Abstract
Anti-inflammatory factor (AIF) is a water soluble extract of three herbs, Panax notoginseng (Burk.) F. H. Chen, Rehmannia glutinosa Libosch and Eleutherococcus senticosus. The present study aimed to investigate the safety and efficacy of herb extracts, AIF, on Korean knee osteoarthritis patients for six weeks. Fifty seven patients with knee osteoarthritis, ranging from 43 to 73 years of age, who fulfilled the "American College of Rheumatology" (ACR) classification of idiopathic osteoarthritis of knee and radiographic criteria were randomly selected and enrolled for the study. After initial screening and resting period, two capsules each of AIF (Each capsule contains; 400 mg) and similar identical placebo were administered twice a day to both groups. Pain intensity at second, fourth, and sixth weeks of study as well as one week after discontinuation of drugs was assessed by using 100 mm visual analogue scale (VAS). Changes in the Korean version of the Western Ontario and McMaster Universities (K-WOMAC) index score were compared at the initiation and completion of the study. VAS assessed by patients were significantly reduced (at visit 2; 54.64±14.72, at visit 4, 37.32±16.58, p< 0.001) after AIF administration. Results showed an improvement in the physical function of K-WOMAC scale which was significantly higher (p=0.013) in AIF than placebo group, and decreases of total K-WOMAC score were also significantly higher (p=0.030) in AIF groups than placebo group. No serious adverse effect was observed, and there was no difference in incidence of adverse effect between AIF and placebo groups. In this population of Korean patients with knee osteoarthritis, AIF was found to be safe, tolerable and effective for symptomatic improvement of pain and physical function.
Keywords: Anti-inflammatory factor, Clinical trial, Herbs, Osteoarthritis, K-WOMAC
INTRODUCTION
Osteoarthritis is a multifactorial disease with a high morbidity rate that is characterized by degradation of matrix and destruction of articular cartilage (Martel-Pelletier et al, 2004). It is one of the most common musculoskeletal disorders and has a substantial economic effect. The prevalence of osteoarthritis increases with age, and it may affect as many as 68% of patients over the age of 65 years (Lawrence et al, 1998; Garstang and Stitik, 2006).
Management of osteoarthritis may be subdivided into non-pharmacological, pharmacological, and surgical forms, which must be tailored according to individual risk factors, and the severity of joint pain and deformity. Moreover, optimal treatment often requires a combination of non-pharmacological and pharmacological modalities (Peat et al, 2001; Zhang et al, 2007).
Of the medical therapies available, analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) have been proven to be highly effective in controlling the symptoms and signs of osteoarthritis, however, they have potential gastrointestinal (GI) adverse effects, and alternatives, such as COX-2 selective NSAIDs like Celecoxib or combinatorial treatment with GI protective agents, are expensive (Ofman, 2002). In osteoarthritis, the degradation of collagen type II is an irreversible process, and it is believed that collagenase-1 and MMP-1 are primarily responsible. Recently, another human collagenase, named collagenase-3 (also called MMP-13), has been identified, and our laboratory showed that it is also involved in the pathophysiology of osteoarthritis (Peat et al, 2001). Furthermore, proinflammatory cytokines, such as IL-1 and TNF-α, have significant effects on chondrocytes. They are able to; (i) increase enzyme synthesis, (ii) inhibit the synthesis of major physiological inhibitors of these enzymes, and (iii) inhibit the synthesis of matrix constituents, such as collagen and proteoglycans.
AIF (Code name, Oscotec Inc., Cheonan, Korea) is a water soluble extract of three herbs, i.e., Panax notoginseng (Burk.) F. H. Chen., Rehmannia glutinosa Libosch and Eleutherococcus senticosus, which have widely been used as herbal medicines in Eastern Asia for more than 2000 years, and their dosage limits have already been characterized. Panax notoginseng (Burk.) F. H. Chen. was selected because it markedly inhibits tumor necrosis factor-alpha (TNF-α) secretion in-vitro (Chang et al, 2007; Ling et al, 2008). Moreover, Rehmannia glutinosa Libosch and Eleutherococcus senticosus are used to treat arthritis. In addition, AIF was previously found to have a suppressive effect on TNF-α, interleukin-1 (IL-1), and matrix metalloproteinase-13 (MMP-13) in a collagen-induced arthritis model (CIA) (Kim et al, 1999; Chang et al, 2005). In preclinical study performed in GLP organization, AIF showed no toxicity in general toxicology.
