Abstract
Astragalus Membranaceus (AM) is a useful Korean herb that has been clinically prescribed for stress-related illness. The objective of the present study was to examine the anti-stress effects of AM on repeated stress-induced alterations of anxiety, learning and memory in rats. Restraint stress was administered for 14 days (2h/day) and AM (400mg/kg) given by oral administration, in the AM group, for the same period. Starting on the eighth day, the rats were tested for spatial memory on the Morris water maze test (MW) and for anxiety on the elevated plus maze (EPM). Changes of expression on immunohistochemistry were studied for cholineacetyl transferase (ChAT) and tyrosine hydroxylase (TH) in the brain. The results showed that the rats treated with AM had significantly reduced stress-induced deficits on learning and memory on the spatial memory tasks. In addition, the ChAT immunoreactivities were increased. In the EPM, treatment with AM increased the time spent in the open arms (p<0.001) compared to the control group. In addition, AM treatment also normalized increases of TH expression in the LC (p<0.001). In conclusion, administration of AM improved spatial learning and memory and reduced stress-induced anxiety. Thus, the present results suggest that AM is able to recover behavioral and neurochemical impairments induced by stress.
Keywords: Astragalus Membranaceus, Morris water maze, Elevated plus maze
INTRODUCTION
Restraint stress is an easy and well-known method used to induce chronic physical and emotional stress (Chrousos, 1998). The emotional and physiological responses to repeated restraint stress are initiated by activation of the hypothalamic-pituitary adrenal axis (HPA), which results in the release of catecholamines and stress hormones such as glucocorticoids from the adrenal glands (Chrousos, 1998). Elevated levels of glucocorticoids result in both short and long-term negative effects on the brain and behavior (Pare, 1964; Chrousos, 1998). At the behavioral level, exposure to stress has been reported to increase anxiety-related behavior, and to impair learning and memory (Luine et al., 1994; File, 1980). Many animal studies have demonstrated that chronic stress, as well as chronic corticosterone treatment, results in learning and memory deficits on several behavioral tasks, including the water-maze and passive-avoidance tests, and impairment was accompanied by hippocampal damage (Luine, 1994; Bondnoff et al., 1995; Sousa et al., 1998; Bisagno et al., 2004; Coburn-Litvak et al., 2004; Krugers et al., 2006; Mclay and Klinski, 2008). In addition, Ader and Cohen (1993) and McEwen and Stellar (1993) reported that repeated stress is a risk factor for psychosomatic psychiatric illness, such as anxiety (Ader and Cohen, 1993; McEwen and Stellar, 1993).
Astragalus Membranaceus (AM) is used mainly as a tonic in traditional Korean medicine. The biological and pharmacological properties of AM cover a wide spectrum of actions. AM has been prescribed for centuries for general weakness, chronic illness, and to increase overall vitality. The main constituents of AM include polysaccharides, saponins, flavonoids, amino acids, and trace elements. Currently much of the human research on AM has reported that it and other active ingredients are useful for treating immune deficiencies (Mills and Bone, 2000). However, few studies have reported that treatment with AM improves deficits in learning and memory and decrease anxiety like behavior in repeatedly stressed rats.
The aim of the present study was therefore to investigate the effects of AM on repeated restraint stress induced anxiety and memory loss in rats by evaluating their performance on the elevated plus maze and Morris water maze. In addition, changes in ChAT and TH expression in the brain were evaluated in order to elucidate the possible mechanisms involved in the anti-stress effects identified.
METHODS
Subjects and stress procedures
Male Sprague Dawley rats at the age of 8 weeks (Orient, Inc. Korea) were used for the study. The rats were housed under controlled temperature (22~24℃) conditions with a 12 h light/dark cycle. The lights were on from 8:00 to 20:00. Food and water were made available ad libitum. All experiments were approved by the Catholic University Institutional Animal Care and Use Committee. They were allowed at least 1 week to adapt to their environment before the experiments. The female rats were randomly divided into three groups (n=6 per group): the nonstressed group (normal), the stressed group (control), and the stressed and Astragalus Membranaceus treatment group (AM). The rats were stressed daily for two weeks using the restraint cone. The AM group was treated daily with AM extract (400 mg/kg, p.o.) for two weeks, and the other groups were given sterile saline. Immobilization was started 30 min after the treatments.
