to viral or microbial pathogens. The rats were cared for in accordance with the Guide for the Care and Use of Experimental Animals of Yeungnam Medical Center.
Cell culture
Rats were anesthetized by pentobarbital (50 mg/kg). Thoracic aortas were isolated and connective tissues were removed. VSMC were processed using a 1 mm chop setting in a 10 cm culture dish, and cultured with DMEM containing 50% fetal bovine serum (FBS) and 1% antibiotics (penicillin 10,000 U/ml, streptomycin 10,000 µl/ml) and were incubated in a CO2 incubator (95% CO2, 5% O2, 37℃) for 7 days. Cells from passage 4 to 10 were used for the experiments.
Cell proliferation
To measure proliferation, VSMC from SHR and WKY were seeded at 10×104 cell/ml. Cell numbers were determined with a hemocytometer after 0, 1, 2, and 3 days in response to stimulation with 10% FBS. Cell proliferation was also analyzed using the MTT assay. VSMC were seeded on 24 well plates (1×104 cells/well) and cultured for 3 days in DMEM containing 10% FBS. VSMC were serum-starved for 48 h and then stimulated with reagents. After different treatments, 50 µl aliquots of 1 mg/ml MTT solution was added to each well (0.1 mg/well) and incubated for 4 h. Supernatants were aspirated, crystals dissolved in 200 µl dimethyl sulfoxide (DMSO), and 100 µl was placed in 96-well plates. Light absorbance at 570 nm was read on a Microplate Reader (Bio-Rad). Experiments were repeated three times, each in triplicate.
Western blot analysis
Cells were lysed in PRO-PREP protein extract solution. The sample was centrifuged at 13,000×g for 5 min at 4℃. Protein concentrations were determined by the Bradford method. An equal volume of 2× sample buffer was added to aliquots of the supernatant fraction from the lysates and the mix was boiled for 5 min. 30 micrograms of protein were loaded per lane and resolved by 10% SDS-PAGE for 1 hour 30 min at 30 mA. The separated proteins were transferred to PVDF membranes (Millipore) for 1 h at 100 V with a SD Semi-dry Transfer Cell (Bio-Rad). The membranes were blocked with 5% skim milk in 1× PBS containing 0.05% Tween 20 (PBS-T) for 1 h at room temperature. The membranes were then incubated with antibodies against HO-1, cyclin D, p21, and β-actin. Proteins were detected with a horseradish peroxidase-coupled secondary antibody using an ECL system.
FACs analysis
For cell cycle analysis, cells were harvested 48 h after stimulation in the absence or presence of aprotinin (1 µM to 10 µM), washed, then fixed in 95% ethanol overnight at 4℃, incubated with RNase A (50 µg/ml) for 30 min at 37℃ and then incubated with PI (50 µg/ml) for 30 min at 37℃. The intracellular PI fluorescence intensity of each 10,000 cells was measured in each sample using a flow cytometer (Becton Dickinson, San Jose, CA).
Transfection of VSMC
VSMC were seeded on 60 mm dishes and cultured in DMEM containing 10% FBS. A day before transfection, 1×104 cells in 60 mm plates were incubated in growth medium without antibiotics so that cells were 90~95% confluent at the time of transfection. Transfection complexes were formed as follows: mouse HO-1 DNA (µg): Lipofectamine 2,000 (µl) ratio from 1 : 0.5 to 1 : 2.5. 100 µl of complexes were added to each well containing cells and medium. The cells were then incubated at 37℃ in a CO2 incubator for 3 h.
Statistical analysis
Results are expressed as mean±SEM from at least three independent experiments. For comparison between multiple groups, statistical significance was tested by the Mann-Whitney test using SPSS version 12.0.
