Abstract
This study was undertaken to elucidate the underlying mechanisms of ATP depletion-induced membrane transport dysfunction and cell death in renal proximal tubular cells. ATP depletion was induced by incubating cells with 2.5 mM potassium cyanide (KCN)/0.1 mM iodoacetic acid (IAA), and membrane transport function and cell viability were evaluated by measuring Na+-dependent phosphate uptake and trypan blue exclusion, respectively. ATP depletion resulted in a decrease in Na+-dependent phosphate uptake and cell viability in a time-dependent manner. ATP depletion inhibited Na+-dependent phosphate uptake in cells, when treated with 2 mM ouabain, a Na+ pump-specific inhibitor, suggesting that ATP depletion impairs membrane transport functional integrity. Alterations in Na+-dependent phosphate uptake and cell viability induced by ATP depletion were prevented by the hydrogen peroxide scavenger such as catalase and the hydroxyl radical scavengers (dimethylthiourea and thiourea), and amino acids (glycine and alanine). ATP depletion caused arachidonic acid release and increased mRNA levels of cytosolic phospholipase A2 (cPLA2). The ATP depletion-dependent arachidonic acid release was inhibited by cPLA2 specific inhibitor AACOCF3. ATP depletion-induced alterations in Na+-dependent phosphate uptake and cell viability were prevented by AACOCF3. Inhibition of Na+-dependent phosphate uptake by ATP depletion was prevented by antipain and leupetin, serine/cysteine protease inhibitors, whereas ATP depletion-induced cell death was not altered by these agents. These results indicate that ATP depletion-induced alterations in membrane transport function and cell viability are due to reactive oxygen species generation and cPLA2 activation in renal proximal tubular cells. In addition, the present data suggest that serine/cysteine proteases play an important role in membrane transport dysfunction, but not cell death, induced by ATP depletion.
Keywords: ATP depletion, Membrane transport, cPLA2, Serine/cysteine proteases
INTRODUCTION
Renal ischemia is a common cause of acute renal failure. Despite numerous experimental and clinical studies on renal ischemia, the prognosis for patients with ischemic acute renal failure remains poor (Devarajan, 2006). Ischemia/reperfusion injury is characterized by ATP depletion, followed by subsequent activation of a number of critical alterations in metabolism (Dagher, 2000; Devarajan et al, 2003; Nangaku & Eckardt, 2007).
Although ischemia in vitro and in vivo has been known to induce generate reactive oxygen species (ROS) (Weinberg, 1991; Goto et al, 1999; Kim et al, 2002), the role of ROS in the pathogenesis of ischemia-induced cell injury is not fully understood. Several workers have postulated that ROS play a critical role in renal cell injury induced by hypoxia in vitro (Paller, 1994; Kim et al, 2002) and ischemia in vivo (Paller, 1994; Devarajan, 2006), whereas, others have reported that ROS do not play any significant role in the pathogenesis of cell injury induced by hypoxia in vitro (Borkan & Schwartz, 1989; Watabe & Nakaki, 2007) and ischemia in vivo (Joannidis et al, 1990; Kim et al, 1999; Nistico et al, 2008). We previously observed that the role of ROS in antimycin A-induced cell death is different between cultured renal proximal tubular cells and renal cortical slices (Kim et al, 2002). The cell death in cortical slices was prevented by antioxidants, whereas the cultured cell death was not altered.
Accumulation of unesterified fatty acids, especially arachidonic acid, occurs during ischemic or hypoxic injury in the kidney (Matthys et al, 1984; Humes et al, 1989; Bunnachak et al, 1994). This accumulation of free fatty acids is attributed to the degradation of membrane phospholipids by activation of phospholipase A2 (PLA2) (Bonventre, 1993; Portilla & Creer, 1995). Although activation of PLA2 has been suggested to be an important mediator of ischemic or hypoxic tubular cell injury (Bonventre, 1993; Portilla et al, 1994; Choi et al, 1995; Choi et al, 1999), the role of PLA2 activation in hypoxic cell injury remains controversial. PLA2 exerts cytoprotective effect (Zager et al, 1996) or no effect (Schnellmann et al, 1994) against hypoxic- and antimycin A-induced proximal tubular cell injury. Most studies have been focused on the role of PLA2 activation in cell death during renal ischemia, but its role in hypoxia-mediated membrane transport dysfunction remains unclear.
Cysteine proteases have been implicated in ischemic and toxicant-induced cell death in various cell types including the kidney (Nicotera et al, 1989; Weinberg, 1991; Yang & Schnellmann, 1996; Seyfried et al, 2001). However, very little has been known on the role of cysteine proteases in hypoxia-induced membrane transport dysfunction.
The present study was, therefore, undertaken to elucidate the role of ROS, PLA2 activation, and cysteine proteases in membrane transport dysfunction and cell death induced by ATP depletion, an in vitro model of ischemia, in primary cultured renal proximal tubular cells. Previous studies have shown that renal tubular cells respond with a sensitivity similar to both KCN/IAA and hypoxia (Lash et al, 1996). The data obtained herein indicated that the membrane transport dysfunction and cell death induced by ATP depletion are dependent on ROS generation and PLA2 activation. In addition, the membrane transport dysfunction, but not the cell death, was prevented by cysteine protease inhibitors.