In this study, we conducted a clinical trial on AIF for the treatment of osteoarthritis and compared its symptomatic outcomes with those of placebo treated group.
METHODS
Study population
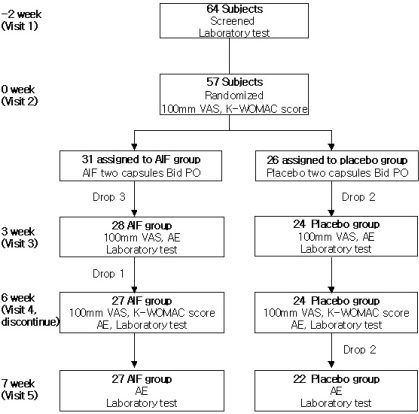
Subjects were recruited at Catholic University Medical Center through bulletin board and poster advertisements. A total 64 patients (ages ranging from 43 to 73 years) with osteoarthritis of the knee, according to the "American College of Rheumatology" (ACR) classification of idiopathic osteoarthritis of knee with radiographic criteria of grade I and grade II, were enrolled (Kellgren and Lawrence, 1957; Altman et al, 1986). All study subjects were required to stop all analgesic medication and physical therapy at least for 1 week prior to commencement of study. Female patients at risk of becoming pregnant were excluded, as were patients with serious comorbid illnesses, such as cardiovascular, gastrointestinal, infectious, autoimmune, rheumatologic, or neoplastic diseases or abnormal laboratory findings according to a screening test (Fig. 1).
Fig. 1.
Schema of patients in the study.
Study design
This randomized, double blind, placebo-controlled study was conducted over the period of 7 weeks, and the study was approved by the institutional review board of the Catholic University of Daegu School of Medicine. All patients provided written informed consent. Furthermore, the study was performed in accordance with the Declaration of Helsinki and its recommendations.
During pre-study screening, subjects underwent complete history taking and a physical examination. Laboratory tests included; simple radiography of the knee, complete blood cell count, blood chemistry, urinalysis, and a urine pregnancy test (premenopausal women). Simple radiography of the affected knee was also performed. Radiographic scores were classified as; grade 1 - possible osteophyte, grade 2 - definite osteophyte and possible narrowing of the joint space, grade 3 - multiple osteophytes and definite narrowing of joint space, and grade 4 - severe sclerosis and definite deformity of bone contour (Kellgren and Lawrence, 1957).
Supplements administration
After initial screening and resting period, the 57 participants were randomly assigned to one of two groups: AIF and placebo, respectively. The AIF participants were treated with the formulation which contained 200 mg of AIF, 192 mg of corn starch, 4 mg of HPMC, 4 mg of magnesium stearate in each capsule. The placebo participants were treated with the formulation of 392 mg of corn starch, 4 mg of HPMC, 4 mg of magnesium stearate in each capsule. The all groups were administered two capsules bid for 6 weeks. The contents of standard material in the AIF were as follows: Ginsenoside Rb1, 19.49±3.89 mg/g, stachyose 0.87±0.17 mg/g and eleutheroside E 0.07±0.014 mg/g.
During study period, systemic or intra-articular steroids, oral anticoagulants, and prolonged use of antacids or drugs affecting gastrointestinal motility were prohibited. AIF and placebo were administered by a pharmacist who was unaware of the study details. We suggested that the participants keep to a normal diet, and prohibited any medication or herbal preparation without permission.
Outcome measures and assessment of efficacy
Subjects in the AIF and placebo groups were assessed at weeks 0 (baseline), 3 and 6 after initiation. AEs after completion of AIF administration were assessed again at seventh week. Pain intensities at weeks 0, 3, and 6 were assessed using a 100 mm visual analogue scale (VAS) by patients, and K-WOMAC scores at weeks 0 and 6 were compared by consensus between two investigators (SH Park, JY Choe) (Bae et al, 2001).
All AEs that occurred during the study period were recorded and classified using guideline of Spiker et al. (1992).