RESULTS
Aprotinin inhibits VSMC proliferation of SHR
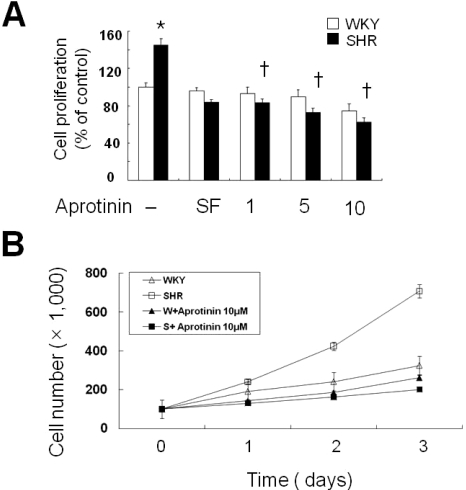
First, we confirmed that VSMC from SHR proliferated faster than those of WKY. VSMC were cultured in medium supplemented with aprotinin (1, 5, 10 µM) for 24 h. Aprotinin treatment significantly decreased VSMC proliferation of SHR compared with WKY cells (Fig. 1A). To confirm these data, we tested VSMC proliferation in medium supplement with aprotinin (10 µM) by determining cell numbers over a period of 3 days. Aprotinin treatment significantly decreased cell populations by almost three-fold at 3 days compared with WKY (Fig. 1B).
Fig. 1.
Aprotinin treatment inhibits VSMC proliferation of SHR. VSMC were treated with aprotinin (1~10 µM) for 24 h, and cell proliferation determined by MTT assay (A). Bars represent the mean±SEM of four different experiments. *p<0.01 compared with WKY control, †p<0.01 compared with SHR control. Cell were treated with or without 10 µM and cell numbers determined after 0, 1, 2 and 3 days by cell counts (B). Bars represent the mean±SEM of three independent experiments.
Aprotinin inhibits cell cycle progression in VSMC from SHR through HO-1
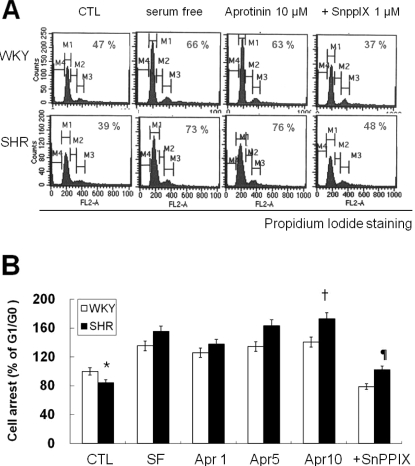
To understand the underlying mechanism of aprotinin-blocked proliferation, VSMC were serum starved and then stimulated with 10% FBS. Cell cycle progression was examined using PI staining and flow cytometry. After 10% FBS stimulation for 24 h, 47% of VSMC from SHR were at the G1/G0 phase, whereas 58% of VSMC were at the G1/G0 phase in WKY. The remaining cells were in the S or G2/M phase. More VSMC from SHR (77%) were at the G1/G0 boundary compared with WKY (63%) after aprotinin (10 µM) treatment. Aprotinin inhibits proliferation of VSMC through activation of HO-1 (Lee et al., 2009), and we could block this inhibit with the HO-1 inhibitor, SnPPIX (1 µM) (Fig. 2). These results indicate that aprotinin-induces HO-1 to inhibit VSMC proliferation by slowing down cell cycle progression and arresting cells at the G1/G0 phase in VSMC from SHR.
Fig. 2.
Aprotinin treatment inhibits cell cycle progression of VSMC from SHR through HO-1. VSMC were treated with 10 µM aprotinin or 10 µM aprotinin plus 1 µM SnppIX for 24 h, and then analyzed for DNA content by flow cytometry. The percentages of cells in G1 phase are graphically represented (A). The data are representative of three separate experiments (B). *p<0.01 compared with WKY control, †P<0.001 compared with SHR control, ¶p<0.001 compared with 10 µM aprotinin-treated SHR. Bars represents the mean±SEM of four independent experiments.
Aprotinin increases HO-1 and p21 but decreases cyclin D levels in VSMC of SHR
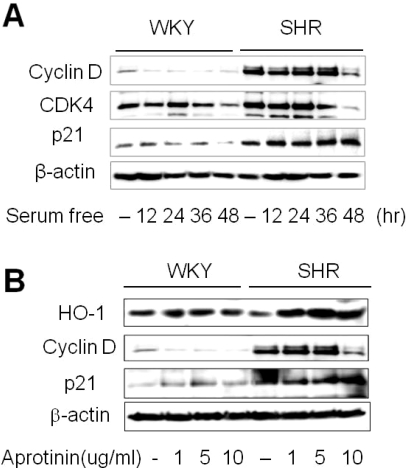
To assess cell cycle arrest, VSMC were growth arrested by serum starvation for 48 h. The basal level of cyclin D in VSMC of SHR was higher than WKY, and downregulated by serum starvation (Fig. 3A). Cyclin D association with cyclin dependent kinase (CDK)-2, CDK-4, and CDK-6 is important for early G1 progression, and CDK-4 levels were downregulated by serum starvation (Dulic, 1992). VSMCs from SHR showed higher levels of the CDK inhibitor, p21. We next tested the effect of aprotinin on key downstream regulators of the G1/G0 transition. Aprotinin (10 µM) treatment reduced cyclin D but increased p21 and HO-1 levels in VSMC from SHR (Fig. 3B). Together, these observations suggest that aprotinin-induced HO-1 could inhibit VSMC proliferation.