METHODS
Culture of rabbit proximal tubular cells
Proximal tubular cells were isolated as previously described (Kim et al, 2002). In brief, adult New Zealand male white rabbits were killed by cervical dislocation. Their kidneys were removed immediately, cleaned of fat and debris, and washed with sterile antibiotic-supplemented medium. The kidneys were perfused with phosphate buffered saline (pH 7.4) through the renal artery and subsequently with DMEM/F12 containing 0.5% (wt/vol.) iron oxide until the kidneys turned a grey-black in color. The cortex was removed and homogenized with 4 strokes of a sterile glass homogenizer. The homogenate was passed through a series of sterile nylon mesh sieves (254 and 85 µm: TETCO, Inc., Depew, NY). Tubules and glomeruli retained on the 85-µm sieve were suspended in a tube containing DMEM/F12 medium and magnetic stirring bar. Glomeruli containing iron oxide were attracted to the magnetic stirring bar. The stirring bar was removed from the solution. The isolated proximal tubules were incubated briefly in DMEM/F12 medium containing 80 µg/ml of collagenase A and 0.025% soybean trypsin inhibitor. Then the dissociated tubules were washed by centrifugation, resuspended in DMEM/F12 medium, and transferred into tissue culture plates. Proximal tubule cells were grown on 24-well tissue culture plates in DMEM/F12 medium supplemented with bovine insulin (5 µg/ml), human transferrin (5 µg/ml) and hydrocortisone (5×10-8 M). The cultures were maintained in a humidified 95% air/5% CO2 incubator at 37℃. Culture medium was changed every 24 hr and 48 hr before each experiment. All experiments started 10~13 days after plating when a confluent monolayer culture was achieved.
ATP depletion
For ATP depletion, cells were treated with 2.5 mM potassium cyanide (KCN)/0.1 mM iodoacetic acid (IAA) in a glucose-free medium. The composition of the incubation medium was 115 mM NaCl, 5 mM KCl, 25 mM NaHCO3, 2 mM NaH2PO4, 1 mM MgSO4, and 1 mM CaCl2 (pH 7.4). Following the incubation, phosphate uptake and cell viability were measured as described below. Unless otherwise stated, cells were treated with KCN/IAA for 120 min at 37℃.
Measurement of Na+-dependent phosphate uptake
The uptake of solutes was determined in cell monolayers grown on 24 well plates. After an exposure to ATP depletion, the reaction buffer was removed and washed twice with the uptake buffer containing the following (in mM): 137 NaCl, 5.4 KCl, 2.8 CaCl2, 1.2 MgSO4, and 10 Hepes (pH 7.4). The cells were incubated for 60 min at 37℃ in the uptake buffer containing 5 µM [32P]-phosphate. At the end of the incubation, the cells were washed three times with ice-cold uptake buffer and solubilized in 0.5 ml of 0.2% Triton X-100. Aliquots of each sample were transferred to scintillation vials and the radioactivity was counted in a liquid scintillation counter (TRI-CARB 2100TR, Packard, USA). Protein was measured by the method of Bradford (Bradford, 1976).
Measurement of cell viability
The cells viability was determined by a trypan blue exclusion assay. Cells were harvested using 0.025% trypsin, incubated with 4% trypan blue solution, and were counted using a hemocytometer under light microscope. Cells failing to exclude the dye were considered nonviable, and the number of nonviable cell was expressed as a percentage of the total cells.
Measurement of ROS production
The intracellular generation of ROS was measured using 2',7'-dichlorofluorescein diacetate (DCFH-DA). The non-fluorescent ester penetrates into the cells and is hydrolyzed to DCFH by the cellular esterases. The probe (DCFH) is rapidly oxidized to the highly fluorescent compound 2',7'-dichlorofluorescein (DCF) in the presence of cellular peroxidase and ROS such as hydrogen peroxide or fatty acid peroxides. Cells were cultured in 35-mm tissue culture petri dishes. The culture medium was removed, and the cells were collected from flasks using trypsin-EDTA solution. The cells were washed twice with DMEM/F12, and were suspended in glucose-free HBSS for fluorescence analysis. The reaction was carried out in a fluorescent cuvette. The cells were preincubated for 10 min at 37℃ in fluorescent cuvette containing 3 ml of glucose-free HBSS with 20 µM DCFH-DA (from a stock solution of 20 mM DCFH-DA in ethanol). After the preincubation, the cells were treated with KCN/IAA and incubated up to 120 min during which the fluorescent intensity was monitored on a spectrofluorometer (SPEX1681, SPEX Co., USA) with excitation wave length of 485 nm and emission wave length of 530 nm. The net increase in DCF fluorescence (arbitrary units) was calculated by taking the difference between the values before and after addition of KCN/IAA.
Measurement of arachidonic acid (AA) release.
Confluent cells were incubated with 3H-AA (2 µCi/ml) in DMEM/F12 for 20 hr. After incubation, cells were gently washed three times with HBSS (pH 7.4) to remove unbound 3H-AA. To measure ATP depletion-induced AA release, cells were exposed to KCN/IAA for appropriate times. The medium was removed and centrifuged, and the radioactivity in 400 µl of supernatant was measured in a liquid scintillation counter (Packard Tricarb A2100). Cells were solubilized with 0.1% deoxycholate and the radioactivity in 400 µl of cell lysate was measured. The amount of 3H-AA released into the medium was expressed as a percent of total (cell-associated plus released).
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from cells with Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Equal amounts of total RNA were reverse transcribed by using a oligo (dT) primer and reverse transcriptase (Promega, Madison, WI, USA), and the synthesized cDNA was then used as a template for the PCR reaction. PCR primers were used to amplify cPLA2 (forward 5'-AACATTCAGGCAGCAGAGGA-3' and reverse 5'-CGTAGGTCGCACAATCCAA-3') and GAPDH (forward 5'-GGGTGTGAACCACGAGAAAT-3' and reverse 5'-TTCAGCTCTGGGATGACCTT-3'). PCR was carried out under the following conditions: the initial denaturation 95℃ for 3 min, followed by 35 cycles of denaturation at 95℃ for 30 s, annealing for 30 s, extension at 72℃ for 30 s and additional extension at 72℃ for 5 min. The PCR products were analyzed by 1.2% agarose gel electrophoresis.