Preparation of herbal extracts
The Astragalus Membranaceus was purchased from an oriental drug store (Jungdo Inc. Seoul, Korea). The specimens were deposited at the herbarium located in the College of Oriental Medicine, Kyung Hee University. The dried Astragalus Membranaceus samples (200 g) were immersed in a 10-fold volume of dH2O, boiled at 80℃ for 1 h, and then the water extract was collected. The process was repeated once, and the extracts were combined and concentrated with a rotary evaporator and vacuum-dried to yield 9.1% (w/w) of the extract.
Elevated plus maze
The plus-maze apparatus was constructed with black wood. It consisted of two open-arms (the arms extended from a central 50×10 cm space) and two enclosed arms (50×10×40 cm). The arms extended from a central platform (10×10 cm). The apparatus was elevated 50 cm above the floor. The animals were transported to the testing room at least 1 hr prior to starting the experiment.
After all of the stress sessions, the rats were individually placed in the central platform facing a closed arm and they were allowed to explore the maze for a 5-min test period. The duration of time spent in the open arms and closed arms were the behavioral measures that were recorded for each rat. The apparatus was wiped clean with a damp sponge and dried with paper towels between tests.
Morris water maze test
training on the MWM task in a swimming pool (1.8 m diameter and 0.5 m high, filled with milky water at a temperature in the 22±2℃ range) for 7 d. A 12 cm diameter round platform was hidden in a constant location (the quadrant center) within the pool with its top surface submerged 1.5 cm below the water level. The rats were trained to locate the hidden island during four trials per day for 6 d. After the 6 d, they were started in the quadrant opposite to the target and were forced to swim for 60 s in the pool without a platform. The spatial memory of the rats was assessed as the latency time. The time spent searching for the platform in the training quadrant, i.e., the previous location of the platform, was recorded and used as a measure of memory retention. A video camera was mounted on the ceiling above the pool and was connected to a video-recorder and tracking device (S-MART; Pan-Lab, Barcelona, Spain), which permitted on-line and off-line automated tracking of the path taken by the animal (Morris, 2000).
Immunohistochemistry
After the behavioral tests were completed, the animals were anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and then perfused transcardially with 100 ml of saline followed by 500 ml of a 4% solution of formaldehyde prepared in phosphate buffer. The brains were then removed, post-fixed in the same fixative for two to three hours at 4℃ and then placed overnight at 4℃ in PBS containing 20% sucrose. The following day, the brain was cut into coronal sections that were sliced to 30 µm-thicknesses. The sections were processed for ChAT immunoreactivity using sheep ChAT polyclonal antibodies (ChAT, concentration 1:2,000; Chemicon international, Temecula, CA.,USA) and tyrosine hydroxylase (TH) immunoreactivity using mouse-TH monoclonal antibodies (TH, concentration 1:2,000; Zymed Laboratories INC. San Francisco, CA., USA). The sections were then processed by a conventional avidin-biotin-peroxidase method (Vector Laboratories, Burlingame, CA, U.S.A.). The tissue was developed using diaminobenzadine (Sigma U.S.A.) as the chromogen. The sections were mounted on gelatine-coated slides, air-dried and coverslipped for microscopic analysis. A microrectangular grid (200×200 µm) was placed according to the atlas of Paxinos and Watson (Paxinos et al., 1985) under the light microscope (×100 magnification) to measure the cells.
Statistical analysis
Statistical comparisons were performed on the results of the behavioral and histochemical studies using the one-way ANOVA, repeated measures of the two-way ANOVA, and the LSD post hoc test. All of the results are presented as the means±S.E.M. SPSS 15.0 software for Windows was used for the statistical analysis. The significance level was set at a p<0.05.
RESULTS
Effects of AM on the elevated plus maze
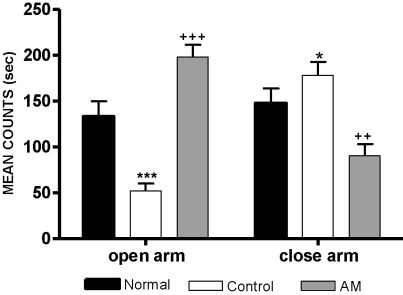
As shown in Fig. 1, the one-way ANOVA performed for the time spent in the closed arms and the time spent in the open arms of the elevated maze revealed significant group differences. The LSD test indicated that the control group spent significantly less time in the open arms [F2,15=27.402, p<0.001] and increased time in the closed arms [F2,15=5.094, p<0.05] compared to the normal group. After administration of AM, the time spent in the open arms was significantly increased and the time spent in the closed arms was decreased in the AM group compared to the control group (p<0.001, in the open arms and p<0.01, in the closed arms).
Fig. 1.