Fig. 3.
Aprotinin increases HO-1 and p21 but decreases cyclin D levels. Quiescent VSMC were stimulated with 10% FBS then serum starved for the indicated time, and cell lysates were processed for the detection of cyclin D, CDK4, and p21 by Western blot (A). Cells were treated with aprotinin (1~10 µM) for 48 h, and cell lysates were processed for the detection of HO-1, cyclin D, and p21 by western blot (B). The blots are representative of three independent experiments.
Aprotinin inhibited proliferation of VSMC from SHR through HO-1
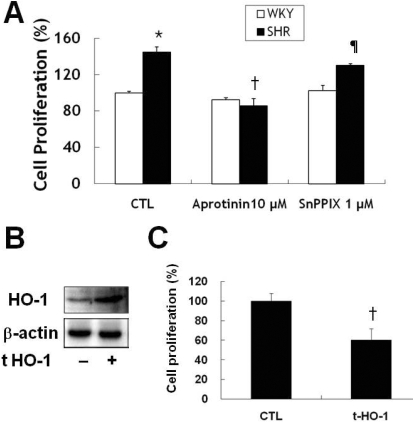
Aprotinin induced HO-1 inhibited VSMC proliferation after stimulation with 10% FBS. VSMC from SHR proliferated faster than those from WKY, and aprotinin blocked this proliferation (Fig. 4A). The HO-1 inhibitor, SnPPIX (1 µM) reversed the anti-proliferative effect of aprotinin to a greater extent in SHR than WKY, indicating that HO-1 activity may be different in the two types of rats. To confirm a direct role for HO-1 in VSMC proliferation, we transfected mouse HO-1 DNA into VSMC from SHR. This ectopic HO-1 expression significantly suppressed cell proliferation (Fig. 4B, C).
Fig. 4.
Aprotinin treatment inhibits VSMC proliferation of SHR through HO-1. VSMC were treated with 10 µM aprotinin or 10 µM aprotinin plus 1 µM SnppIX for 24 h, and cell proliferation determined by MTT assay (A). *p<0.01 compared with WKY control, †p<0.001 compared with SHR control, ¶p<0.001 compared with 10 µM aprotinin-treated SHR. Cells were transfected with mouse HO-1 DNA for 3 h and cell lysates were processed for the detection of HO-1 by Western blot (B). The blots are representative of three individual experiments. Cells were transfected with mouse HO-1 DNA for 3 h, incubated with 10% FBS for 24 h, and then cell proliferation was determined by MTT assay (C). †p<0.001 compared with SHR control. Bars represent the mean±SEM of four independent experiments.
DISCUSSION
Aprotinin is a broad-spectrum serine protease inhibitor extracted from bovine lung tissue. It is widely used to reduce blood loss and the need for transfusion after cardiac operations requiring cardiopulmonary bypass (CBP) (Bidstrup et al., 1989). However, the effects of aprotinin are not uniformly observed and may be due to the aprotinin dose utilized (Englberger et al., 2002). Although the efficacy of aprotinin as a hemostatic agent is indisputable, its safety has been questioned (Mangano et al., 2006). Recently, McEvoy and colleagues indicated that clinically relevant aprotinin concentrations in an intact murine model of ischemia reperfusion induces differential effects on left ventricular contractility, cytokine release, and oxidative stress (McEvoy et al., 2008). Another report demonstrated less postoperative bleeding, blood product transfusion, and early extubation with aprotinin use (Ngaage et al., 2008).