Statistical analysis
Statistical analysis was performed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL). The analysis was performed on a per-protocol (PP) and intention to treat (ITT) basis. Repeated measures two factor analysis was used to compare temporal differences. Intra-group differences were analyzed using the paired t-test, and the sample t-test was used to analyze inter-group differences. Treatment efficacies were compared using the Chi-square (χ2) test. Inter-group K-WOMAC score changes were compared using the two sample t-test. Discontinued case data were analyzed using LOCF (Last-Observation-Carried-Forward) analysis.
RESULTS
Baseline characteristics of study population
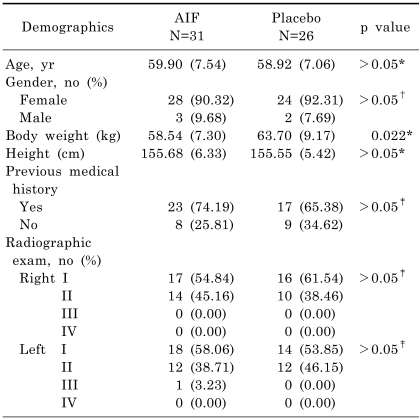
Sixty four patients were recruited, and 57 patients were randomly allocated to either AIF (31 patients) or placebo (26 patients) groups. Seven of the original 64 patients were not enrolled due to screening failure (three patients) or consent withdrawal (four patients) (Fig. 1). Baseline characteristics, such as age, gender, and medical history, were similar in the two groups (Table 1). Radiographic severities of both knee joints were also similar.
Table 1.
Patient baseline characteristics
Values are means (SD). *t-test, †Fisher's exact test, ‡Chi-square test.
Adverse events
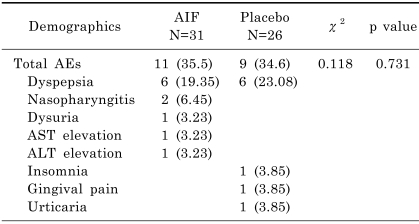
The most common adverse events (AEs) in the AIF group were gastrointestinal in nature, such as dyspepsia, and 6 patients in both groups were affected (Table 2). In the AIF group, 2 patients withdrew due to an AE; 1 patient for dyspepsia and 1 patient due to a sense of dysuria. In the placebo group, 2 patients withdrew; 1 for dyspepsia and 1 for insomnia. Liver enzyme elevation was observed in 1 patient transiently less than twice the upper normal limit, and returned to normal at sixth week. No serious AEs were reported.
Table 2.
Summary of adverse events (AEs)
Values are number (%) of patients. AST, alanine aminotransferase; ALT, aspartate aminotransferase.
Effectiveness for relieving pain
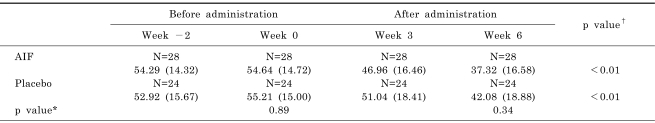
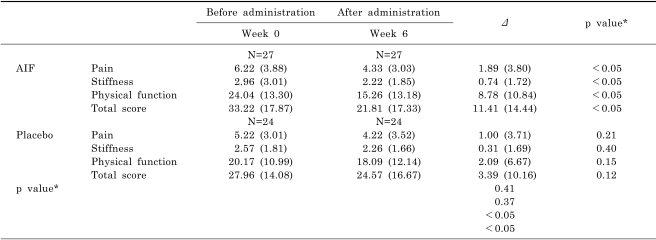
Repeated measures two factor analysis showed no interaction between the two groups in terms of longitudinal VAS changes (data not shown). Decreases in VAS scores were significant in both study groups (p<0.01 between week 0 and week 6), and the rate of response to AIF was 4.76 percentage points higher at 6 weeks than placebo group, however no significant difference was found between the two groups (p=0.89 at week 0, 0.34 at week 6) because of significantly high placebo effect in the placebo group between week 0 and 6 (p<0.01) (Table 3). Assessments of K-WOMAC scores at weeks 0 and 6 showed significant improvements in pain, stiffness, physical function and total scores in the AIF group only. AIF was found to be significantly more effective for improving physical function and total K-WOMAC scores than the placebo (Table 4).