Time spent in the open and closed arms in the elevated plus maze maze. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of elevated plus maze maze were analyzed by performing separate one-way ANOVA among the groups were followed by LSD test. Each value represents the mean±S.E.M. *p<0.05, ***p<0.001 compared to normal group and ++p<0.01, +++p<0.001 compared to control group, respectively.
Effects of AM on the Morris water maze test
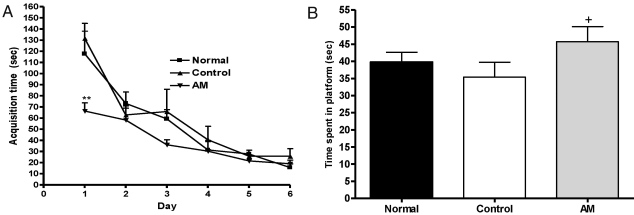
Fig. 2A shows the mean group latencies recorded to reach the hidden platform in the MWM for all groups over 6-days [F2,15=35.574, p<0.05]. On the first day, the AM group had a significant decrease in the time spent searching for the platform compared to the control group (p<0.01). However, after the second day, there were no statistically significant differences among the groups. There was a slight trend for a significant interaction effect on the distance traveled to reach the platform.
Fig. 2.
(A) Changes of the latency time during 6 d of the acquisition test in the Morris water maze test. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. Repeated measures of two-way ANOVA of swimming time among the groups following by LSD test. Each value represents the mean±S.E.M. **p<0.01 compared to normal group. (B) The latency time on the 7th day of the retention test in the Morris water maze test. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of retention test were analyzed by performing separate measures of one-way ANOVA of swimming time among the groups were followed by LSD test. Each value represents the mean±S.E.M. +p<0.05 compared to control group.
To examine the spatial memory, on the seventh day of retention testing, the time spent swimming to the platform was compared among the groups as seen in Fig. 2B. The times spent around the platform were significantly different among the groups [F2,15=4.859, p<0.01]; the AM group spent more time around the platform than the control group (p<0.05).
Effects of AM on the cholinergic system
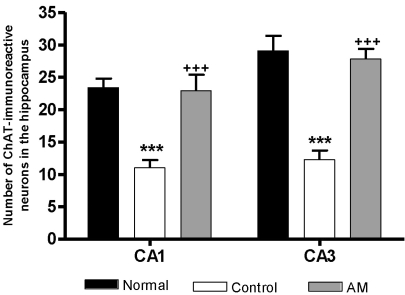
The results of the evaluations of the ChAT immunoreactive cells per section, from different hippocampal formations, are shown in Fig. 3. The number of ChAT neurons in the CA1 area was 23.4±1.4 in the normal group, 11.0±1.2 in the control group, and 22.9±2.5 in the AM group [F2,23=16.6, p<0.001]; these results show a two-fold increase in the number of ChAT neurons in the AM group compared to the controls (p<0.001). The ChAT immunoreactive cells in the CA3 area were: 29.1±2.3 in the normal group, 12.3±1.4 in the control group, and 27.8±1.6 in the AM group [F2,23=20.1, p<0.01]. Thus, the number of ChAT positive neurons in the AM group was increased by 236.7% compared to the control group (p<0.001).
Fig. 3.
Number of choline acetyltransferase (ChAT) immunostained nuclei in the different hippocampal areas of the experimental groups after 8 d of the behavior test. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments. The results of ChAT-reactivity were analyzed by performing separate one-way ANOVA of neurons among the groups were followed by LSD test. Each value represents the mean±S.E.M. ***p<0.001 compared to normal group and +++p<0.001 compared to control group.
Effects of AM on the catecholaminergic system
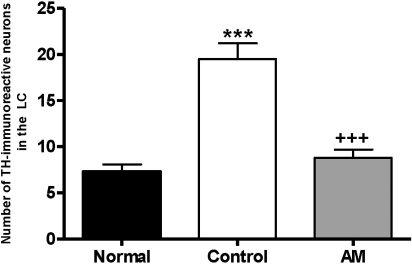
The results of the TH immunoreactive cells per section, from the locus coeruleus, are shown in Fig. 4. The TH immunoreactive cells in the LC area were: 7.3±0.8 in the normal group, 19.5±1.7 in the control group, and 8.8±0.9 in the AM group [F2,28=30.6, p<0.001]. Thus, the number of TH positive neurons in the AM group was decreased by 45.0% compared to the control group (p<0.001).
Fig. 4.