We recently reported that aprotinin reduced iNOS expression, cell proliferation, and reactive oxygen species through inducing HO-1 in VSMC under inflammatory conditions (Lee et al., 2009). Here, we investigated the effect of aprotinin treatment on the growth of VSMC in SHR. We first demonstrated that aprotinin induces G1/G0 arrest but not apoptosis, as measured using the MTT assay and DNA content estimation through FACS analysis. Cell proliferation was decreased by an average of 29% after 4 days of 10 µM aprotinin in VSMC of SHR (Fig. 1B). Aprotinin treatment significantly increased the fraction of cells in G1/G0 phases (Fig. 2B), down-regulated cyclin D, and upregulated p21. Interestingly, aprotinin treatment induced HO-1 protein in VSMC from SHR and reduced proliferation (Fig. 3B), consistent with our previous findings that hemin-induced HO-1 expression reduced the proliferation of VSMC of SHR under inflammatory conditions (Jeon et al., 2009) such as hypertension. To confirm the effects of HO-1 directly, we transfected cells with mouse HO-1 DNA to specifically inhibit proliferation (Fig. 4C). Pretreatment with SnPPIX, an HO-1 inhibitor, blocked the effects of aprotinin on cell proliferation (Fig. 4A) and cell cycle arrest (Fig. 2B), while SnPPIX alone had no effect.
Upregulation of p21 protein, HO-1 derived CO, and bilirubin-dependent modulation of ERK1/2 phosphorylation underlie HO-1 mediated inhibition of cell proliferation (Peyton et al., 2002; Taille et al., 2003). CDK inhibitor negatively regulates cell proliferation (Dong et al., 2004). Deletion of the p21 gene in mouse VSMC abolished the inhibitory effect of HO-1 on cell proliferation (Duckers et al., 2001). Consistent with these reports, we found that inhibition of VSMC proliferation by aprotinin was accompanied by an increased expression of p21 protein in SHR only (Fig. 3B).
In conclusion, aprotinin inhibited VSMC proliferation by decelerating cell cycle progression through arresting cells at G0/G1 phase. The induction of HO-1 mediates the inhibitory effect of aprotinin on cell growth in SHR. Changes in the level of p21 and cyclin D might also be involved in aprotinin-mediated inhibition of VSMC proliferation.
ACKNOWLEDGEMENT
This research was funded to AVDRC (R13-2005-005-01003-0) by the Korea Science and Engineering Foundation.
ABBREVIATIONS
- VSMC
vascular smooth muscle cell
- SHR
spontaneous hypertensive rats
- WKY
Wistar-Kyoto rats
- CDK
cyclin-dependent kinases
- HO-1
heme oxygenase-1
- SnPPIX
tin protoporphyrin IX
- MTT
3-(4,5 dimethylthiazole 2yl)-2,5-diphenyltetrazolium bromide thiazoyl blue
- PI
propidium iodide
- CBP
cardiopulmonary bypass
- iNOS
inducible nitric oxide synthase
- PVDF
polyvinylidene fluoride
- DMEM
dulbecco modified Eagle's minimal essential medium
- FBS
fetal bovine serum
- PBS
phosphate buffered saline
- DMSO
dimethyl sulfoxide
- SDS-PAGE
sodium dodecyl sulfate poly acrylamide gel electrophoresis
References
- 1.Bidstrup BP, Royston D, Sapsford RN, Taylor KM. Reduction in blood loss and blood use after cardiopulmonary bypass with high dose aprotinin (Trasylol) J Thorac Cardiovasc Surg. 1989;97:364–372. [PubMed] [Google Scholar]
- 2.Chang T, Wu L, Wang R. Inhibition of vascular smooth muscle cell proliferation by chronic hemin treatment. Am J Physiol Heart Circ Physiol. 2008;295:H999–H1007. doi: 10.1152/ajpheart.01289.2007. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, Chi SL, Borowsky AD, Fan Y, Weiss RH. Cytosolic p21Waf1/Cip1 increases cell cycle transit in vascular smooth muscle cells. Cell Signal. 2004;16:263–269. doi: 10.1016/s0898-6568(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 4.Duckers HJ, Boehm M, True al., Yet SF, San H, Park JL, Clinton Webb R, Lee ME, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 5.Dulic V, Lees E, Reed SI. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 6.Englberger L, Kipfer B, Berdat PA, Nydegger UE, Carrel TP. Aprotinin in coronary operation with cardiopulmonary bypass: does "low-dose" aprotinin inhibit the inflammatory response? Ann Thorac Surg. 2002;73:1897–1904. doi: 10.1016/s0003-4975(02)03535-x. [DOI] [PubMed] [Google Scholar]