Table 3.
Changes of VAS scores as assessed by patients
Values are means (SD). *two sample t-test, †Week 0, Week 6. paired t-test, LOCF.
Table 4.
Changes of K-WOMAC scale scores
Values are means (SD). *two sample t-test (AIF - Placebo), †Week 0, Week 6. paired t-test, Δ Week 0- Week 6.
DISCUSSION
Degeneration of articular cartilage is a characteristic of the pathogenesis of Osteoarthritis. This degeneration is due to enzymatic breakdown of collagen and proteoglycan, and MMPs play a dominant role.
During the clinical stage of osteoarthritis, morphological changes include variable degree of synovial inflammation, which in turn produces inflammatory mediators like IL-1 and TNF-α that play pivotal roles in mediating the pathophysiological mechanisms of osteoarthritis. As mentioned above, the MMP family play a key role in the disease process, and collagenase and stromelysin (the latter of which is responsible for proteoglycan degradation) in particular play primary roles in the degradation of the extracellular matrix (Lawrence and Helmick, 1998).
In this randomized, double blind, placebo-controlled clinical trial in patients with radiologically confirmed osteoarthritis of the knee, we investigated the efficacy of AIF. AIF is composed of three herbal constituents, including Panax notoginseng (Burk.) F. H. Chen., Rehmannia glutinosa Libosch, and Eleutherococcus senticosus, which have traditionally been used in East Asia for many ages and have known for analgesic, anti-inflammatory, and anti-osteolytic effects. Based on its suppressive effects on matrix metalloproteinase (MMP)-13 and TNF-α in a collagen-induced arthritis mouse model (Chang, 2005), we conducted this clinical trial to determine the effects of new herbal formulation of these herbs. The recommended daily doses of these three herbs in the compendium of oriental medicine are 9 g, 15 g, and 27 g equivalent to extracts in four capsules of AIF, 904 mg, 723.2 mg, 180.8 mg of three herbs, respectively, which were administered to patients. In view of the doses administered and the absence of adverse events related to these three herbs in preclinical studies, we believe that the trial dose is safe.
The optimal management of osteoarthritis requires an adequate combination of non-pharmacological and pharmacological treatment modalities. Because of the well-known toxicity profiles of NSAID and analgesics, supplementary drugs or health food supplements, such as avocado/soybean, diacerein, glucosamine and chondroitin, have widely been used (McAlindon et al, 2000; Pelletier et al, 2000; Christensen et al, 2008).
Because of the high rate of response to placebo in pain perception (60.1%), it is usually difficult to discern significant results by comparing the subjective pain via exploratory analysis as VAS (Clegg et al, 2006). In the present experiment, there was no significant difference in VAS between groups because of significantly high placebo effect in placebo group between week 0 and 6 (p<0.01), nevertheless, the administration of AIF for 6 weeks improved VAS, since the rate of response to AIF was 4.76 percentage points higher at 6 weeks than placebo group, and significantly improved K-WOMAC scores.
In conclusion, AIF was shown to be effective at improving pain and knee function in osteoarthritis patients, as assessed by using the VAS and K-WOMAC scale, and was found to have an acceptable safety profile. Therefore, it may be an applicable candidate as supplementary drugs for symptomatic osteoarthritis patients. Long term evaluation using different doses of AIF with large numbers of participants is recommended to accurately and precisely determine the effects of this formulation on prevention and treatment of osteoarthritis.
ACKNOWLEDGEMENTS
We would like to thank Oscotec Inc. for financial support for this study.