Number of Tyrosine hydroxylase (TH) immunostained nuclei in the locus coerleus areas of the experimental groups. The AM group was daily treated with the AM extract (400 mg/kg, p.o.) for 2 weeks, and other groups were given sterile saline. Immobilization began 30 min after the treatments.The results of TH-reactivity were analyzed by performing separate one-way ANOVA of neurons among the groups were followed by LSD test. Each value represents the mean±S.E.M. ***p<0.001 compared to normal group and +++p<0.001 compared to control group.
DISCUSSION
The main findings of this study were that treatments with the crude extract of AM reduced repeated stress induced anxiety and memory loss. Moreover, treatment with AM significantly suppressed the increases of TH expression and enhanced the ChAT expression in the stressed rats.
The restraint stress used in this study is widely used in studies on the molecular and behavioral effects of chronic stress, and has been very helpful in improving our understanding of stress-related brain pathology and changes in cognition (Chrousos, 1998). Restraint elicits a variety of physiological stress responses; the magnitude of these responses can be decreased or increased by prior stress history (Ader and Cohen, 1993; Chrousos, 1998; Wang et al., 2008). To establish a practical animal model for chronic stress, we examined the behavioral traits, anxiety and memory loss, as stress-assessment parameters. We found that 2 h~14 days of restraint stress significantly induced anxiety and memory loss, and that repeated restraint stress changed the expression of neurotransmitters in the brain.
The degeneration of the cholinergic innervations from the basal forebrain to the hippocampal formation, in the temporal lobe, is thought to be one of the factors involved in the determination of the progression of memory decay, both during normal aging and AD (Morris, 2000). The best available marker for cholinergic neurons in the basal forebrain is the ChAT activity. ChAT synthesizes the neurotransmitter acetylcholine in the basal forebrain, cortex, hippocampus, and amygdala. A significant reduction in ChAT activity has been reported in the postmortem brains of mentally impaired patients. In addition, Gottesfeld et al. (1978). showed a decrease in ChAT activity in the hippocampus after repeated immobilization stress (Gottesfeld et al., 1978). However, in the present study, treatment with AM prevented stress-induced loss of ChAT-immunoreactive neurons, suggesting that AM exerts beneficial effects on cholinergic neurotransmission in the brain by increasing ChAT activity in the hippocampus. The behavioral and neurochemical results of this study indicate that the memory-enhancing effects of AM may be mediated by cholinergic mechanisms in the rat brain.
Most norepinephrine (NE)-containing neurons in the brain are concentrated in the nucleus locus coeruleus (LC) (Dinan and Aston-Jones, 1984; Sabban, 2007). These neurons are part of a widespread network with a broad range of functions that extends throughout the neuroaxis and accounts for about 70% of all brain NE in primates (Dinan and Aston-Jones, 1984). Activation of the LC produces intense anxiety, hypervigilance and an inhibition of exploratory behavior (Redmond and Huang, 1982; Dinan and Aston-Jones, 1984; Foote et al., 1991). Based on these data, it has been proposed that clinical anxiety or depression may be the result of alterations in the activity of the LC noradrenergic system. The LC is considered a crucial site for the CNS stress response. An abundance of data collected using different techniques has shown that the corticotropin releasing hormone can modulate the rate of discharge of NE and TH in LC neurons (Valentino and Foote, 1987; Melia et al., 1992; Valentino et al., 1993; Ma S et al., 2008). In this study, the repeated restraint stress caused a 35.0% increase in the TH-ir cells in the LC. However, treatment with AM significantly reduced the expected increases of the TH-ir cells in the LC. These results suggest that treatment with AM effectively decreased over-expressed TH-ir neurons in the LC.
In conclusion, the results of this study show that extract of AM possesses anti-stress properties in rats. However, further studies are necessary to confirm and extend these results. These findings provide evidence that AM is useful for stress reduction under certain circumstances.
ACKNOWLEDGEMENTS
This study was supported by the Seoul R&D Program (10829) and the Cognitive Neuroscience Program of the Korea Ministry of Science and Technology (M10644000017-06N4400-01710), Republic of Korea.