Chemicals
Catalase, dimethylthiourea (DMTU), thiourea, antipain, leupetin, and ouabain were purchased from Sigma-Aldrich Chemical (St. Louis, MO). AACOCF3 was purchased from Calbiochem (San Diego, CA, USA). DCFH-DA was obtained from Molecular Probes (Eugene, OR, USA). All other chemicals were of the highest commercial grade available.
Statistical analysis
Data are expressed as mean±SEM. Comparison between two groups was made using the unpaired t test. Multiple group comparisons were done using one-way analysis of variance followed by the Turkey post hoc test. p<0.05 was considered statistically significant.
RESULTS
Reduction of phosphate uptake and cell viability by ATP depletion
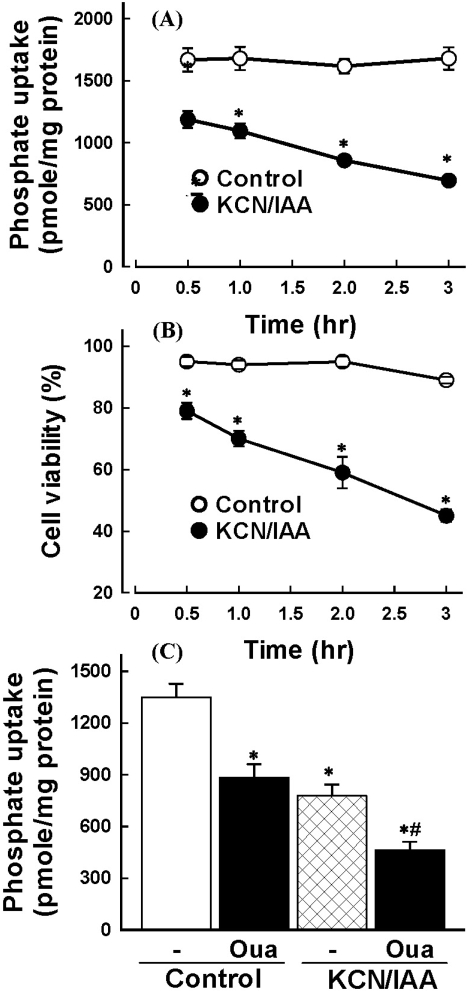
To determine the time course of ATP depletion-induced alterations in Na+-dependent phosphate uptake and cell viability, cells were exposed to 2.5 mM KCC/0.1 mM IAA for various times (0~3 hr). Fig. 1A and B shows that the Na+-dependent phosphate uptake and cell viability were decreased in a time-dependent manner.
Fig. 1.
Effect of ATP depletion on Na+-dependent phosphate uptake (A) and cell viability (B) in primary cultured renal proximal tubular cells. Cells were exposed to medium with 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) or without (Control) for 0~3 hr at 37℃. Na+-dependent phosphate uptake and cell viability were measured as described in 'Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. *p<0.05 compared with control. (C) Effect of ATP depletion on Na+-dependent phosphate uptake in primary cultured renal proximal tubular cells which were treated with ouabain. Cells were exposed to medium with 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) or without (Control) in the presence or absence of 2 mM ouabain for 120 min at 37℃. Na+-dependent phosphate uptake was measured as described in 'Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. *p<0.05 compared with control; #p<0.05 compared with ouabain alone.
Since phosphate is transported across the brush border membrane via a Na+-dependent active mechanism in renal proximal tubular cells, the driving force of active phosphate uptake is the Na+ gradient across the membrane generated by Na+-pump. Thus, reduction of Na+-dependent phosphate uptake in cells exposed to KCN/IAA could be attributed to a decrease in the driving force by inhibition of Na+-pump rather than impairment of membrane transport functional integrity. To test the possibility, the effect of ATP depletion on Na+-dependent phosphate uptake was examined in the absence or presence of 2 mM ouabain. As shown in Fig. 1C, treatment of primary cultured renal proximal tubular cells with ouabain partially inhibited Na+-dependent phosphate uptake. Treatment of the cells with KCN/IAA in the presence of ouabain significantly attenuated Na+-dependent phosphate uptake further, suggesting that ATP depletion induces membrane transport dysfunction through Na+-pump-independent mechanism.
Role of ROS in ATP depletion-induced alterations in membrane transport function and cell viability
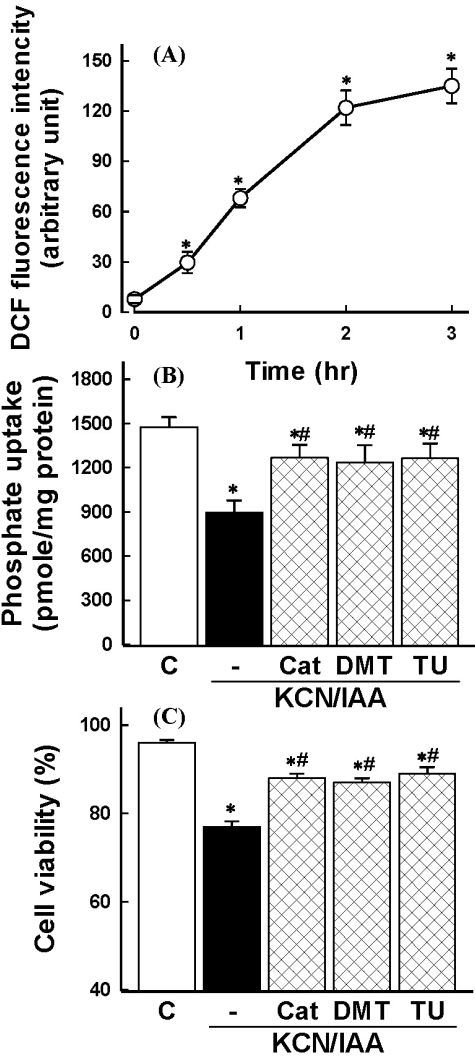
ROS production was examined in cells exposed to ATP depletion using DCFH-DA. ATP depletion induced an increase of ROS generation in a time-dependent manner (Fig. 2A). Involvement of ROS in ATP depletion-induced alterations in Na+-dependent phosphate uptake and cell viability was evaluated using catalase, the hydrogen peroxide scavenger, and the hydroxyl radical scavengers, such as DMTU and thiourea. As shown in Fig. 2B and C, these agents could reverse the ATP depletion-induced alterations, possibly supporting the notion that hydrogen peroxide and hydroxyl radicals are responsible for reduction in Na+-dependent phosphate uptake and cell viability induced by ATP depletion.