ABBREVIATIONS
- AIF
antiinflammatory factor
- NSAIDs
non-steroidal antiinflammatory drugs
- TNF
tumor necrosis factor
- WOMAC
Western Ontario and McMaster University
References
- 1.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 2.Bae SC, Lee HS, Yun HR, Kim TH, Yoo DH, Kim SY. Cross-cultural adaptation and validation of Korean Western Ontario and McMaster Universities (WOMAC) and Lequesne osteoarthritis indices for clinical research. Osteoarthritis Cartilage. 2001;9:746–750. doi: 10.1053/joca.2001.0471. [DOI] [PubMed] [Google Scholar]
- 3.Chang SH, Choi Y, Park JA, Jung DS, Shin J, Yang JH, Ko SY, Kim SW, Kim JK. Anti-inflammatory effects of BT-201, an n-butanol extract of Panax notoginseng, observed in vitro and in a collagen-induced arthritis model. Clin Nutr. 2007;26:785–791. doi: 10.1016/j.clnu.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Chang SH, Sung HC, Choi Y, Ko SY, Lee BE, Baek DH, Kim SW, Kim JK. Suppressive effect of AIF, a water extract from three herbs, on collagen-induced arthritis in mice. Int Immunopharmacol. 2005;5:1365–1372. doi: 10.1016/j.intimp.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Christensen R, Bartels EM, Astrup A, Bliddal H. Symptomatic efficacy of avocado-soybean unsaponifiables (ASU) in osteoarthritis (OA) patients: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2008;16:399–408. doi: 10.1016/j.joca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, Bradley JD, Bingham CO, Weisman MH, Jackson CG, Lane NE, Cush JJ, Moreland LW, Schumacher HR, Jr., Oddis CV, Wolfe F, Molitor JA, Yocum DE, Schnitzer TJ, Furst DE, Sawitzke AD, Shi H, Brandt KD, Moskowitz RW, Williams HJ. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354:795–808. doi: 10.1056/NEJMoa052771. [DOI] [PubMed] [Google Scholar]
- 7.Garstang SV, Stitik TP. Osteoarthritis: epidemiology, risk factors, and pathophysiology. Am J Phys Med Rehabil. 2006;85:S2–S11. doi: 10.1097/01.phm.0000245568.69434.1a. [DOI] [PubMed] [Google Scholar]
- 8.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HM, An CS, Jung KY, Choo YK, Park JK, Nam SY. Rehmanniaglutinosa inhibits tumour necrosis factor-alpha and interleukin- 1 secretion from mouse astrocytes. Pharmacol Res. 1999;40:171–176. doi: 10.1006/phrs.1999.0504. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, Heyse SP, Hirsch R, Hochberg MC, Hunder GG, Liang MH, Pillemer SR, Steen VD, Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Ling S, Nheu L, Dai A, Guo Z, Komesaroff P. Effects of four medicinal herbs on human vascular endothelial cells in culture. Int J Cardiol. 2008;128:350–358. doi: 10.1016/j.ijcard.2007.05.111. [DOI] [PubMed] [Google Scholar]
- 12.Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 2004;12(Suppl A):S31–S33. doi: 10.1016/j.joca.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 13.McAlindon TE, LaValley MP, Gulin JP, Felson DT. Glucosamine and chondroitin for treatment of osteoarthritis: a systematic quality assessment and meta-analysis. Jama. 2000;283:1469–1475. doi: 10.1001/jama.283.11.1469. [DOI] [PubMed] [Google Scholar]
- 14.Ofman JJ, MacLean CH, Straus WL, Morton SC, Berger ML, Roth EA, Shekelle P. A metaanalysis of severe upper gastrointestinal complications of nonsteroidal antiinflammatory drugs. J Rheumatol. 2002;29:804–812. [PubMed] [Google Scholar]
- 15.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelletier JP, Yaron M, Haraoui B, Cohen P, Nahir MA, Choquette D, Wigler I, Rosner IA, Beaulieu AD. Efficacy and safety of diacerein in osteoarthritis of the knee: a double-blind, placebo-controlled trial. The Diacerein Study Group. Arthritis Rheum. 2000;43:2339–2348. doi: 10.1002/1529-0131(200010)43:10<2339::AID-ANR23>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 17.Spiker B, Kraemer HC, Scott DT, Gross RT. Design issues in a randomized clinical trial of a behavioral intervention: Insights from the infant health and development program. J Dev Behav Pediatr. 1992;12:386–393. [PubMed] [Google Scholar]
- 18.World Medical Association Declaration of Helsinki: Recommendations Guiding Medical Doctors in Biomedical Research Involving Human Subjects. Available at: http://www.net. [DOI] [PubMed]
- 19.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P. OARSI Recommendations for the management of hip and knee osteoarthritis. part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]