ABBREVIATIONS
- AM
Astragalus Membranaceus
- ChAT
choline acetyltransferase
- TH
tyrosine hydroxylase
References
- 1.Ader R, Cohen N. Psychomeuroimmunology: conditioning and stress. Annu Rev Psychol. 1993;44:53–85. doi: 10.1146/annurev.ps.44.020193.000413. [DOI] [PubMed] [Google Scholar]
- 2.Bisagno V, Grillo CA, Piroli GG, Giraldo P, MaEwen B, Luine VN. Chronic stress alters amphetamine effects on behavior and synaprophysin levels in female rats. Pharmacol Biochem Behav. 2004;78:541–550. doi: 10.1016/j.pbb.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticoserone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrousos GP. Stressors, stress, and neuroendocrinology integration of the adaptive response. The 1997 Hans Selye Memorial lecture. 1998;851:311–335. doi: 10.1111/j.1749-6632.1998.tb09006.x. [DOI] [PubMed] [Google Scholar]
- 5.Coburn-Litvak PS, Tata DA, Gorby HE, McCloskey DP, Richardson G, Anderson BJ. Chronic corticosterone affects brain weight, and mitochondrial, but nor glial volume fraction in hippocampal area CA3. Neuroscience. 2004;124:429–438. doi: 10.1016/j.neuroscience.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Dinan TG, Aston-Jones G. Acute haloperidol increases impulse activity of brain noradrenergic neurons. Brain Res. 1984;307:359–362. doi: 10.1016/0006-8993(84)90495-5. [DOI] [PubMed] [Google Scholar]
- 7.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlrodiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 8.Foote SL, Berridge CW, Adams LM, Pineda JA. Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog Brain Res. 1991;88:521–532. doi: 10.1016/s0079-6123(08)63831-5. [DOI] [PubMed] [Google Scholar]
- 9.Gottesfeld Z, Kvetnansky R, Kopin IJ, Jacobowitz DM. Effects of repeated immobilization stress on glutamate decarboxylase and choline acetyltransferase in discrete brain regions. Brain Res. 1978;152:374–378. doi: 10.1016/0006-8993(78)90267-6. [DOI] [PubMed] [Google Scholar]
- 10.Krugers HJ, Goltstein PM, van der Linden S, Joels M. Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. Eur J neurosci. 2006;23:3051–3055. doi: 10.1111/j.1460-9568.2006.04842.x. [DOI] [PubMed] [Google Scholar]
- 11.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversibal impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 12.Ma S, Mifflin SW, Cunningham JT, Mortilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitarydrenal stress reactivity and Fos induction in the rat locus coerleus in response to subsequent immobilization stress. Neuroscience. 2008;154:1639–1647. doi: 10.1016/j.neuroscience.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS, Stellar E. Stress and the individual:mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- 14.Mclay R, Klinski A. Changes in prescription habits with the introduction of generic fluoxetine. Mil Med. 2008;173:100–104. doi: 10.7205/milmed.173.1.100. [DOI] [PubMed] [Google Scholar]
- 15.Melia KR, Rasmussen RZ, Terwillinger JW, Haycook EJ, Nestler RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase the rat locus coeruleus: Effects of chronic stress and drug treatment. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 16.Mills S, Bone K. Principles and practice of phytotherapy. 1st ed. Edinburgh, Scotland: Churchill Livingstone; 2000. PART2: PRACTICAL CLINICAL GUIDES: Dosage Issues. Herbal Approaches to Pathological States. Herbal Approaches to System Dysfunctions. A Systematic Approach to Herbal Prescribing; pp. 27–60. [Google Scholar]
- 17.Morris RG. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 18.Pare WP. The effects of chronic environmental stress and stomach ulceration, adrenal function and consummatory behavior in the rat. J Psychol. 1964;57:143–151. doi: 10.1080/00223980.1964.9916683. [DOI] [PubMed] [Google Scholar]
- 19.Paxinos G, Watson C, Pennisi M. Bregma, lamda and the interaural midpoint in stereotaxic surgery with rats of different sex, Strain and Weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 20.Redmond DE, Jr, Huang YH. The primate locus coerleus and effects of clonidine on opiate withdrawal. J Clin Psychiatry. 1982;43:25–29. [PubMed] [Google Scholar]
- 21.Sabban EL. Catecholamines in stress: molecular mechanisms of gene expression. Endocr Regul. 2007;41:61–73. [PubMed] [Google Scholar]
- 22.Sousa N, Almeida OF, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 1998;2:237–249. doi: 10.3109/10253899809167288. [DOI] [PubMed] [Google Scholar]
- 23.Valentino RJ, Foote SL. Corticotropin-releasing factor distrups sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- 24.Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotrophin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang YT, Tan QR, Sun LL, Cao J, Dou KF, Xia B, Wang W. Possible therapeutic effect of a traditional chinese medicine, sinisan, on chronic restraint stress related disorders. Neurosci Lett. 2008;449:215–219. doi: 10.1016/j.neulet.2008.10.100. [DOI] [PubMed] [Google Scholar]