Fig. 2.
(A) Time course of reactive oxygen species (ROS) generation in primary cultured renal proximal tubular cells during exposure to ATP depletion. Cells were exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) for various times and ROS generation was measured. Shown is the net increase of DCF fluorescence (arbitrary units) calculated by subtracting the values for the control cells from the corresponding values for KCN/IAA-treated cells. Data are mean±SEM of three independent experiments performed in duplicate. *p<0.05 compared with zero (0) time. (B-C) Effect of radical scavengers on inhibition of phosphate uptake (B) and cell death (C) induced by ATP depletion. Cells were exposed to KCN/IAA in the presence or absence of 500 units/ml catalase (Cat), 30 mM dimethylthiourea (DMTU), or 30 mM thiourea (TU) for 3 hr at 37℃. Na+-dependent phosphate uptake and cell viability were measured as described in 'Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. *p<0.05 compared with control; #p<0.05 compared with KCN/IAA alone.
Effect of amino acids on ATP depletion-induced cell injury
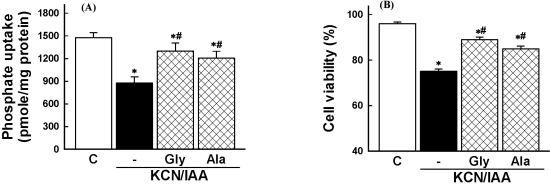
It has been demonstrated that several amino acids such as glycine and alanines protect cells from hypoxic injury induced by oxygen deprivation (Weinberg, 1991; Wetzels et al, 1993; Choi et al, 1999). To determine whether amino acids prevent the ATP depletion-induced cell injury, the effect of glycine and alanine on the cell injury was investigated. As shown in Fig. 3, the reduction of Na+-dependent phosphate uptake and cell death which were induced by ATP depletion was attenuated by these amino acids.
Fig. 3.
Effects of amino acids on inhibition of phosphate uptake (A) and cell death induced by ATP depletion, (B) in primary cultured renal proximal tubular cells. Cells were exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) in the presence or absence of 5 mM glycine (Gly) or 5 mM alanine (Ala) for 3 hr at 37℃. Na+-dependent phosphate uptake and cell viability were measured as described in 'Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. *p<0.05 compared with control; #p<0.05 compared with KCN/IAA alone.
Role of PLA2 activation in ATP depletion-induced alterations in membrane transport function and cell viability
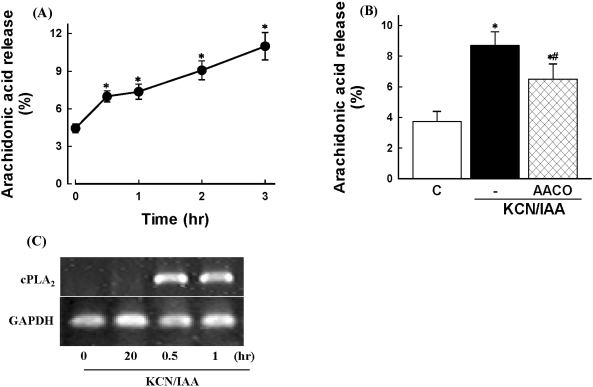
To determine if ATP depletion induces PLA2 activation, [3H]AA release was measured in prelabeled cells. ATP depletion caused an increase of AA release in a time-dependent manner (Fig. 4A). The AA release by ATP depletion was inhibited by the PLA2 inhibitor AACOCF3 (Fig. 4B). Similar results were also obtained from RT-PCR of cPLA2 mRNA. As shown in Fig. 4C, cPLA2 mRNA was increased 30 min after ATP depletion. These data suggest that ATP depletion induces cPLA2 activation in renal proximal tubular cells.
Fig. 4.
Effects of ATP depletion on arachidonic acid release in primary cultured renal proximal tubular cells. Cells were prelabeled with [3H]arachidonic acid for 20 hr, washed, and exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) for various times (A) or for 3 hr in the presence or absence of 20 µM AACOCF3 (B). Data are mean±SEM of four independent experiments performed in duplicate. *p<0.05 compared with zero time (A) and control (B); #p<0.05 compared with KCN/IAA alone. (C) RT-PCR analysis of cPLA2 mRNA in cells subjected to ATP depletion. Cells were exposed to KCN/IAA for 0.2, 0.5, and 1 hr, and mRNA levels of were measured as described in 'Materials and Methods'.