- 7.Hunter T. Braking the cycle. Cell. 1993;75:839–841. doi: 10.1016/0092-8674(93)90528-x. [DOI] [PubMed] [Google Scholar]
- 8.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 9.Jeon EM, Choi HC, Lee KY, Chang KC, Kang YJ. Hemin inhibits hypertensive rat vascular smooth muscle cell proliferation through regulation of cyclin D and p21. Arch Pharm Res. 2009;32:375–382. doi: 10.1007/s12272-009-1310-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee DH, Choi HC, Lee KY, Kang YJ. Aprotinin inhibits vascular smooth muscle cell inflammation and proliferation via induction of HO-1. Korean J Physiol Pharmacol. 2009;13:123–130. doi: 10.4196/kjpp.2009.13.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy JH. Pharmacologic preservation of the hemostatic system during cardiac surgery. Ann Thorac Surg. 2001;72:S1814–S1820. doi: 10.1016/s0003-4975(01)03218-0. [DOI] [PubMed] [Google Scholar]
- 12.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 13.McEvoy MD, Taylor AG, Zavadzkas JA, Mains IM, Ford RL, Stroud RE, Jeffords LB, Beck CU, Reeves ST, Spinale FG. Aprotinin exerts differential and dose-dependent effects on myocardial contractility, oxidative stress, and cytokine release after ischemia-reperfusion. Ann Thorac Surg. 2008;86:568–575. doi: 10.1016/j.athoracsur.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndisang JF, Wu L, Zhao W, Wang R. Induction of heme oxygenase-1 and stimulation of cGMP production by hemin in aortic tissues from hypertensive rats. Blood. 2003;101:3893–3900. doi: 10.1182/blood-2002-08-2608. [DOI] [PubMed] [Google Scholar]
- 15.Ndisang JF, Zhao W, Wang R. Selective regulation of blood pressure by heme oxygenase-1 in hypertension. Hypertension. 2002;40:315–321. doi: 10.1161/01.hyp.0000028488.71068.16. [DOI] [PubMed] [Google Scholar]
- 16.Ngaage DL, Cale AR, Cowen ME, Griffin S, Guvendik L. Aprotinin in primary cardiac surgery: operative outcome of propensity score-matched study. Ann Thorac Surg. 2008;86:1195–1202. doi: 10.1016/j.athoracsur.2008.06.048. [DOI] [PubMed] [Google Scholar]
- 17.Peyton KJ, Reyna SV, Chapman GB, Ensenat D, Liu XM, Wang H, Schafer AI, Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002;99:4443–4448. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]
- 18.Resink TJ, Scott-Burden T, Baur U, Bühler FR. Increased proliferation fate and phosphoinositide turnover in cultured smooth muscle cells from spontaneously hypertensive rats. J Hypertens Suppl. 1987;5:S145–S148. [PubMed] [Google Scholar]
- 19.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 20.Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 21.Taillé C, Almolki A, Benhamed M, Zedda C, Mégret J, Berger P, Leséche G, Fadel E, Yamaguchi T, Marthan R, Aubier M, Boczkowski J. Heme oxygenase inhibits human airway smooth muscle proliferation via a bilirubin-dependent modulation of ERK1/2 phosphorylation. J Biol Chem. 2003;278:27160–27168. doi: 10.1074/jbc.M300364200. [DOI] [PubMed] [Google Scholar]
- 22.Tanner FC, Greutert H, Barandier C, Frischknecht K, Lüscher TF. Different cell cycle regulation of vascular smooth muscle in genetic hypertension. Hypertension. 2003;42:184–188. doi: 10.1161/01.HYP.0000082360.65547.7C. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Shamloul R, Wang X, Meng Q, Wu L. Sustained normalization of high blood pressure in spontaneously hypertensive rats by implanted hemin pump. Hypertension. 2006;48:685–692. doi: 10.1161/01.HYP.0000239673.80332.2f. [DOI] [PubMed] [Google Scholar]
- 24.Westaby S. Aprotinin in perspective. Ann Thorac Surg. 1993;55:1033–1041. doi: 10.1016/0003-4975(93)90149-c. [DOI] [PubMed] [Google Scholar]