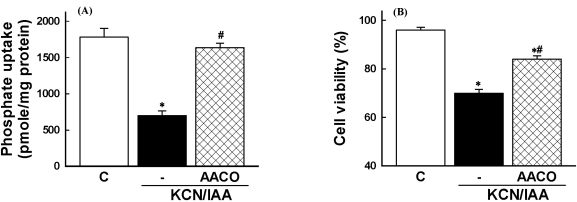
Involvement of PLA2 activation in ATP depletion-induced membrane transport dysfunction and cell death was evaluated using the PLA2 inhibitor. Thus, cells were exposed to ATP depletion in the presence of AACOCF3, a trifluoromethyl ketone analogue of arachidonyl acid which inhibits the 85-kD cPLA2 (Street et al, 1993). As shown in Fig. 5, the reduction of Na+-dependent phosphate uptake and the cell death was attenuated by the inhibitor. These data indicate that PLA2 is involved in ATP depletion-induced alterations in membrane transport function and cell viability.
Fig. 5.
Effects of phospholipase A2 inhibitor on inhibition of Na+-dependent phosphate uptake (A) and cell death (B), induced by ATP depletion, in primary cultured renal proximal tubular cells. Cells were exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) in the presence or absence of 20 µM AACOCF3 for 3 hr at 37℃. Na+-dependent phosphate uptake and cell viability were measured as described in 'Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. #p<0.05 compared with control; #p<0.05 compared with KCN/IAA alone.
Effect of serine/cysteine protease inhibitors on ATP depletion-induced alterations in membrane transport function and cell viability
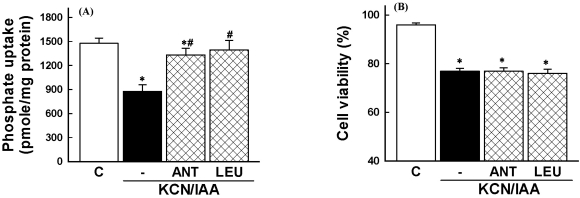
To examine the role of serine/cysteine proteases in ATP depletion-induced cell injury, cells were exposed to ATP depletion in the presence of serine/cysteine protease inhibitors (antipain and leupetin). Fig. 6 shows that these inhibitors prevented the ATP depletion-induced impairment in Na+-dependent phosphate uptake, but the cell death was not affected by these inhibitors (Fig. 6).
Fig. 6.
Effects of serine/cysteine protease inhibitors on inhibition of phosphate uptake (A) and cell death (B), induced by ATP depletion, in primary cultured renal proximal tubular cells. Cells were exposed to 2.5 mM potassium cyanide/0.1 mM iodoacetic acid (KCN/IAA) in the presence or absence of 50 µM antipain (ANT) or 100 µM leupetin (LEU) for 3 hr at 37℃. Na+-dependent phosphate uptake and cell viability were measured as described in 'Materials and Methods'. Data are mean±SEM of four independent experiments performed in duplicate. *p<0.05 compared with control; #p<0.05 compared with KCN/IAA alone.
DISCUSSION
Besides oxygen deprivation, an alternative method to induce ATP depletion is to use metabolic inhibitors, such as simultaneous treatment of cells with potassium cyanide (KCN) and iodoacetic acid (IAA). Such an experimental approach has extensively been used in studies of toxicological effects of ATP depletion in hepatocytes (Gores et al, 1989). By inhibiting ATP generation by both oxidative phosphorylation and glycolysis, rapid and substantial depletion of intracellular ATP can be achieved (Gores et al, 1988; Lash et al, 1996).
Although ROS generation has been implicated in the pathogenesis of ischemic acute renal failure (Halliwell et al, 1992; Devarajan, 2006), several studies have shown that ROS do not mediate the ischemic injury (Weinberg et al, 1987; Kim et al, 1999). Previous studies have shown that ROS generation plays an important role in ATP depletion- induced cell death (Hagar et al, 1996; Kim et al, 2002). However, it is unclear whether ROS generation by ATP depletion is responsible for membrane transport dysfunction. The present study demonstrated that ATP depletion induced ROS generation and ROS scavengers prevented the reduction of Na+-dependent phosphate uptake and cell death by ATP depletion (Fig. 2). These data suggest that ATP depletion-induced alterations in membrane transport function and cell viability are mediated by ROS generation.
Cytoprotective effect of amino acids such as glycine and alanine against ischemia/reperfusion injury in vivo has been reported in various tissues and organs including kidney, liver, and heart (Weinberg, 1991; Yin et al, 2002; Zhong et al, 2003; Habib et al, 2006). Amino acids also prevent hypoxic/ATP depletion-induced injury in vitro in renal epithelial cells (Pan et al, 2005; Zager et al, 2007). Consistent with these reports, the present study showed that impairment of Na+-dependent phosphate uptake and cell death was prevented by addition of glycine and alanine (Fig. 3).
Increased accumulation of free fatty acids, particularly unsaturated fatty acids such as arachidonic acid, is believed to mediate cell injury in renal proximal tubules (Humes et al, 1989; Wetzels et al, 1993). The initial step of the arachidonic acid cascade is mediated by PLA2 that hydrolyses the sn-2 fatty acyl ester bond of glycerophospholipids to free fatty acid and lysophospholipids. At present, three main types of PLA2 are identified, namely, the 85-kD cytosolic Ca2+-dependent PLA2 (cPLA2), cytosolic Ca2+-independent PLA2 (iPLA2), and the secretary form of PLA2 (sPLA2) (Denis et al, 1994). PLA2 is activated during hypoxia in vitro (Portilla et al, 1992; Choi et al, 1995) and ischemia in vivo (Goto et al, 1999). However, the role of PLA2 in hypoxic injury remains incompletely understood. Many reports have shown that activation of PLA2 plays an important role in the pathogenesis of membrane injury during hypoxia which is induced by oxygen deprivation or chemicals (Portilla et al, 1994; Choi et al, 1995; Wang et al, 1996; Edelstein et al, 1997). By contrast, Zager group (1993; 1996) found that PLA2 exert cytoprotective effect against renal proximal tubules subjected to hypoxia/reoxygenation- and ATP depletion-mediated injury. In the present study, we examined the role of PLA2 in ATP depletion-induced cell injury using the cPLA2 specific inhibitor AACOCF3. Exposure of cells to ATP depletion induced arachidonic acid release and an increase of mRNA levels of cPLA2, which was inhibited by the cPLA2 inhibitor AACOCF3 (Fig. 4), suggesting that ATP depletion causes cPLA2 activation.
Activation of PLA2 has been implicated in an important biochemical mechanism responsible for accelerated phospholipids hydrolysis during renal ischemia (Nakamura et al, 1991; Weinberg, 1991). In this context, the membrane transport function may affect the activation of PLA2. Indeed, our data showed that the cPLA2 specific inhibitor completely prevented the impaired Na+-dependent phosphate uptake by ATP depletion, while it partially prevented the ATP depletion-induced cell death (Fig. 5). These results indicate that activation of cPLA2 is involved in ATP depletion- induced cell injury.
Proteases in lysosomes, in the cytosol or associated with the cell membrane could contribute to cell injury during ischemia (Weinberg, 1991). It has been reported that serine/ cysteine protease inhibitors prevent ischemia-induced cell injury (Bolli et al, 1983; Weinberg, 1991; Seyfried et al, 2001). However, our data indicated that serine/cysteine protease inhibitors, antipain and leupetin, exerted the protective effect against membrane transport dysfunction induced by ATP depletion, while the cell death was not altered by these inhibitors (Fig. 6). Although the underlying mechanism of differential effect of serine/cysteine protease inhibitors on membrane transport dysfunction and cell death remains to be defined, the data that these inhibitors did not affect the ATP depletion-induced cell death are consistent with those reported by other investigators in renal proximal tubular cells and hepatocytes (Nieminen et al, 1992; Yang & Schnellmann, 1996). Since antipain and leupetin are also protease inhibitors active against calpain (Weinberg, 1991), cytoprotective effect of these inhibitors may be attributed to the ability to inhibit calpain rather than lysosomal proteases. Calpains are intracellular Ca2+-dependent cysteine proteases that are released in the extracellular milieu by tubular epithelial cells following renal ischemia (Goll et al, 2003; Frangie et al, 2006). Calpains are considered as a key mediator of ischemia/reperfusion injury (Chatterjee et al, 2005; Frangie et al, 2006) and calpain inhibitors prevent cell death induced by hypoxia/reoxygenation and ATP depletion in renal proximal tubular cells (Liu et al, 2001; Liu et al, 2002).
In conclusion, ATP depletion with KCN/IAA reduced Na+-dependent phosphate uptake and cell death dependent on ROS generation, which was prevented by some amino acids and cPLA2 inhibitor. The serine/cysteine protease inhibitors ameliorated the reduction of Na+-dependent phosphate uptake induced by ATP depletion, but not the cell death.
ABBREVIATIONS
- KCN
potassium cyanide
- IAA
iodoacetic acid
- cPLA2
cytosolic phospholipase A2
- ROS
reactive oxygen species
- DCFH-DA
2',7'-dichlorofluorescein diacetate
- DMTU
dimethylthiourea
References
- 1.Bolli R, Cannon RO, Speir E, Goldstein RE, Epstein SE. Role of cellular proteinases in acute myocardial infarction. I. Proteolysis in nonischemic and ischemic rat myocardium and the effects of antipain, leupeptin, pepstatin and chymostatin administered in vivo. J Am Coll Cardiol. 1983;2:671–680. doi: 10.1016/s0735-1097(83)80307-6. [DOI] [PubMed] [Google Scholar]
- 2.Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 3.Borkan SC, Schwartz JH. Role of oxygen free radical species in in vitro models of proximal tubular ischemia. Am J Physiol. 1989;257:F114–F125. doi: 10.1152/ajprenal.1989.257.1.F114. [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 5.Bunnachak D, Almeida AR, Wetzels JF, Gengaro P, Nemenoff RA, Burke TJ, Schrier RW. Ca2+ uptake, fatty acid, and LDH release during proximal tubule hypoxia: effects of mepacrine and dibucaine. Am J Physiol. 1994;266:F196–F201. doi: 10.1152/ajprenal.1994.266.2.F196. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee PK, Todorovic Z, Sivarajah A, Mota-Filipe H, Brown PA, Stewart KN, Mazzon E, Cuzzocrea S, Thiemermann C. Inhibitors of calpain activation (PD150606 and E-64) and renal ischemia-reperfusion injury. Biochem Pharmacol. 2005;69:1121–1131. doi: 10.1016/j.bcp.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Choi KH, Edelstein CL, Gengaro P, Schrier RW, Nemenoff RA. Hypoxia induces changes in phospholipase A2 in rat proximal tubules: evidence for multiple forms. Am J Physiol. 1995;269:F846–F853. doi: 10.1152/ajprenal.1995.269.6.F846. [DOI] [PubMed] [Google Scholar]
- 8.Choi WR, Ko SH, Cho SI, Woo JS, Jung JS, Lee SH, Kim YK. Role of phospholipase A2 in hypoxia-induced renal cell injury. Korean J Physiol Pharmacol. 1999;3:93–100. [Google Scholar]
- 9.Dagher PC. Modeling ischemia in vitro: selective depletion of adenine and guanine nucleotide pools. Am J Physiol Cell Physiol. 2000;279:C1270–C1277. doi: 10.1152/ajpcell.2000.279.4.C1270. [DOI] [PubMed] [Google Scholar]
- 10.Denis I, Pointillart A, Lieberherr M. Effects of growth hormone and insulin-like growth factor-I on the proliferation and differentiation of cultured pig bone cells and rat calvaria cells. Growth Regul. 1994;4:123–130. [PubMed] [Google Scholar]
- 11.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 12.Devarajan P, Mishra J, Supavekin S, Patterson LT, Steven Potter S. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab. 2003;80:365–376. doi: 10.1016/j.ymgme.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Edelstein CL, Ling H, Schrier RW. The nature of renal cell injury. Kidney Int. 1997;51:1341–1351. doi: 10.1038/ki.1997.183. [DOI] [PubMed] [Google Scholar]
- 14.Frangie C, Zhang W, Perez J, Dubois YC, Haymann JP, Baud L. Extracellular calpains increase tubular epithelial cell mobility. Implications for kidney repair after ischemia. J Biol Chem. 2006;281:26624–26632. doi: 10.1074/jbc.M603007200. [DOI] [PubMed] [Google Scholar]
- 15.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 16.Gores GJ, Flarsheim CE, Dawson TL, Nieminen AL, Herman B, Lemasters JJ. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am J Physiol. 1989;257:C347–C354. doi: 10.1152/ajpcell.1989.257.2.C347. [DOI] [PubMed] [Google Scholar]
- 17.Gores GJ, Nieminen AL, Fleishman KE, Dawson TL, Herman B, Lemasters JJ. Extracellular acidosis delays onset of cell death in ATP-depleted hepatocytes. Am J Physiol. 1988;255:C315–C322. doi: 10.1152/ajpcell.1988.255.3.C315. [DOI] [PubMed] [Google Scholar]
- 18.Gores GJ, Nieminen AL, Wray BE, Herman B, Lemasters JJ. Intracellular pH during "chemical hypoxia" in cultured rat hepatocytes. Protection by intracellular acidosis against the onset of cell death. J Clin Invest. 1989;83:386–396. doi: 10.1172/JCI113896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto S, Nakamura H, Morooka H, Terao Y, Shibata O, Sumikawa K. Role of reactive oxygen in phospholipase A2 activation by ischemia/reperfusion of the rat kidney. J Anesth. 1999;13:90–93. doi: 10.1007/s005400050032. [DOI] [PubMed] [Google Scholar]
- 20.Habib MM, Hodgson HJ, Davidson BR. The role of glycine in hepatic ischemia-reperfusion injury. Curr Pharm Des. 2006;12:2953–2967. doi: 10.2174/138161206777947605. [DOI] [PubMed] [Google Scholar]
- 21.Hagar H, Ueda N, Shah SV. Role of reactive oxygen metabolites in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Am J Physiol. 1996;271:F209–F215. doi: 10.1152/ajprenal.1996.271.1.F209. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants, and human disease: Where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 23.Humes HD, Nguyen VD, Cieslinski DA, Messana JM. The role of free fatty acids in hypoxia-induced injury to renal proximal tubule cells. Am J Physiol. 1989;256:F688–F696. doi: 10.1152/ajprenal.1989.256.4.F688. [DOI] [PubMed] [Google Scholar]
- 24.Joannidis M, Gstraunthaler G, Pfaller W. Xanthine oxidase: evidence against a causative role in renal reperfusion injury. Am J Physiol. 1990;258:F232–F236. doi: 10.1152/ajprenal.1990.258.2.F232. [DOI] [PubMed] [Google Scholar]
- 25.Kim SY, Kim CH, Yoo HJ, Kim YK. Effects of radical scavengers and antioxidant on ischemic acute renal failure in rabbits. Renal Failure. 1999;21:1–11. doi: 10.3109/08860229909066965. [DOI] [PubMed] [Google Scholar]
- 26.Kim YK, Lee SK, Ha MS, Woo JS, Jung JS. Differential role of reactive oxygen species in chemical hypoxia-induced cell injury in opossum kidney cells and rabbit renal cortical slices. Exp Nephrol. 2002;10:275–284. doi: 10.1159/000063702. [DOI] [PubMed] [Google Scholar]
- 27.Lash LH, Tokarz JJ, Chen Z, Pedrosi BM, Woods EB. ATP depletion by iodoacetate and cyanide in renal distal tubular cells. J Pharmacol Exp Ther. 1996;276:194–205. [PubMed] [Google Scholar]
- 28.Lash LH, Tokarz JJ, Chen Z, Pedrosi BM, Woods EB. ATP depletion by iodoacetate and cyanide in renal distal tubular cells. J Pharmacol Exp Ther. 1996;276:194–205. [PubMed] [Google Scholar]
- 29.Liu X, Harriman JF, Schnellmann RG. Cytoprotective properties of novel nonpeptide calpain inhibitors in renal cells. J Pharmacol Exp Ther. 2002;302:88–94. doi: 10.1124/jpet.302.1.88. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Rainey JJ, Harriman JF, Schnellmann RG. Calpains mediate acute renal cell death: role of autolysis and translocation. Am J Physiol Renal Physiol. 2001;281:F728–F738. doi: 10.1152/ajprenal.2001.281.4.F728. [DOI] [PubMed] [Google Scholar]
- 31.Matthys E, Patel Y, Kreisberg J, Stewart JH, Venkatachalam M. Lipid alterations induced by renal ischemia: pathogenic factor in membrane damage. Kidney Int. 1984;26:153–161. doi: 10.1038/ki.1984.149. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura H, Nemenoff RA, Gronich JH, Bonventre JV. Subcellular characteristics of phospholipase A2 activity in the rat kidney. Enhanced cytosolic, mitochondrial, and microsomal phospholipase A2 enzymatic activity after renal ischemia and reperfusion. J Clin Invest. 1991;87:1810–1818. doi: 10.1172/JCI115202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med. 2007;85:1325–1330. doi: 10.1007/s00109-007-0278-y. [DOI] [PubMed] [Google Scholar]
- 34.Nicotera P, McConkey DJ, Dypbukt JM, Jones DP, Orrenius S. Ca2+-activated mechanisms in cell killing. Drug Metab Rev. 1989;20:193–201. doi: 10.3109/03602538909103536. [DOI] [PubMed] [Google Scholar]
- 35.Nieminen AL, Gores GJ, Bond JM, Imberti R, Herman B, Lemasters JJ. A novel cytotoxicity screening assay using a multiwell fluorescence scanner. Toxicol Appl Pharmacol. 1992;115:147–155. doi: 10.1016/0041-008x(92)90317-l. [DOI] [PubMed] [Google Scholar]
- 36.Nistico R, Piccirilli S, Cucchiaroni ML, Armogida M, Guatteo E, Giampa C, Fusco FR, Bernardi G, Nistico G, Mercuri NB. Neuroprotective effect of hydrogen peroxide on an in vitro model of brain ischaemia. Br J Pharmacol. 2008;153:1022–1029. doi: 10.1038/sj.bjp.0707587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paller MS. The cell biology of reperfusion injury in the kidney. J Investig Med. 1994;42:632–639. [PubMed] [Google Scholar]
- 38.Pan C, Bai X, Fan L, Ji Y, Li X, Chen Q. Cytoprotection by glycine against ATP-depletion-induced injury is mediated by glycine receptor in renal cells. Biochem J. 2005;390:447–453. doi: 10.1042/BJ20050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Portilla D, Creer MH. Plasmalogen phospholipid hydrolysis during hypoxic injury of rabbit proximal tubules. Kidney Int. 1995;47:1087–1094. doi: 10.1038/ki.1995.155. [DOI] [PubMed] [Google Scholar]
- 40.Portilla D, Mandel LJ, Bar-Sagi D, Millington DS. Anoxia induces phospholipase A2 activation in rabbit renal proximal tubules. Am J Physiol. 1992;262:F354–F360. doi: 10.1152/ajprenal.1992.262.3.F354. [DOI] [PubMed] [Google Scholar]
- 41.Portilla D, Shah SV, Lehman PA, Creer MH. Role of cytosolic calcium-independent plasmalogen-selective phospholipase A2 in hypoxic injury to rabbit proximal tubules. J Clin Invest. 1994;93:1609–1615. doi: 10.1172/JCI117141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnellmann RG, Yang X, Carrick JB. Arachidonic acid release in renal proximal tubule cell injuries and death. J Biochem Toxicol. 1994;9:211–217. doi: 10.1002/jbt.2570090406. [DOI] [PubMed] [Google Scholar]
- 43.Seyfried DM, Veyna R, Han Y, Li K, Tang N, Betts RL, Weinsheimer S, Chopp M, Anagli J. A selective cysteine protease inhibitor is non-toxic and cerebroprotective in rats undergoing transient middle cerebral artery ischemia. Brain Res. 2001;901:94–101. doi: 10.1016/s0006-8993(01)02289-2. [DOI] [PubMed] [Google Scholar]
- 44.Street IP, Lin HK, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Harrison SD, Lemasters JJ, Herman B. Contribution of pH-dependent group II phospholipase A2 to chemical hypoxic injury in rat hepatocytes. FASEB J. 1996;10:1319–1325. doi: 10.1096/fasebj.10.11.8836046. [DOI] [PubMed] [Google Scholar]
- 46.Watabe M, Nakaki T. ATP depletion does not account for apoptosis induced by inhibition of mitochondrial electron transport chain in human dopaminergic cells. Neuropharmacology. 2007;52:536–541. doi: 10.1016/j.neuropharm.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg JM. The cell biology of ischemic renal injury. Kidney Int. 1991;39:476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg JM, Davis JA, Abarzua M, Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 1987;80:1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wetzels JF, Wang X, Gengaro PE, Nemenoff RA, Burke TJ, Schrier RW. Glycine protection against hypoxic but not phospholipase A2-induced injury in rat proximal tubules. Am J Physiol. 1993;264:F94–F99. doi: 10.1152/ajprenal.1993.264.1.F94. [DOI] [PubMed] [Google Scholar]
- 50.Wetzels JF, Yu L, Wang X, Kribben A, Burke TJ, Schrier RW. Calcium modulation and cell injury in isolated rat proximal tubules. J Pharmacol Exp Ther. 1993;267:176–180. [PubMed] [Google Scholar]
- 51.Yang X, Schnellmann RG. Proteinases in renal cell death. J Toxicol Environ Health. 1996;48:319–332. doi: 10.1080/009841096161221. [DOI] [PubMed] [Google Scholar]
- 52.Yin M, Zhong Z, Connor HD, Bunzendahl H, Finn WF, Rusyn I, Li X, Raleigh JA, Mason RP, Thurman RG. Protective effect of glycine on renal injury induced by ischemia-reperfusion in vivo. Am J Physiol Renal Physiol. 2002;282:F417–F423. doi: 10.1152/ajprenal.00011.2001. [DOI] [PubMed] [Google Scholar]
- 53.Zager RA, Burkhart KM, Conrad DS, Gmur DJ, Iwata M. Phospholipase A2-induced cytoprotection of proximal tubules: potential determinants and specificity for ATP depletion-mediated injury. J Am Soc Nephrol. 1996;7:64–72. doi: 10.1681/ASN.V7164. [DOI] [PubMed] [Google Scholar]
- 54.Zager RA, Johnson AC, Lund S, Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol. 2007;292:F304–F312. doi: 10.1152/ajprenal.00237.2006. [DOI] [PubMed] [Google Scholar]
- 55.Zager RA, Schimpf BA, Gmur DJ, Burke TJ. Phospholipase A2 activity can protect renal tubules from oxygen deprivation injury. Proc Natl Acad Sci USA. 1993;90:8297–8301. doi: 10.1073/pnas.90.17.8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]